Abstract
Purpose
Left ventricular (LV) performance is often quantified by echocardiography in critically ill patients. Pulse wave analysis (PWA) systems can also monitor cardiac function but in a continuous fashion. We compared echocardiographic and PWA-derived indices of LV function.
Methods
We enrolled 70 critically ill patients equipped with invasive arterial pressure monitoring who required echocardiography. We simultaneously assessed LV ejection fraction (LVEF), the rate of LV pressure rise during systole (dP/dt MAX) obtained with echocardiography (EC-dP/dt MAX), the ratio of effective arterial elastance to LV end-systolic elastance (E a/E es) determined by echocardiography, the dP/dt MAX estimated from the arterial pressure waveform (AP-dP/dt MAX) and the cardiac cycle efficiency (CCE) using PWA.
Results
Mean LVEF was 53 ± 18 % and CCE 0.16 ± 0.26. CCE was correlated linearly with LVEF (r = 0.88, 95 % CI 0.81 to 0.92, P < 0.001), and the dP/dt MAX values from the two techniques were linearly correlated (r = 0.93, 95 % CI 0.87 to 0.96, P < 0.001). There was minimal bias between the techniques for measurement of dP/dt MAX (23.7 mmHg/ms; 95 % CI −23.6 to 71.0). E a/E es and CCE were inversely correlated (r = −0.81, 95 % CI −0.88 to −0.71, P < 0.001). A CCE value of <0.07 predicted LVEF <40 % with a sensitivity of 0.93 and a specificity of 0.96 (AUC 0.98, 95 % CI 0.90 to 1.0, P < 0.001). A CCE value of >0.12 predicted LVEF ≥50 % with a sensitivity of 0.96 and a specificity of 0.82 (AUC 0.94, 95 % CI 0.87 to 1.0, P < 0.001). A CCE value <0.12 predicted E a/E es ≥1.3 with a sensitivity of 0.93 and a specificity of 0.89 (AUC 0.94, 95 % CI 0.83 to 1.0, P < 0.001).
Conclusions
PWA-derived variables provide relevant information on cardiac contractility and performance in critically ill patients. PWA provides an easy method for online hemodynamic evaluation in critically ill patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular monitoring is frequently used in critically ill patients. Measurements of cardiac output and filling pressures (or volumes) provide a global evaluation of cardiovascular function, but evaluation of left ventricular (LV) contractile performance remains difficult [1]. Echocardiography has emerged as an effective diagnostic tool in the assessment of LV function. Among the various indices that can be obtained by echocardiography, LV ejection fraction (LVEF) has become the most widely used to evaluate LV contractility [2, 3]. However, LVEF is affected by loading conditions and is an index of the interaction of the left ventricle and the arterial system. Indeed, LVEF can be derived from the relationship between effective arterial elastance (E a), a measure of the net arterial load imposed on the left ventricle, and LV end-systolic elastance (E es), a load-independent measure of intrinsic LV contractility [4]. As therapeutic interventions may have different effects on LVEF, determination of intrinsic contractility is desirable.
The relationship between E a and E es (i.e., their ratio) is termed arterial-ventricular (A–V) coupling and is a major determinant of cardiovascular performance [5]. Initial studies on E a/E es were performed in animals using high-fidelity ventricular catheters [6] and, subsequently, in humans during cardiac catheterization [7]. Recently, echocardiography has been proposed for the noninvasive measurement of E a/E es [8, 9]. However, this approach is impractical, as it requires off-line analysis and very precise synchronization of the arterial signal and echocardiography device. The rate of LV pressure rise during systole (dP/dt MAX) may also provide an estimate of ventricular contractility [10] and can be measured from the mitral valve regurgitation jet using echocardiography [11].
Whatever their intrinsic capabilities, most of these methods are limited by their intermittent nature. At best, cardiac function can be estimated once and repeated after a therapeutic intervention. However, hemodynamic status can be highly variable in critically ill patients, and continuous monitoring of cardiac function may, therefore, provide important information.
Analysis of the arterial signal can provide information on cardiac performance. The PiCCO continuous cardiac output monitoring system (Pulsion Medical Systems AG, Munich, Germany) provides dP/dt online, but the cardiac function index, a surrogate of LVEF, is only obtained intermittently during recalibration [12]. The PRAM (pressure recording analytical method) Most Care system (Vygon Health, Padua, Italy), an uncalibrated pulse wave analysis system that has been validated for cardiac output measurement in various clinical conditions [13], also measures dP/dt MAX, which represents the maximal value of the first derivative of the radial or femoral pulse wave [14], and cardiac cycle efficiency (CCE), an indirect index of A–V coupling, which represents the dynamic equilibrium between preload, arterial elastance and contractility [15–18]. CCE reflects the sum of the energies required to generate stroke volume (SV), which depends on the interaction between pump function and the arterial system. The factors involved in the arterial component of this process are arterial elastance and reflected waves. Reflected waves are composed of a multitude of wavelets generated by reflection of previous beats at branchpoints (backward waves) and also at the aortic valve (forward waves). The energy generated by backward waves either increases (counted as positive in CCE) or decreases (counted as negative in CCE) heart efficiency, depending on their location according to the dicrotic notch, whereas energy generated by forward wavelets is positive, facilitating ejection. CCE is the ratio of systolic energy to total energy expenditure, and can thus be positive or negative, depending on the sum of the individual energies (see the Electronic supplementary material for physiologic explanation and details of computation).
The aim of this study was to evaluate whether analysis of the arterial pressure waveform could be useful for evaluating some indices of LV function. We hypothesized that the dP/dt MAX estimated from the arterial pressure waveform (AP-dP/dt MAX) would reflect the dP/dt MAX obtained with echocardiography (EC-dP/dt MAX) and that the CCE would reflect the LVEF and E a/E es determined by echocardiography.
Materials and methods
Patients
In this prospective, observational study, patients admitted to a 35-bed university hospital medicosurgical ICU during a 6-month period who were (1) undergoing echocardiography in the setting of circulatory or respiratory failure, and (2) monitored with a standard arterial catheter line (radial or femoral artery) for invasive arterial blood pressure measurement were enrolled. Exclusion criteria were: (1) patients less than 18 years of age; (2) the presence of pathologies that could affect the quality and reliability of the arterial signal (aortic valve pathologies, ascending aorta pathologies, and cardiac arrhythmias); and (3) insufficient echogenicity preventing echocardiographic assessment. Approval from the Institutional Review Board was obtained but informed consent was waived because of the purely observational nature of the study.
Study protocol
In each patient, echocardiographic and arterial waveform measurements were simultaneously obtained during periods of hemodynamic stability, defined as no more than a 5 % variation in heart rate and mean systemic arterial pressure with respect to baseline during the time needed for echocardiography. During measurements, no changes to ventilation, sedation, vasoactive drugs or fluid infusions were made.
Echocardiography measurements
A single experienced examiner (L.B.) performed echocardiography using a 3-MHz transthoracic probe and a dedicated unit (Xario SSA-660A; Toshiba, Japan). LV systolic and diastolic volumes and EF were estimated using the Simpson discs method. LVEF was then calculated as: 100 × [(LV end-diastolic volume − LV end-systolic volume)/LV end-diastolic volume] [3, 19]. EC-dP/dt MAX was evaluated by estimating the mitral valve regurgitation jet, according to the method proposed by Bargiggia et al. [11]. For measurements of E a/E es, we used the simplified method proposed by Chen et al. [8]. E a was approximated from the ratio of end-systolic pressure (ESP) to SV, where ESP was estimated as 0.9 × systolic blood pressure [20] and SV was computed as the product of the velocity–time integral, obtained using pulse wave Doppler in an apical four-chamber view, and the LV outflow tract cross-sectional area, measured in the parasternal long-axis view. E es was calculated as ESP/(end-systolic volume − V 0), in which V 0, the intersection of the end-systolic elastance line with the x axis (the “volume” axis) of a LV pressure–volume loop, can be considered negligible [21–23]. In a secondary analysis, we used the pressure wave dicrotic notch as an alternative way to estimate ESP. However, as we could not directly evaluate the aortic dicrotic notch, which best represents ESP [8], we used the dicrotic notch determined on the peripheral waves as a surrogate for the central dicrotic notch.
Pulse waveform analysis measurements
Pulse waveform analysis was performed with the Most Care device. This device analyzes the arterial signal using a sampling frequency of 1,000 Hz. The high-frequency sampling enables analysis of the shape of the pressure waveform and correct measurement of pressures (systolic, diastolic, mean, dicrotic).
After zeroing the arterial pressure-transducer system and before each measurement, the arterial waveform signal fidelity was checked visually using a fast flush test to assess the adequacy of the damping of the arterial shape [24]. If there was a resonance effect of the catheter-transducer system [24, 25], we either adapted the pulse wave analysis system setting [26] or used the resonance over-shoot eliminator (ROSE; Becton-Dickinson, Franklin Lakes, NJ) if there was excessive under-damping/resonance [14], to maximize the signal-to-noise ratio. The decision to improve the arterial trace quality was taken prior to collecting the pulse waveform analysis data in order to avoid any potential influence of the operators (S.S. and A.D.). Pulse wave analysis measurements were performed with both the investigators blinded to the data obtained from echocardiography and to other hemodynamic measurements except arterial pressure.
As this pulse wave analysis device does not require a dedicated catheter-transducer system, it was connected via a simple cable to the main monitoring system. Because a high-quality signal “without postprocessing” is needed for accurate estimations, we used the unprocessed arterial signal directly from the analog output of the main monitor, for continuous recording of the radial or femoral arterial pressure waveform and computation of AP-dP/dt MAX and CCE. dP/dt MAX was estimated from the arterial pressure waveform as the maximal rate of pressure change over time measured between two consecutive points during the systolic upstroke and is expressed in millimeters Hg per millisecond [14] (Fig. 1, Electronic supplementary material). CCE is a dimensionless variable (i.e., measured in arbitrary units) that ranges from negative values (worst values) to +1 (best value) (see the Electronic supplementary material for details of computation) [15–18]. Briefly, CCE is determined from the morphology of the arterial waveform and computed from the ratio of systolic power (i.e., systolic energetic performance) to the power of the entire heart beat (i.e., total energetic expenditure of the heartbeat). Systolic power and the power of the entire cardiac cycle are obtained using mathematical “power functions” of arterial pressure changes over time [17].
For all pulse wave analysis variables, values (approximately 10–15) were averaged over the time needed for each echocardiography measurement.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the normality of all the variables analyzed. The relationship between EC-dP/dt MAX and AP-dP/dt MAX was assessed using linear correlation analysis. Agreement between EC-dP/dt MAX and EC-dP/dt MAX was determined by the Bland–Altman method [27]. The percentage error was also calculated [28]. The relationship between CCE and LVEF was assessed using linear correlation analysis, and for the relationships between E a/E es and CCE a nonlinear regression model was used. Receiver operating characteristic (ROC) curves were constructed to study the ability of CCE to predict low (<40 %) or normal (≥50 %) LVEF [29], or altered A–V coupling (E a/E es ≥1.3) [30]. A P < 0.05 was considered statistically significant.
Results
In this convenience sample, 70 patients were enrolled. Patient characteristics are shown in Table 1 and hemodynamic and respiratory profiles in Table 2. Eleven patients (16 %) had significant under-damping/resonance of the arterial pressure signal and needed positioning of the ROSE device before measurements. No patient was excluded after performing pulse waveform analysis measurements. Transesophageal echocardiography was used, for clinical purposes, in 15 patients who had inadequate transthoracic recordings.
All patients had LVEF assessed by echocardiography. The mean LVEF was 53 ± 18 % (range 12–90 %). Mean CCE was 0.16 ± 0.26 (range −0.56 to +0.89). There was a good correlation between LVEF and CCE (r = 0.88, 95 % CI 0.81–0.92, P < 0.001; Fig. 1). Further analyses for patients with LVEF ≤40 % (n = 16) and >40 % (n = 54) showed similar correlations between LVEF and CCE (r = 0.59 and r = 0.67, respectively; Figs. 2, 3, Electronic supplementary material).
Relationship between CCE and LVEF. CCE was estimated by pulse wave analysis and LVEF by echocardiography. The correlation coefficient is 0.88 (95 % CI 0.81–0.92, P < 0.001). Black dots represent LVEF <40 %, white dots LVEF ≥40 %. The solid line is the line of regression, dashed lines are the 95 % CI
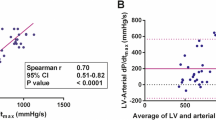
Of the 70 patients, 44 (63 %) had a detectable mitral regurgitation jet so that dP/dt MAX could be estimated by echocardiography. In these patients, the mean EC-dP/dt MAX was 1,240 ± 477 mmHg/s (range 450–2,000 mmHg/s). Mean AP-dP/dt MAX was 1,216 ± 410 mmHg/s (range 400–1,900 mmHg/s). Values of dP/dt MAX from the two techniques were significantly correlated (r = 0.93, 95 % CI 0.87–0.96, P < 0.001; Fig. 2a). The mean bias between EC-dP/dt MAX and AP-dP/dt MAX was 23.7 mmHg/s (95 % CI −23.6 to 71.0) with limits of agreement of −372 to +383 mmHg/s (lower 95 % CI −454 to −290; upper 95 % CI 338 to 502) and a relative percentage error of 28 % (Fig. 2b).
Relationship between dP/dt MAX estimated by echocardiography and pulse wave analysis. a Linear regression: correlation between dP/dt MAX estimated by echocardiography and pulse wave analysis (r = 0.93, 95 % CI 0.87–0.96; P < 0.001) for 44 patients with mitral insufficiency. Solid line line of regression. b Bland–Altman analysis. Solid line mean difference (bias); dashed lines limits of agreement (LoA, bias 1.96 SD). Lower 95 % CI of LoA, −454 to −290; upper 95 % CI of LoA, 338 to 502)
The mean E a/E es was 0.86 ± 0.74 (range 0.07–3.90). E a/E es and CCE were inversely related (r = −0.81, 95 % CI −0.88 to −0.71, P < 0.001; Fig. 3). A similar correlation was observed between CCE and E a/E es when peripheral dicrotic pressures were used to estimate ESP (Fig. 4, Electronic supplementary material).
Relationship between CCE and A–V coupling. CCE was calculated with pulse wave analysis and A–V coupling (E a/E es) estimated by echocardiography. For E es evaluation, ESP was determined as 0.9 × systolic arterial pressure. E a/E es and CCE were inversely related (r = −0.81, 95 % CI −0.88 to −0.71, P < 0.001)
A CCE value <0.07 predicted LVEF <40 % with a sensitivity of 0.93 and a specificity of 0.96. The area under the ROC curve for detecting LVEF <40 % by CCE was 0.98 (95 % CI 0.90–1.0, P < 0.001; Fig. 4a). A CCE value of >0.12 predicted LVEF ≥50 % with a sensitivity of 0.96 and a specificity of 0.82. The area under the ROC curve to detect LVEF ≥50 % by CCE was 0.94 (95 % CI 0.87–1.0, P < 0.001; Fig. 4a). A CCE value <0.12 predicted E a/E es ≥1.3 with a sensitivity of 0.93 and a specificity of 0.89. The area under the ROC curve for detecting E a/E es ≥1.3 by CCE was 0.94 (95 % CI 0.83–1.0, P < 0.001; Fig. 4b).
Prediction of LVEF and A–V coupling by CCE. a ROC curves constructed to study the ability of CCE to predict a LVEF <40 % (solid line) and ≥50 % (dashed line). The area under the ROC curve is 0.98 (95 % CI 0.90–1.0, P < 0.001) for LVEF <40 % and 0.94 (95 % CI 0.87–1.0, P < 0.001) for LVEF ≥50 %. The best cut-off values were a CCE value <0.07 for predicting LVEF <40 % (sensitivity 0.93, specificity 0.96), and a CCE value >0.12 for predicting LVEF ≥50 % (sensitivity 0.98, specificity 0.82). b ROC curve constructed to study the ability of CCE to predict E a/E es ≥1.3. The area under the ROC curve is 0.94 (95 % CI 0.83–1.0, P < 0.001). A CCE value <0.12 predicted E a/E es ≥1.3 with a sensitivity of 0.93 and a specificity of 0.89
We observed similar correlations and agreements between parameters derived from pulse waveform analysis and echocardiography in patients with sepsis (n = 19; 27 %), low systemic vascular resistance (n = 36; 51 %) and norepinephrine infusion (n = 23; 32 %) as in the other patients (Figs. 5–13, Electronic supplementary material). In addition, similar relationships were observed using radial and femoral lines (femoral dP/dt vs. echo dP/dt r 2 = 0.80 and radial dP/dt vs. echo dP/dt r 2 = 0.88).
Finally, data regarding SV values obtained by echocardiography and pulse wave analysis, and from a pulmonary artery catheter, are provided in the Electronic supplementary material Tables 1 and 2.
Discussion
This study showed that arterial pressure waveform analysis can provide useful information on cardiac function in critically ill patients, with reliable estimation of LV contractility and performance. Arterial pressure waveform-derived dP/dt MAX reliably estimated EC-dP/dt MAX, an index of LV contractility, and CCE was a reliable indicator of cardiac function and A–V coupling.
LV dP/dt MAX is an index of LV function, and especially contractility, representing the rate of pressure rise during the isovolumetric contraction period [31]. LV dP/dt MAX occurs before opening of the aortic valve, which limits the influence of afterload [32]. LV dP/dt MAX may thus reflect LV function better than LVEF [1], even though it remains a preload-dependent variable. The estimation of LV dP/dt MAX requires LV catheterization with a high-fidelity pressure catheter, but it can also be estimated from the mitral regurgitant jet [3, 11]. Admittedly, LV dP/dt MAX and peripheral artery-derived dP/dt MAX represent different phases in the cardiac cycle: LV dP/dt MAX is a measure of isovolemic contraction (pre-ejection phase variable), whereas peripheral artery-derived dP/dt MAX is actually measured after aortic valve opening as it occurs during the LV ejection phase (ejection phase variable). Factors affecting vascular tone (elastance, compliance and stiffness) may lead to over- or underestimation of the true LV dP/dt MAX. In addition, the arterial pulse is amplified during travel in the arterial tree [33], which may affect the reliability of peripheral artery-derived dP/dt MAX. However, the relative contributions of left ventricular and arterial factors in peripheral artery-derived dP/dt MAX are not well defined.
Another key point is that the dP/dt MAX is usually estimated with pulse wave analysis over the first 20 ms of the pressure upstroke. However, the dP/dt MAX can occur earlier (e.g., in LV hypertrophy) or later (e.g., in cardiac failure). We used a pulse wave analysis device that is able to measure the highest dP/dt of the pressure upstroke, even when it does not fall during the first 20 ms of the upstroke. Others have used arterial pulse derived dP/dt. Tartiere et al. [34] used applanation tonometry to noninvasively calculate the radial dP/dt MAX and to compare it to LV dP/dt MAX estimated by echocardiography. Good correlation and agreement were found between the two techniques. Using a femoral arterial line in cardiac surgery patients, De Hert et al. [35] reported that changes in femoral dP/dt MAX, estimated by the transpulmonary thermodilution system, accurately reflect changes in LV dP/dt MAX measured by a high-fidelity LV catheter. These data, combined with our results, suggest that peripheral artery dP/dt MAX may be considered a suitable surrogate for LV dP/dt MAX.
The usefulness of dP/dt MAX in critically ill patients has not been established. Nevertheless, in patients with cardiac failure, alterations in dP/dt are associated with mortality however the dP/dt is measured, with a micro-manometer tipped catheter [36], with echo-Doppler [37] or noninvasively from the radial pulse wave [38]. However, some major advantages of peripheral artery-derived dP/dt MAX have to be mentioned. First, compared to invasive LV dP/dt MAX and echocardiographic techniques, it can be obtained in most critically ill patients as they will already have had an arterial catheter inserted. Second, it is feasible even in the absence of mitral valve regurgitation. Finally, it can be used to detect abrupt changes in LV function [39].
LVEF is easily obtained by echocardiography [40] and is often used to assess the contractile performance of the left ventricle [41]. An LVEF less than 40 %, which indicates severely depressed LV systolic function [29], is associated with a markedly increased risk of death [42–44]. Our data show that CCE could serve as an easy bedside tool to detect alterations in LVEF and alert the physician to the need to perform echocardiography. CCE showed a good correlation with LVEF and was also able to discriminate normal, moderately altered and markedly altered LVEF, as CCE values <0.07 and >0.12 diagnosed LVEF <40 and ≥50 %, respectively, with good sensitivity and specificity.
E a and E es are relatively independent of loading conditions, making their relationship a more accurate index of LV function [45]. E a is a measure of the net arterial load that is imposed on the left ventricle. E es indicates how much the LV end-systolic volume will increase in response to an elevation in ESP [46]. E a and E es share common units (i.e., mmHg/ml), and their ratio, E a/E es, known as A–V coupling, represents a direct measure of the interaction between the left ventricle and the arterial system [47]. E a and E es are optimally coupled, for an optimal mechanical efficiency (best possible stroke work for the cardiovascular system), when A–V coupling is approximately 1 [48]. Physiologically, we usually operate with E a = 0.5 E es, a situation that corresponds to the best energetic efficiency (best ratio between stroke work and energy needed for cardiac contraction) [4, 49]. In contrast, in patients with congestive heart failure, E a/E es typically increases to as high as 4.0 [49]. We demonstrated an inverse relationship between CCE and E a/E es. Moreover, a CCE value <0.12 could predict E a/E es ≥1.3 with good sensitivity and specificity. Importantly, E a/E es is an early sign of myocardial impairment in critically ill patients, as the alteration in E a/E es occurs prior to significant LV dysfunction [50]. CCE may thus be proposed as a simple surrogate marker of E a/E es and could serve as a “wake-up call” for an echocardiographic evaluation to assess the actual cardiovascular status.
A number of limitations have to be considered when using the PRAM Most Care system. Under-damped arterial pressure waveforms may affect both the amplitude and morphology of the pressure wave, thus affecting the precision of the tool under study [26, 51–53]. This pulse wave analysis system is highly sensitive to artifacts because its algorithm is exclusively based on the analysis of the pressure wave morphology [13]. Moreover, it is dependent on the operator, who sometimes needs to maximize the quality of the arterial signal [26]. The need for skill and the operator dependency may limit the application of pulse wave analysis in daily clinical practice.
In the present study, we corrected the resonance of the catheter-transducer system by maximizing the signal-to-noise ratio (i.e., we adapted the setting of the pulse wave analysis system) or by using the resonance over-shoot eliminator, ROSE, in patients with an under-damped pressure waveform, using morphologic criteria (i.e., the presence of a double systolic peak or dicrotic notch, and oscillations in the systolic decline and diastolic run-off of the pressure wave) and, after the fast-flush test, revealed an overshoot in the waveform [24, 25] (examples provided in the Electronic supplementary material, Figs. 14–16). Of note, no patient had to be excluded because of an inability to enhance the quality of the pressure wave signal. Aorta and aortic valve pathologies could influence the analysis of the arterial waveform [53]. Cardiac dysrhythmias can also affect the reliability of pulse wave analysis [54]. These conditions were considered as exclusion criteria in the present study. Finally, in hyperdynamic sepsis, where systemic vascular resistance is low, transmission of reflected waves can alter the morphology of the arterial signal [55]. In our trial, results were not affected by the sepsis state, low systemic vascular resistance, or norepinephrine administration.
A main limitation of this study is that we did not evaluate the respective alterations in these variables during hemodynamic changes related to different therapies or to spontaneous variations in the clinical state. Moreover, to estimate E a/E es we used a simplified method [8] and did not use a high-fidelity ventricular pressure catheter [5–7, 45]. The use of echocardiography may be suboptimal as a “gold standard”, but it was the best available comparator, as left heart catheterization would have been too invasive. Finally, these results need to be confirmed in an independent cohort.
Conclusions
We demonstrated that arterial pressure-derived variables provide information on cardiac performance in critically ill patients. Pulse wave analysis cannot replace the information provided by echocardiography, but could provide online hemodynamic evaluation in ICU patients. Low CCE and AP-dP/dt MAX indicate poor cardiac function and if present may be considered an indication for echocardiography.
References
Dittoe N, Stultz D, Schwartz BP, Hahn HS (2007) Quantitative left ventricular systolic function: from chamber to myocardium. Crit Care Med 35:S330–S339
Kirkpatrick JN, Vannan MA, Narula J, Lang RM (2007) Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol 50:381–396
De Backer D (2011) Evaluation of left ventricular systolic function. In: De Backer D, Cholley BP, Slama M, Vieillard-Baron A, Vignon P (eds) Hemodynamic monitoring using echocardiography in the critically Ill. Springer, Heidelberg, pp 79–89
Robotham JL, Takata M, Berman M, Harasawa Y (1991) Ejection fraction revisited. Anesthesiology 74:172–183
Chantler PD, Lakatta EG, Najjar SS (2008) Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol 105:1342–1351
Sagawa K, Suga H, Shoukas AA, Bakalar KM (1977) End-systolic pressure/volume ratio: a new index of ventricular contractility. Am J Cardiol 40:748–753
Burkhoff D, de Tombe PP, Hunter WC, Kass DA (1991) Contractile strength and mechanical efficiency of left ventricle are enhanced by physiological afterload. Am J Physiol 260:H569–H578
Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA (2001) Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38:2028–2034
Cariou A, Pinsky MR, Monchi M, Laurent I, Vinsonneau C, Chiche JD, Charpentier J, Dhainaut JF (2008) Is myocardial adrenergic responsiveness depressed in human septic shock? Intensive Care Med 34:917–922
Little WC (1985) The left ventricular dP/dtmax-end-diastolic volume relation in closed-chest dogs. Circ Res 56:808–815
Bargiggia GS, Bertucci C, Recusani F, Raisaro A, De Servi S, Valdes-Cruz LM, Sahn DJ, Tronconi L (1989) A new method for estimating left ventricular dP/dt by continuous wave Doppler-echocardiography. Validation studies at cardiac catheterization. Circulation 80:1287–1292
Jabot J, Monnet X, Bouchra L, Chemla D, Richard C, Teboul JL (2009) Cardiac function index provided by transpulmonary thermodilution behaves as an indicator of left ventricular systolic function. Crit Care Med 37:2913–2918
Romano SM, Pistolesi M (2002) Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med 30:1834–1841
Romagnoli S, Romano SM, Bevilacqua S, Lazzeri C, Gensini GF, Pratesi C, Quattrone D, Dini D, De Gaudio AR (2011) Dynamic response of liquid-filled catheter systems for measurement of blood pressure: precision of measurements and reliability of the pressure recording analytical method with different disposable systems. J Crit Care 26:415–422
Romano SM, Olivotto I, Chiostri M, Giglioli C, Margheri M, Gensini GF (2006) Minimally invasive and noninvasive hemodynamic monitoring of the cardiovascular system: available options and future perspectives. Curr Cardiol Rev 2:37–39
Gelsomino S, Luca F, Renzulli A, Rubino AS, Romano SM, van der Veen FH, Carella R, Maessen JG, Gensini GF, Lorusso R (2011) Increased coronary blood flow and cardiac contractile efficiency with intraaortic balloon counterpulsation in a porcine model of myocardial ischemia-reperfusion injury. ASAIO J 57:375–381
Romano SM (2012) Cardiac cycle efficiency: a new parameter able to fully evaluate the dynamic interplay of the cardiovascular system. Int J Cardiol 155:326–327
Scolletta S, Ranaldi G, Carlucci F, Franchi F, Romano SM, Biagioli B (2010) Relationship between N-terminal pro-B-type natriuretic peptide (Nt-proBNP) and cardiac cycle efficiency in cardiac surgery. Biomed Pharmacother 64:511–515
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA (1992) Effective arterial elastance as index of arterial vascular load in humans. Circulation 86:513–521
Burkhoff D, Sagawa K (1986) Ventricular efficiency predicted by an analytical model. Am J Physiol 250:R1021–R1027
Nevo E, Marmor M, Lanir Y, Weiss TA, Marmor A (1995) A new methodology for non-invasive clinical assessment of cardiovascular system performance and of ventricular-arterial coupling during stress. Heart Vessels 10:24–34
Cohen-Solal A, Faraggi M, Czitrom D, Le Guludec D, Delahaye N, Gourgon R (1998) Left ventricular-arterial system coupling at peak exercise in dilated nonischemic cardiomyopathy. Chest 113:870–877
Kleinman B, Powell S, Kumar P, Gardner RM (1992) The fast flush test measures the dynamic response of the entire blood pressure monitoring system. Anesthesiology 77:1215–1220
Gardner RM (1981) Direct blood pressure measurement – dynamic response requirements. Anesthesiology 54:227–236
Scolletta S, Taccone FS, Romagnoli S, Landoni G, Giomarelli P (2011) Pressure recording analytical method to measure cardiac output after cardiac surgery: some practical considerations. Br J Anaesth 107:814–815
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15:85–91
Vieillard-Baron A, Charron C, Chergui K, Peyrouset O, Jardin F (2006) Bedside echocardiographic evaluation of hemodynamics in sepsis: is a qualitative evaluation sufficient? Intensive Care Med 32:1547–1552
Antonini-Canterin F, Enache R, Popescu BA, Popescu AC, Ginghina C, Leiballi E, Piazza R, Pavan D, Rubin D, Cappelletti P, Nicolosi GL (2009) Prognostic value of ventricular-arterial coupling and B-type natriuretic peptide in patients after myocardial infarction: a five-year follow-up study. J Am Soc Echocardiogr 22:1239–1245
Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K (1987) Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure–volume relationships. Circulation 76:1422–1436
Choong CY, Herrmann HC, Weyman AE, Fifer MA (1987) Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol 10:800–808
Nichols WW, O’Rourke MF (2005) McDonald’s blood flow in arteries: theoretic, experimental, and clinical principles, 5th edn. Hodder Arnold, New York
Tartiere JM, Logeart D, Beauvais F, Chavelas C, Kesri L, Tabet JY, Cohen-Solal A (2007) Non-invasive radial pulse wave assessment for the evaluation of left ventricular systolic performance in heart failure. Eur J Heart Fail 9:477–483
De Hert SG, Robert D, Cromheecke S, Michard F, Nijs J, Rodrigus IE (2006) Evaluation of left ventricular function in anesthetized patients using femoral artery dP/dt(max). J Cardiothorac Vasc Anesth 20:325–330
Suzuki H, Shimano M, Yoshida Y, Inden Y, Muramatsu T, Tsuji Y, Tsuboi N, Hirayama H, Shibata R, Murohara T (2010) Maximum derivative of left ventricular pressure predicts cardiac mortality after cardiac resynchronization therapy. Clin Cardiol 33:E18–E23
Kolias TJ, Aaronson KD, Armstrong WF (2000) Doppler-derived dP/dt and -dP/dt predict survival in congestive heart failure. J Am Coll Cardiol 36:1594–1599
Tartière JM, Tabet JY, Logeart D, Tartière-Kesri L, Beauvais F, Chavelas C, Cohen Solal A (2008) Noninvasively determined radial dP/dt is a predictor of mortality in patients with heart failure. Am Heart J 155:758–763
Thiele RH, Durieux ME (2011) Arterial waveform analysis for the anesthesiologist: past, present, and future concepts. Anesth Analg 113:766–776
Bergenzaun L, Gudmundsson P, Ohlin H, During J, Ersson A, Ihrman L, Willenheimer R, Chew MS (2011) Assessing left ventricular systolic function in shock: evaluation of echocardiographic parameters in intensive care. Crit Care 15:R200
Schiller NB (2003) Ejection fraction by echocardiography: the full monty or just a peep show? Am Heart J 146:380–382
Mock MB, Ringqvist I, Fisher LD, Davis KB, Chaitman BR, Kouchoukos NT, Kaiser GC, Alderman E, Ryan TJ, Russell RO Jr, Mullin S, Fray D, Killip T III (1982) Survival of medically treated patients in the coronary artery surgery study (CASS) registry. Circulation 66:562–568
The Multicenter Postinfarction Group (1983) Risk stratification and survival after myocardial infarction. N Engl J Med 309:331–336
Pilote L, Silberberg J, Lisbona R, Sniderman A (1989) Prognosis in patients with low left ventricular ejection fraction after myocardial infarction. Importance of exercise capacity. Circulation 80:1636–1641
Suga H (2003) Cardiac energetics: from E(max) to pressure–volume area. Clin Exp Pharmacol Physiol 30:580–585
Little WC, Pu M (2009) Left ventricular-arterial coupling. J Am Soc Echocardiogr 22:1246–1248
Sunagawa K, Maughan WL, Burkhoff D, Sagawa K (1983) Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 245:H773–H780
Starling MR (1993) Left ventricular-arterial coupling relations in the normal human heart. Am Heart J 125:1659–1666
Asanoi H, Sasayama S, Kameyama T (1989) Ventriculoarterial coupling in normal and failing heart in humans. Circ Res 65:483–493
Prabhu SD (2007) Altered left ventricular-arterial coupling precedes pump dysfunction in early heart failure. Heart Vessels 22:170–177
Gibbs NC, Gardner RM (1988) Dynamics of invasive pressure monitoring systems: clinical and laboratory evaluation. Heart Lung 17:43–51
Camporota L, Beale R (2010) Pitfalls in haemodynamic monitoring based on the arterial pressure waveform. Crit Care 14:124
Vincent JL, Rhodes A, Perel A, Martin GS, Della RG, Vallet B, Pinsky MR, Hofer CK, Teboul JL, de Boode WP, Scolletta S, Vieillard-Baron A, De BD, Walley KR, Maggiorini M, Singer M (2011) Clinical review: update on hemodynamic monitoring – a consensus of 16. Crit Care 15:229
Maj G, Monaco F, Landoni G, Barile L, Nicolotti D, Pieri M, Melisurgo G, Pappalardo F, Zangrillo A (2011) Cardiac index assessment by the pressure recording analytic method in unstable patients with atrial fibrillation. J Cardiothorac Vasc Anesth 25:476–480
Hatib F, Jansen JR, Pinsky MR (2011) Peripheral vascular decoupling in porcine endotoxic shock. J Appl Physiol 111:853–860
Acknowledgments
We wish to thank Vygon Health (Padua, Italy) for kindly providing the Most Care device, but all other costs were covered by departmental funds only.
Conflicts of interest
S. Scolletta has received lecture fees and material for studies from Vygon, and grants and material for studies from Edwards Lifesciences; D. De Backer has received honoraria for lectures and grants for studies from Edwards Lifesciences. The other authors declare they have no conflicts of interest related to this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scolletta, S., Bodson, L., Donadello, K. et al. Assessment of left ventricular function by pulse wave analysis in critically ill patients. Intensive Care Med 39, 1025–1033 (2013). https://doi.org/10.1007/s00134-013-2861-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2861-8