Abstract
Purpose
To develop and validate a new simulation system to predict the optimal femoral insertion site of the medial patellofemoral ligament (MPFL) using preprogrammed conditions of graft length change.
Methods
A computed tomography scan was performed for constructing bone surface models of the patella and the femur. Lateral radiographs of the knee and axial radiographs of the patellofemoral joint at knee flexion were used for constructing a three-dimensional patellofemoral joint model by 3D–2D image matching at each knee flexion angle. To determine the optimal femoral insertion site, the following three conditions were devised: (1) MPFL behaves isometric from 0° to 60°, (2) MPFL is most taut at full extension, and (3) MPFL is slack at more than 60° of knee flexion. Every condition was applied to the femoral bone surface model, and the area that fulfilled all three conditions was defined as the optimal femoral insertion site of MPFL. Twenty knees of ten healthy volunteers were assessed to verify the simulation. Comparison between simulated optimal femoral insertion and anatomical insertion was done.
Results
The mean simulated optimal femoral insertion was close to the anatomical insertion site. There were no significant differences in the proximal–distal position and anterior–posterior position between the simulated insertion and the anatomical insertion sites.
Conclusion
The system can be useful for predicting the optimal femoral insertion site as a part of a preoperative plan for MPFL reconstruction, and it may help surgeons to determine the optimal femoral insertion site during MPFL reconstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anatomical and biomechanical studies have shown that the medial patellofemoral ligament (MPFL) is the primary restraint against lateral patellar displacement among the medial patellar stabilizers [1, 3, 5, 12, 15, 22]. In recent years, reconstruction of the MPFL with an autologous or artificial graft has been successfully performed for the treatment of patients with patellar instability [4, 13, 14]. The attachment site of the graft is one of the important factors affecting the results of MPFL reconstruction. It has been reported that the length change pattern of the graft is mostly affected by the location of femoral fixation, and length change patterns greatly differ if the femoral fixation point is moved even by 5 mm from the normal femoral attachment site [6, 12, 17]. In addition, a malpositioned femoral attachment site can lead to iatrogenic medial subluxation, medial patellofemoral articular overload, deficits in range of motion and recurrent lateral instability [2, 6]. Therefore, to obtain good clinical outcomes after MPFL reconstruction, it is important to identify the optimal femoral graft fixation site that provides the desired change in graft length. However, during the course of surgery, it is not easy for a surgeon to determine the optimal femoral attachment site and establish the desired length change of the graft.
So far there have been no systems that can predict the optimal femoral graft fixation site before surgery. Therefore, in this study, new simulation system was developed to predict the optimal site for femoral insertion of the MPFL using preprogrammed conditions of the graft length change. Furthermore, validation of this new simulation system was performed. Our working hypothesis was that the optimal femoral insertion site determined by our new system would be the same as the anatomical femoral insertion site in normal knees.
Our new simulation system will be the first system to predict length behaviours of the reconstructing MPFL and the optimal femoral insertion site before surgery.
Materials and methods
Reproducing the patellofemoral joint
A computed tomography (CT; Aquilion™ 64, Toshiba Medical Systems, Otawara, Japan) scan was performed for all the volunteers with the knees in full extension. For creating bone surface models of the patella and the femur, a section thickness of 1.25 mm was used. The contour of the patella and the femur was extracted from the CT data using a free open-source software (3D Slicer, http://www.slicer.org) [7]. A three-dimensional (3D) mathematical model was constructed using consecutive CT images, which consisted of a 3D co-ordinate system and triangular patches. The 3D model was loaded in MATLAB ® (MathWorks Inc., MA, USA) for further analysis.
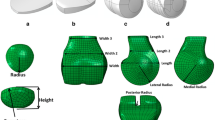
An initial 3D co-ordinate system was defined based on the direction of scanning and the position of the knee. Therefore, the anatomical co-ordinate system was reconstructed so that it could be applied in a simulation programme. The patellar anatomical co-ordinate system was defined as follows: the most medial and lateral points of the 3D patellar model were determined in the initial 3D co-ordinate system, and the line joining these two points was defined as the x axis. The line perpendicular to the x axis and parallel to the line passing through the most distal point was defined as the z axis. The line perpendicular to the x axis and the z axis was defined as y axis. The origin of the co-ordinate axes was the midpoint of each of the axes (Fig. 1a). The femoral anatomical co-ordinate system was defined as follows: the sphere approximations of the posterior condyle were defined according to the medial and lateral condyles. The line passing through the centres of the sphere approximations was defined as the x axis. The origin of the axis was the midpoint of the centres of the sphere approximations. The line parallel to the bone axis in the distal shaft of the femur and passing through the origin was defined as the z axis. The line perpendicular to the x axis and the z axis and passing through the origin was defined as the y axis (Fig. 1b).
Lateral radiographs of the knee and axial radiographs of the patellofemoral joint at 30°, 60°, 90° and 120° of knee flexion were used for constructing the 3D patellofemoral joint model. 3D–2D image matching was semi-automatically performed by using biplane radiographs using MATLAB® at each knee flexion angle. Based on the CT data obtained at knee extension, the position of the patellofemoral joint at 0° of knee flexion was obtained. The data of the reproduced 3D patellofemoral motion during knee flexion were saved in MATLAB® (Fig. 2).
Derivation of the virtual ligament route
To calculate the femoral insertion site of the MPFL, patellar insertion site was defined at the most medial aspect of the patella. In this study, the distances between the patellar insertion site and every point on the medial surface of the femur, at intervals of 1 mm, were calculated at each angle of knee flexion. To determine the shortest running route of the virtual ligament, we developed a derivation method such that the virtual ligament could trace the femoral surface along the shortest route (Fig. 3). By calculating the length of the virtual ligament during knee motion, each femoral insertion site provided data regarding the length change patterns of the ligament.
Programming three conditions of the length change pattern
To calculate the optimal femoral insertion site, three conditions were devised based on previous studies regarding MPFL length change [1, 15, 17, 20, 23]. First, we defined the length of the virtual ligament at 30° of knee flexion as the standard length and expressed the length change under strain as follows:
ε strain of the virtual ligament; L length of the virtual ligament at each flexion of the knee; L 30 length of the virtual ligament at 30° knee flexion.
The following three conditions were programmed into the simulation system:
-
1.
MPFL behaves nearly isometric (<5 %) from 0° to 60°.
$$ \varepsilon_{1} < 5 \, (\% ) $$ -
2.
MPFL is most taut at full extension.
$$ 0 < \varepsilon_{2} < 5 \, \left( \% \right) $$ -
3.
MPFL becomes slack at more than 60° of knee flexion.
$$ \varepsilon_{3} < 0 \, \left( \% \right) $$
The simulated optimal femoral insertion sites that fulfilled all three conditions was determined by our new system and compared with the anatomical insertion sites.
Analysing the simulated femoral insertion and anatomical femoral insertion sites
To analyse the validation of the simulation, we examined 20 knees of 10 healthy volunteers (mean age, 33 years; age range, 24–45 years). None of these subjects showed any symptoms or objective pathologic findings related to the patellofemoral joint. The average height of the volunteers was 174.3 cm (range 160–180 cm), and the average weight was 67.9 kg (range 55–82 kg). The study design was approved by the Ethics Committee of Kobe University Graduate School of Medicine, and all volunteers provided informed consent to participate in this study.
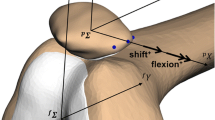
To evaluate the location of simulated femoral insertion sites, lateral views of the femur were used. To measure the anterior–posterior diameter of medial femoral condyle, a line between the medial and lateral trochlea and a tangential line to posterior condyle were drawn parallel to femoral bone axis. The distance between the two lines was defined as the diameter of the medial femoral condyle (c). The proximal–distal ratio (a/c (%)) and the anterior–posterior ratio (b/c (%)) were calculated [21] (Fig. 4). The mean simulated femoral insertion site was compared to the anatomical femoral insertion site, which was described by Smirk et al. to be distal to the adductor tubercle [8, 17]. To verify the validity of the preprogrammed conditions, the length change of MPFL at the anatomical femoral insertion site was examined using the derivation method. All distances were measured by digital scale with 0.1 mm as the minimum unit.
Medial aspect of lateral view in the femoral bone model. The proximal–distal ratio [a/c (%)] and anterior–posterior ratio [b/c (%)], which were measured by considering the anterior–posterior diameter of the medial femoral condyle as a standard. The circle is centre of the calculated optimal femoral insertion
Statistical analysis
The study aimed to include at least 19 knees, which was expected to yield power >0.95, based on α < 0.05 and assuming a large effect size (d = 0.80) using G power 3.1.5 (http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3/).
All values were expressed as the mean ± SD. Student’s t test was used to compare the simulated insertion and anatomical insertion sites for all 20 participants. The length change pattern of MPFL at the anatomical insertion site was analysed using the analysis of variance (ANOVA) test followed by the Tukey’s post hoc procedure. Statistical significance was defined by an α level of 0.05 and a β level of 80 %. All statistical analyses were performed using the free software package G power 3 (http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3/).
Results
To visualize the results, each condition was applied to the femoral bone surface model using a colour map (Fig. 5a–c). The area that fulfilled all the conditions in the simulation system was defined as the optimal site for femoral insertion of MPFL (Fig. 6).
Colour maps showing the strain (ε) calculated from the programmed conditions of the length change pattern. a The isometricity of the MPFL from 0° to 60°. The blue-coloured area indicates the highest isometricity. b The strain of the MPFL at full extension. The MPFL is most taut at full extension. The blue or light-blue-coloured areas indicate the strain at full extension. c The slackness of the MPFL at more than 60° of knee flexion. The blue-coloured area indicates the slackness
When the location was expressed as a ratio relative to the diameter of the femoral condyle, the mean proximal–distal and anterior–posterior positions of the simulated femoral insertion were located at 53.6 ± 5.4 and 37.7 ± 3.4 %, respectively. The mean proximal–distal and anterior–posterior positions of the anatomical femoral insertion were located at 54.4 ± 3.1 and 38.9 ± 3.7 %, respectively. There were no significant differences in the proximal–distal position and anterior–posterior position between the simulated insertion and the anatomical insertion sites.
Next the length of the virtual MPFL at the anatomical point was examined. The mean MPFL lengths at the anatomical femoral insertion site were 62.4 ± 4.6, 61.9 ± 3.7, 60.0 ± 3.7, 57.3 ± 3.8 and 54.0 ± 3.7 mm at 0°, 30°, 60°, 90° and 120° of the knee flexion (Fig. 7). No significant differences in the length were observed during 0° to 60° knee flexion. At the anatomical insertion site, the mean strain between 0° and 30° (ε = [(L 0 − L 30)/L 30] × 100) was 0.7 ± 3.5 %, and the mean strain between 30° and 60° (ε = [(L 60 − L 30)/L 30] × 100) was 3.1 ± 2.0 %. The strains between 0° and 30° and between 30° and 60° were less than 5 %.
Length change pattern of the MPFL at the anatomical femoral insertion site. The MPFL was most taut at 0° and nearly isometric up to 60°. The MPFL length decreased significantly from 60° to 90° and 90° to 120°. There were significant differences in the mean length between 0° and 90°, and between 0° and 120°
The system can also calculate the length change pattern at any point on the femur, without any special equipment, by using only CT and radiography data (Fig. 8).
Length change in the distance between the three patellar attachment sites and the femoral attachment sites in 1 case (45-year-old male). FA refers to the anatomical femoral attachment of the MPFL, 1 cm distal to the adductor tubercle. All femoral sites were 5 mm apart. The superior bundle is seen at the supra-medial corner, the inferior bundle is located at the most medial aspect of the patella, and the medial bundle is located halfway between the superior and inferior bundles
Discussion
The most important contribution of this study was that a simulation system that can predict the optimal femoral insertion site of MPFL, using preprogrammed conditions of graft length change, was developed.
In recent years, the length change pattern of the MPFL in anatomical insertion has become clear [9, 10, 17, 18, 20, 23]. The MPFL is a primary passive soft tissue restraint against lateral patellar displacement from 0° to 30° of knee flexion [1, 12, 24]. The patella is engaged in the trochlea, and the MPFL is gradually slack above 60° of knee flexion. An open MRI study conducted by Higuchi et al. [10] revealed that the length change pattern presented a slight change up to a flexion angle of 60° and a decrease at over 60° in normal knees. Smirk et al. [17] showed that the MPFL remained isometric (<5 mm) at 80° and became slack with increasing knee flexion in cadaveric knees. In some studies, the length of the MPFL at 120° knee flexion was approximately 10 mm shorter than that at full extension [17, 18]. In our study, the conditions for the length change pattern were formulated and programmed into the simulation system based on the results of these previous studies.
In the present study, the simulated femoral insertion site determined by using the programmed conditions was almost identical to the anatomical insertion site, suggesting the validity of our new system. Stephen et al. [20] reported that the MPFL attachment was 40 % posterior, 50 % from the distal in relation to the size of the medial femoral condyle in eight cadaveric knees. Nomura et al. reported that the femoral attachment site was located at 39 % of the antero-posterior length of the medial condyle from the posterior edge in 20 cadaveric knees [20]. These insertion sites were close to our simulated femoral insertion site and the anatomical insertion site in the present study, further supporting the validity of our simulation system.
Several studies have reported the relationship between the femoral insertion site and the length change of the MPFL [17, 20, 21]. These studies have shown that proximal malpositioning increases the distance between the attachment points on the femur and the patella during knee flexion and that only a 5-mm error in the position of the femoral insertion site can dramatically change the length of the MPFL. Similar to previous studies, we also found that proximal malpositioning of the femoral point increased the distance between the attachment points on the femur and the patella during knee flexion, whereas distal malpositioning caused an increased length change during knee extension (Fig. 8), suggesting that caution must be exercised when determining the femoral insertion site during MPFL reconstruction.
Biomechanical differences between anatomical and malpositioned femoral insertion have been reported using normal knees [6, 19]. And results of these studies have been a basis of putting the graft at anatomical femoral insertion in MPFL reconstruction. Bollier et al. [2] reported five cases of femoral tunnel malpositioning after MPFL reconstruction. They emphasized that it is important to understand that most of the previous studies that examined MPFL tension and length changes had been performed in patients with a normal anatomy, and that biomechanical properties may be different in patients with patella alta or trochlea dysplasia. Servien et al. [16] also referred to useless comparison of tunnel positioning in the patients with patellofemoral instability against a standardized ‘normal’ population, because patella alta and trochlea dysplasia may alter the insertions of the MPFL with corresponding alterations in MPFL kinematics. Additionally, during MPFL reconstruction in such patients, it is not easy for a surgeon to determine the optimal femoral attachment site and establish the desired length change of the graft. Currently, there has been no system that predicts the length behaviour of the MPFL before surgery. Therefore, it would be helpful for surgeons to predict length behaviours of the reconstructing MPFL before surgery.
There are a few limitations of the current study. First, we used 20 knees from 10 volunteers. It might not be appropriate to use bilateral knees of one subject due to anatomical similarity of the knees. Second, the patellar attachment of the MPFL was broad, and the MPFL had two functional bands of fibres [1, 11, 23]. Although the patellar attachment can be chosen in the simulation system (Fig. 8), the most medial aspect of the patella was used because of its simplicity in the co-ordinate system. Third, the accuracy of the simulation system was not evaluated in this study. Fourth, the femoral anatomical insertion site was determined to be located distal to the adductor tubercle [17], which may be different from the true anatomical insertion site. Further studies using cadaveric knees are necessary to assess the accuracy of our simulation system.
The clinical relevance of this study is that our new system is related to preoperative planning for MPFL reconstruction. Although this study is the first step to verify the simulation system using normal knees, our new simulation system can be applied to patients with patellar instability as a part of a preoperative plan for MPFL reconstruction, and it may help to determine the optimal femoral insertion site during MPFL reconstruction.
Conclusions
New simulation system was developed to determine the optimal femoral insertion site of the MPFL and calculate the length change pattern at any point on the femur, without using special equipment, by using only CT and radiography data. In normal knees, the mean optimal femoral insertion site determined by the simulation system using the programmed conditions was close to the anatomical insertion site, and the length change pattern at the anatomical insertion site was sufficient under our defined conditions, indicating the validity of system. The simulation system could be applied to patients with patellar instability as a part of a preoperative plan for MPFL reconstruction.
References
Amis AA, Firer P, Mountney J, Senavongse W, Thomas NP (2003) Anatomy and biomechanics of the medial patellofemoral ligament. Knee 10(3):215–220
Bollier M, Fulkerson J, Cosgarea A, Tanaka M (2011) Technical failure of medial patellofemoral ligament reconstruction. Arthroscopy 27(8):1153–1159
Conlan T, Garth WP Jr, Lemons JE (1993) Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am 75(5):682–693
Deie M, Ochi M, Sumen Y, Adachi N, Kobayashi K, Yasumoto M (2005) A long-term follow-up study after medial patellofemoral ligament reconstruction using the transferred semitendinosus tendon for patellar dislocation. Knee Surg Sports Traumatol Arthrosc 13(7):522–528
Desio SM, Burks RT, Bachus KN (1998) Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med 26(1):59–65
Elias JJ, Cosgarea AJ (2006) Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med 34(9):1478–1485
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30(9):1323–1341
Fujino K, Tajima G, Yan J, Kamei Y, Maruyama M, Takeda S, Kikuchi S, Shimamura T (2013) Morphology of the femoral insertion site of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-013-2797-0
Ghosh KM, Merican AM, Iranpour-Boroujeni F, Deehan DJ, Amis AA (2009) Length change patterns of the extensor retinaculum and the effect of total knee replacement. J Orthop Res 27(7):865–870
Higuchi T, Arai Y, Takamiya H, Miyamoto T, Tokunaga D, Kubo T (2010) An analysis of the medial patellofemoral ligament length change pattern using open-MRI. Knee Surg Sports Traumatol Arthrosc 18(11):1470–1475
Kang HJ, Wang F, Chen BC, Su YL, Zhang ZC, Yan CB (2010) Functional bundles of the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc 18(11):1511–1516
Nomura E, Horiuchi Y, Kihara M (2000) Medial patellofemoral ligament restraint in lateral patellar translation and reconstruction. Knee 7(2):121–127
Nomura E, Horiuchi Y, Kihara M (2000) A mid-term follow-up of medial patellofemoral ligament reconstruction using an artificial ligament for recurrent patellar dislocation. Knee 7(4):211–215
Nomura E, Inoue M, Kobayashi S (2007) Long-term follow-up and knee osteoarthritis change after medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Am J Sports Med 35(11):1851–1858
Nomura E, Inoue M, Osada N (2005) Anatomical analysis of the medial patellofemoral ligament of the knee, especially the femoral attachment. Knee Surg Sports Traumatol Arthrosc 13(7):510–515
Servien E, Fritsch B, Lustig S, Demey G, Debarge R, Lapra C, Neyret P (2011) In vivo positioning analysis of medial patellofemoral ligament reconstruction. Am J Sports Med 39(1):134–139
Smirk C, Morris H (2003) The anatomy and reconstruction of the medial patellofemoral ligament. Knee 10(3):221–227
Steensen RN, Dopirak RM, McDonald WG 3rd (2004) The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med 32(6):1509–1513
Stephen JM, Kaider D, Lumpaopong P, Deehan DJ, Amis AA (2014) The effect of femoral tunnel position and graft tension on patellar contact mechanics and kinematics after medial patellofemoral ligament reconstruction. Am J Sports Med 42(2):364–372
Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA (2012) The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med 40(8):1871–1879
Tateishi T, Tsuchiya M, Motosugi N, Asahina S, Ikeda H, Cho S, Muneta T (2011) Graft length change and radiographic assessment of femoral drill hole position for medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 19(3):400–407
Tuxoe JI, Teir M, Winge S, Nielsen PL (2002) The medial patellofemoral ligament: a dissection study. Knee Surg Sports Traumatol Arthrosc 10(3):138–140
Victor J, Wong P, Witvrouw E, Sloten JV, Bellemans J (2009) How isometric are the medial patellofemoral, superficial medial collateral, and lateral collateral ligaments of the knee? Am J Sports Med 37(10):2028–2036
Zaffagnini S, Colle F, Lopomo N, Sharma B, Bignozzi S, Dejour D, Marcacci M (2013) The influence of medial patellofemoral ligament on patellofemoral joint kinematics and patellar stability. Knee Surg Sports Traumatol Arthrosc 21(9):2164–2171
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oka, S., Matsushita, T., Kubo, S. et al. Simulation of the optimal femoral insertion site in medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 22, 2364–2371 (2014). https://doi.org/10.1007/s00167-014-3192-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-014-3192-1