Abstract
Purpose
Tourniquets are still widely used in total knee arthroplasty (TKA), although they may be associated with several adverse effects. An observer-blinded, randomized, controlled trial was performed to evaluate the effects of tourniquet use in TKA.
Methods
Fifty participants who underwent staged bilateral TKA were recruited for this study. The first-side TKA was randomly allocated to either long-duration tourniquet use or short-duration tourniquet use followed by a 3-month washout period and crossover to the other tourniquet strategy for the opposite-side TKA. Blood loss was monitored perioperatively. The operating time, allogeneic blood transfusion rate, thigh pain, knee pain, limb swelling, clinical outcome as measured by the Likert-type Western Ontario and McMaster University (WOMAC) score, straight leg raising and knee active range of motion (ROM) were also recorded.
Results
The long-duration tourniquet group exhibited reduced total blood loss [−99.1 ml, 95 % confidence interval (CI) −168.1 to −30.1, P = 0.0411] and intraoperative blood loss (−225.2 ml, 95 % CI −369.5 to −80.9, P = 0.0071) compared with the short-duration tourniquet group. However, there were greater postoperative blood loss (69.6 ml, 95 % CI 21.1 to 118.2, P = 0.0282) and hidden blood loss (52.8 ml, 95 % CI 10.5 to 95.1, P = 0.0332) in the long-duration tourniquet group. The short-duration tourniquet group showed better outcomes for thigh and knee pain, limb swelling, WOMAC score at 6-week follow-up, straight leg raising and knee ROM. Similar allogeneic blood transfusion rates were observed for both groups.
Conclusion
Total and intraoperative blood losses were reduced with the long-duration tourniquet use, whereas the short-duration tourniquet use would reduce postoperative and hidden blood losses without increasing the allogeneic blood transfusion rate. In addition, short-duration tourniquet use would result in faster recovery and less pain during the early rehabilitation period following TKA.
Level of evidence
I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tourniquets are widely used during total knee arthroplasty (TKA) to provide better visualization and to facilitate the cementing technique and other surgical procedures [26]. Moreover, the use of tourniquets may reduce the operating time [9], which may then decrease the incidence of infection [3].
However, tourniquets may also be associated with several disadvantages, including thigh pain, limb swelling, nerve palsy, muscle injury, postoperative stiffness and deep vein thrombosis (DVT) [3, 4, 9, 18, 19, 22, 28]. Additionally, whereas various studies have demonstrated that tourniquets effectively reduce blood loss [7, 29], others have demonstrated that tourniquets increase blood loss [1, 17, 28].
Several randomized controlled trials (RCTs) and meta-analyses comparing the effects of tourniquet use have been published [2, 3, 14, 22, 26, 28, 31]; however, no medical consensus regarding tourniquet use has been reached. Heterogeneous data were reported for various trials. Furthermore, most of these studies were not based on a crossover design. Therefore, we conducted an observer-blinded, randomized, controlled, crossover trial in which all the patients received staged bilateral TKA employing two different durations of tourniquet use with a 3-month washout period between the interventions, allowing each patient to serve as his or her own control to minimize confounding variables between the groups. In this study, the effect of tourniquet use on blood loss reduction and its impact on functional and clinical outcomes were evaluated.
The hypothesis was that applying long-duration tourniquet use would reduce the intraoperative blood loss but might increase the postoperative blood loss and the hidden blood loss, while applying short-duration tourniquet use would result in faster recovery and less pain during the early postoperative rehabilitative period.
Materials and methods
Before this study began, approval was obtained from the Ethics Committee of Peking University People’s Hospital, Beijing, China [Approval Number: (2013) IRB-Clinical-No. 17], and the study was registered at ClinicalTrials.gov (NCT02429713). Participants were recruited consecutively between January 2013 and June 2014 during a period of approximately 1.5 years. All patients provided written consent and were enrolled in the study in accordance with the Consolidated Standards of Reporting Trials (CONSORT) and the Helsinki Declaration.
Fifty patients who were scheduled for staged bilateral TKA were recruited. The inclusion criteria were being 50 years or older and being classified as American Society of Anesthesiologists (ASA) class 1 or 2. The exclusion criteria included coagulopathy, uncontrolled hypertension, peripheral vascular disease, previous knee surgery and body mass index (BMI) >35.
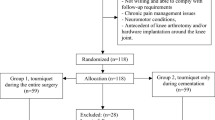
For each patient, the first-side TKA was randomly allocated to either long-duration tourniquet use (tourniquet inflated just before the incision and deflated after the hardening of the cement) or short-duration tourniquet use (tourniquet inflated just before the cement application and deflated after its hardening) followed by a 3-month washout period and crossover to the other tourniquet strategy for the opposite-side TKA operation (see Fig. 1).
The randomization sequence was generated by an independent statistician via a computer-generated random-number table. The randomization numbers were contained in sealed, opaque envelopes and were retained by office staff who were not involved in outcome measurements. The observers collecting the data after surgery were uninvolved in the experimental operations and were unaware of the intervention assignments.
Each patient received the same protocol of intraoperative tranexamic acid injection, spinal anaesthesia, postoperative pain treatment and rehabilitation. Both groups had an appropriately sized thigh tourniquet applied. The tourniquet was inflated with the patient’s leg fully extended to a pressure that equaled the systolic blood pressure plus 100 mmHg for both groups.
All the operations were performed by the same surgeon using the same type of cemented prosthesis (NexGen, Complete Knee Solution, Zimmer, Warsaw, IN, USA). A medial parapatellar approach was used in all the arthroplasties. An intramedullary guide was used for both the femoral and tibial cuts. Anchorage holes were drilled into the tibial plateau to increase the contact area between bone and cement. High-pressure pulsatile lavage was used to remove blood and to provide better cement interdigitation. For both groups, blood collected during the operation was re-infused. A non-suction wound drainage system was used in both groups and removed 48 h after surgery. After the joint capsule was closed, tranexamic acid (1 g) was injected into the capsule to control bleeding.
Oral rivaroxaban (10 mg per day) was administered 12 h after surgery and was continued for 2 weeks after the operation as thrombosis prophylaxis. Absent patient contraindications, an oral non-steroidal anti-inflammatory drug (celecoxib, 200 mg twice a day) was routinely prescribed for pain control. Fluid replacement was standardized in both groups. The criteria for blood transfusion included haemoglobin (Hb) < 8 g/dl or Hb < 9 g/dl along with symptoms of anaemia, such as tachycardia, dyspnoea, hypotension and angina pectoris. Postoperative rehabilitation began on the second postoperative day and followed a standard protocol including muscle power training, passive and active range-of-motion training, and walking. This protocol was begun on postoperative day 2.
Outcome measurements
The primary outcome was perioperative blood loss. We tested the level of Hb and haematocrit (Hct) preoperatively and on days 1, 2, and 5, postoperatively. The decreases in Hb and Hct were defined as the difference between the lowest values and the preoperative baseline values. The blood volume of each patient was calculated according to a formula published by Nadler and colleagues which takes into account patient weight, height and sex [21]. Thus, multiplying the blood volume by the change in Hct yields the calculated total blood loss [23]. In addition, the volumes of intraoperative blood loss, postoperative blood loss, blood transfusion and hidden blood loss were also recorded. The increased weight of the gauze pads plus the volume in the aspirator bottle excluding the rinse represented the intraoperative blood loss. The postoperative blood loss was calculated by measuring the drainage volume and by weighing the dressings. Hidden blood loss was calculated according to a formula published by Gross [8], which is calculated by subtracting the intraoperative and postoperative blood losses from the calculated total blood loss and then adding the volume of blood re-infused or transfused.
Postoperative visual analog scale (VAS) scores were obtained for thigh and knee pain. Patients were asked to rate the pain in their thigh and knee on a scale of 0–10, with 0 representing no pain and 10 representing the worst pain imaginable. Thigh circumference (10 cm proximal to the superior border of the patella), knee circumference (midpoint of the patella) and calf circumference (20 cm proximal to the medial malleolus) were recorded to assess the degree of limb swelling. The degree of limb swelling was presented as a percentage compared with the preoperative measure. To ensure the reliability of our results, we used an oil pen that slowly fades over time to preoperatively demarcate the patients’ skin sites where we measured the circumference; this demarcation served as a reference for the sites postoperatively. Notably, this measurement should be performed when there is no tension over the skin. That is, when the observer releases the elastic tape, the two ends of the tape should not be retracted by limb tissue at the measured site. Clinical outcome assessments included the Likert-type Western Ontario and McMaster University (WOMAC) pain scale (ranging from 0 to 20, with lower scores indicative of better outcome), physical function (ranging from 0 to 68) and knee stiffness (ranging from 0 to 8) subscales at the 6-week follow-up visit. The WOMAC translation into Chinese has been validated in Chinese patients [30]. Knee active range of motion (ROM) was measured in the supine position using a long-arm goniometer. The time needed to achieve straight leg raise was also recorded. Special attention was paid to whether symptomatic pulmonary embolism (PE) or DVT occurred. Occult DVT was detected by a regular bilateral lower extremity deep venous color Doppler ultrasound examination.
Statistical analysis
Continuous variables are reported as the mean and the standard deviation (SD), and categorical variables are reported as proportions. Comparisons between groups were determined using an independent t test if the data were normally distributed; otherwise, a Mann–Whitney U test was used. The Chi square test was used to analyse categorical variables. The effects of long-duration tourniquet use and short-duration tourniquet use were compared using a paired t test if the data were normally distributed; otherwise, the Wilcoxon signed-rank test was used. Parameters that were measured in the same group but at different times were analysed with the repeated-measures general linear model. Two-sided P values of <0.05 indicated statistical significance.
Based on our previous study, the SD of blood loss was assumed to be 110 ml. It was estimated that we would need to enroll 38 patients to detect a reduction in the blood loss of 50 ml with the long-duration tourniquet use as compared with the short-duration tourniquet use, with statistical power (1 minus the β value) of 80 %, allowing for a type I (α) error of 0.05. Allowing for a loss to follow-up of 20 %, 48 patients were required to undergo randomization.
Results
Fifty participants were enrolled in this trial. Twenty-five of these participants were assigned to the long-duration tourniquet group for their first operation, whereas the other 25 participants were first assigned to the short-duration tourniquet group (Fig. 1). No participants were lost to follow-up.
At baseline, no significant differences in demographic data were noted between the two groups. The operating time was reduced in the long-duration tourniquet group (Table 1).
The mean total blood loss was reduced in the long-duration tourniquet group compared with the short-duration group, and the difference was significant [−99.1 ml, 95 % confidence interval (CI) −168.1 to −30.1, P = 0.0411]. Similarly, intraoperative blood loss was also reduced in the long-duration tourniquet group (−225.2 ml, 95 % CI −369.5 to −80.9, P = 0.0071]. However, the long-duration tourniquet group exhibited greater postoperative blood loss (69.6 ml, 95 % CI 21.1 to 118.2, P = 0.0282) and hidden blood loss (52.8 ml, 95 % CI 10.5 to 95.1, P = 0.0332) compared with the short-duration tourniquet group (Table 2). Three patients in the short-duration tourniquet group and two patients in the long-duration tourniquet group received allogeneic blood transfusions. These patients were transfused due to low Hb levels (Hb < 8 g/dl). The number of allogeneic blood transfusions in each group was similar (Table 2).
The short-duration tourniquet group exhibited reduced thigh and knee pain (assessed by VAS scores) on days 1 and 2 as well as 1 week postoperatively compared with the long-duration tourniquet group (Fig. 2a, b). The short-duration tourniquet group also exhibited reduced thigh and knee circumferences compared with the long-duration tourniquet group (Fig. 2c, d). No significant difference between the groups was noted with respect to calf circumference (Fig. 2e).
a, b Depict thigh pain and knee pain on postoperative day 1, day 2, 1 week (1 W), 2 weeks (2 W) and 6 weeks (6 W). Pain was assessed using VAS scores. VAS scores were lower in the short-duration tourniquet group. c–e Depict thigh swelling, knee swelling and calf swelling, respectively, on postoperative day 1, day 2, 1 weeks, 2 weeks and 6 weeks. Limb swelling was assessed by measuring the rate of limb circumference increase. Vertical bars represent SD. The asterisks indicate values that were significantly different between the two groups (P < 0.05)
There was more significant improvement in WOMAC scores in the short-duration tourniquet group at the 6-week follow-up (Table 3). The short-duration tourniquet group also exhibited better outcomes in terms of the active knee ROM postoperatively and the time interval required for patients to achieve straight leg raise (Table 3).
As investigated by ultrasound on postoperative day 7, no patients in either group exhibited DVT. However, two patients in the long-duration tourniquet group exhibited intramuscular vein thrombosis without obvious symptoms. No excessive oozing of blood or wound complications were noted in either group postoperatively.
Discussion
The most important finding of the present study was that although reduced total and intraoperative blood loss were observed with long-duration tourniquet use, the short-duration tourniquet use would reduce the postoperative and hidden blood loss and not increase the incidence of allogeneic blood transfusion. In addition, short-duration tourniquet use would result in faster recovery and less pain during the early rehabilitation period following TKA.
The debate continues as to whether tourniquet use can reduce blood loss. Various studies [2, 7, 14, 25, 29] claim that the tourniquet effectively reduces blood loss; however, results from other studies show no reduction in blood loss. Numerous studies [1, 12, 31] reported no significant difference in the amount of blood loss with or without tourniquet use. Moreover, various studies demonstrate even greater blood loss [17, 28] when using a tourniquet. Despite adequate blinding and randomization, patients in the two intervention groups may differ at baseline with regard to key characteristics such as total blood volume, muscle strength and coagulation function. However, since this was a crossover trial, these differences should have had minimal effects on the results.
It was demonstrated in our study that the mean total blood loss and intraoperative blood loss were significantly higher in the short-duration tourniquet group, which were consistent with some previous studies [2, 7, 14, 25, 29]. Furthermore, as Sehat et al. reported [24], hidden or unmeasured blood loss could often be substantial during TKA, which we therefore considered. Our study demonstrated that postoperative blood loss and hidden blood loss were increased in the long-duration tourniquet group. There are multiple reasons why blood loss may be greater in the long-duration tourniquet group. First, the ischaemic conditions caused by the use of a tourniquet could induce sustained local reactive hyperaemia that lasts several hours after tourniquet deflation. This hyperaemia would subsequently promote more haemorrhage into the traumatized tissue and bleeding from the incision site during the postoperative period. Second, bleeding into the local tissues was promoted by the increased fibrinolytic activity associated with tourniquet-induced ischaemia [1]. Third, according to our own experiences, this latter condition is convenient for surgical haemostasis because the bleeding structure is clearly observed without a tourniquet. Conversely, bleeding vessels might be missed when the tourniquet is inflated during TKA surgery.
In our trial, the short-duration tourniquet group was associated with better clinical outcomes, less pain and less limb swelling during the early stage of rehabilitation. Similar results were reported by Zhang et al. [31] and Ejaz et al. [6]. A possible explanation for improved outcomes among patients in the short-duration tourniquet group is that the direct compression of the tourniquet on tissue and reperfusion injury may increase pain, which would influence patients’ postoperative rehabilitation [15]. In the long-duration tourniquet group, the postoperative blood loss was increased. This lost blood may escape into the soft tissue, potentially resulting in limb swelling. Additional swelling may hinder patients’ early postoperative rehabilitation exercises. Increased swelling also increases the soft tissue tension, thus decreasing oxygen tension [16]. Furthermore, as Dennis et al. [5] reported, patients who underwent TKA using a tourniquet had diminished quadriceps strength during the first 3 months after TKA. Loss of lower quadriceps strength may result in reduced recovery of the active ROM of the knee and instability when walking and climbing stairs.
Thromboembolism is one of the most serious postoperative complications following TKA. For the long-duration tourniquet group, thromboembolism potentially increases the risk of DVT due to stasis of venous blood in the lower limb and possible damage to blood vessels [2]. However, in our study, DVT was not detected in either of the two groups. Chemical prophylaxis with rivaroxaban and early physiotherapy may be of great benefit. Two meta-analyses also found no difference in DVT or PE occurrence with the use of the tourniquet application during TKA surgery [2, 26]. Moreover, various additional tourniquet-related complications have been reported in the literature, including nerve palsy [10], vascular injuries [2], rhabdomyolysis [25] and subcutaneous fat necrosis [27]. Some studies have also reported changes in intraoperative patellofemoral tracking caused by tourniquet use [11, 13, 20]. None of these complications occurred during our trial.
The operating time mainly depends on the surgical technique used by the surgeons. The application of a tourniquet during TKA is thought to effectively provide a relative bloodless field, which saves time in surgery [31]. In our trial, the operating time of the long-duration tourniquet group decreased by approximately 9 min, which had little influence on our clinical practice. Furthermore, Ejaz et al. [6] reported that the visibility of the surgical field was not impaired when a tourniquet was not used. Similarly, we found that control of intraoperative bleeding was not an obstacle with short-duration tourniquet use.
There were some limitations with our study. First, testing was continued for only 6 weeks postoperatively. However, the focus of this report was to investigate the effects of a tourniquet on early TKA recovery. Second, we studied relatively low-risk patients (ASA class 1 or 2). Complications such as nerve palsy, vascular injuries and DVT could occur with prolonged tourniquet use if high-risk patients such as older patients or those with calcified arteries were included. Third, deep venous color Doppler ultrasound is an operator-dependent examination that relies on operator experience and technique. A future trial with more precise diagnostic testing to detect thrombosis, such as echoscope or venography, is needed to reach a more definitive conclusion.
According to our study, the postoperative and hidden blood losses were reduced with the short-duration tourniquet use (tourniquet inflated just before the cement application and deflated after its hardening) in TKA. Furthermore, faster recovery, less limb swelling and less pain during the early rehabilitation period were observed with the short-duration tourniquet use.
Conclusion
Total and intraoperative blood losses were reduced with the long-duration tourniquet use. However, short-duration tourniquet use was associated with lower postoperative blood loss, hidden blood loss and would lead to faster recovery and less pain during the early rehabilitation period following TKA.
References
Aglietti P, Baldini A, Vena LM, Abbate R, Fedi S, Falciani M (2000) Effect of tourniquet use on activation of coagulation in total knee replacement. Clin Orthop Relat Res 371:169–177
Alcelik I, Pollock RD, Sukeik M, Bettany-Saltikov J, Armstrong PM, Fismer P (2012) A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty 27(3):331–340
Burg A, Dudkiewicz I, Heller S, Salai M, Velkes S (2009) The effects of using a tourniquet in total knee arthroplasty: a study of 77 patients. J Musculoskelet Res 12(03):137–142
Butt U, Ahmad R, Aspros D, Bannister GC (2011) Factors affecting wound ooze in total knee replacement. Ann R Coll Surg Engl 93(1):54–56
Dennis DA, Kittelson AJ, Yang CC, Miner TM, Kim RH, Stevens-Lapsley JE (2015) Does tourniquet use in TKA affect recovery of lower extremity strength and function? A randomized trial. Clin Orthop Relat Res. doi:10.1007/s11999-015-4393-8
Ejaz A, Laursen AC, Kappel A, Laursen MB, Jakobsen T, Rasmussen S, Nielsen PT (2014) Faster recovery without the use of a tourniquet in total knee arthroplasty. Acta Orthop 85(4):422–426
Fukuda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A (2007) Effect of tourniquet application on deep vein thrombosis after total knee arthroplasty. Arch Orthop Trauma Surg 127(8):671–675
Gross JB (1983) Estimating allowable blood loss: corrected for dilution. Anesthesiology 58(3):277–280
Hernandez AJ, Almeida AM, Favaro E, Sguizzato GT (2012) The influence of tourniquet use and operative time on the incidence of deep vein thrombosis in total knee arthroplasty. Clinics (Sao Paulo) 67(9):1053–1057
Horlocker TT, Hebl JR, Gali B, Jankowski CJ, Burkle CM, Berry DJ, Zepeda FA, Stevens SR, Schroeder DR (2006) Anesthetic, patient, and surgical risk factors for neurologic complications after prolonged total tourniquet time during total knee arthroplasty. Anesth Analg 102(3):950–955
Husted H, Toftgaard Jensen T (2005) Influence of the pneumatic tourniquet on patella tracking in total knee arthroplasty: a prospective randomized study in 100 patients. J Arthroplasty 20(6):694–697
Katsumata S, Nagashima M, Kato K, Tachihara A, Wauke K, Saito S, Jin E, Kawanami O, Ogawa R, Yoshino S (2005) Changes in coagulation-fibrinolysis marker and neutrophil elastase following the use of tourniquet during total knee arthroplasty and the influence of neutrophil elastase on thromboembolism. Acta Anaesthesiol Scand 49(4):510–516
Komatsu T, Ishibashi Y, Otsuka H, Nagao A, Toh S (2003) The effect of surgical approaches and tourniquet application on patellofemoral tracking in total knee arthroplasty. J Arthroplasty 18(3):308–312
Kvederas G, Porvaneckas N, Andrijauskas A, Svensen CH, Ivaskevicius J, Mazunaitis J, Marmaite U, Andrijauskas P (2013) A randomized double-blind clinical trial of tourniquet application strategies for total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21(12):2790–2799
Ledin H, Aspenberg P, Good L (2012) Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion. Acta Orthop 83(5):499–503
Li B, Wen Y, Liu D, Tian L (2012) The effect of knee position on blood loss and range of motion following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 20(3):594–599
Li B, Wen Y, Wu H, Qian Q, Lin X, Zhao H (2009) The effect of tourniquet use on hidden blood loss in total knee arthroplasty. Int Orthop 33(5):1263–1268
Liu D, Gillies RM, Gillies K, Graham D (2012) Effect of tourniquet use on quadriceps in total knee arthroplasty. J Bone Joint Surg 94(Suppl 23):94
Lohmann-Jensen R, Holsgaard-Larsen A, Emmeluth C, Overgaard S, Jensen C (2014) The efficacy of tourniquet assisted total knee arthroplasty on patient-reported and performance-based physical function: a randomized controlled trial protocol. BMC Musculoskelet Disord 15:110
Lombardi AV Jr, Berend KR, Mallory TH, Dodds KL, Adams JB (2003) The relationship of lateral release and tourniquet deflation in total knee arthroplasty. J Knee Surg 16(4):209–214
Nadler SB, Hidalgo JH, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51(2):224–232
Olivecrona C, Blomfeldt R, Ponzer S, Stanford BR, Nilsson BY (2013) Tourniquet cuff pressure and nerve injury in knee arthroplasty in a bloodless field: a neurophysiological study. Acta Orthop 84(2):159–164
Sehat KR, Evans R, Newman JH (2000) How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee 7(3):151–155
Sehat KR, Evans RL, Newman JH (2004) Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 86(4):561–565
Tai TW, Chang CW, Lai KA, Lin CJ, Yang CY (2012) Effects of tourniquet use on blood loss and soft-tissue damage in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 94(24):2209–2215
Tai TW, Lin CJ, Jou IM, Chang CW, Lai KA, Yang CY (2011) Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 19(7):1121–1130
Tamvakopoulos GS, Toms AP, Glasgow M (2005) Subcutaneous thigh fat necrosis as a result of tourniquet control during total knee arthroplasty. Ann R Coll Surg Engl 87(5):W11–W13
Tetro AM, Rudan JF (2001) The effects of a pneumatic tourniquet on blood loss in total knee arthroplasty. Can J Surg 44(1):33–38
Vandenbussche E, Duranthon LD, Couturier M, Pidhorz L, Augereau B (2002) The effect of tourniquet use in total knee arthroplasty. Int Orthop 26(5):306–309
Xie F, Li SC, Goeree R, Tarride JE, O’Reilly D, Lo NN, Yeo SJ, Yang KY, Thumboo J (2008) Validation of Chinese Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) in patients scheduled for total knee replacement. Qual Life Res 17(4):595–601
Zhang W, Li N, Chen S, Tan Y, Al-Aidaros M, Chen L (2014) The effects of a tourniquet used in total knee arthroplasty: a meta-analysis. J Orthop Surg Res 9(1):13
Acknowledgments
We acknowledge Guangshan Sui MS, Ke Yuan MS, and Jiebing Li MS for their assistance with inpatient testing; Bin Wang Ph.D., for assistance with study coordination, including the randomization of participants and the statistical analysis; and Yuankun Xu Ph.D., for assistance with labVIEW programming. This study was supported by Peking University People’s Hospital Research and Development Funds (Project No. RDC2013-06). Zhichang Li MD was in charge of this Hospital Fund programme and made a contribution similar to the first author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, K., Ni, S., Li, Z. et al. The effects of tourniquet use in total knee arthroplasty: a randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc 25, 2849–2857 (2017). https://doi.org/10.1007/s00167-015-3964-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3964-2