Abstract
Purpose
To identify the prognostic factors associated with mortality in heat-related illness.
Methods
Multi-center observational cohort-study in 16 emergency departments (ED) belonging to the teaching hospital network of the Paris area. The cohort comprised all patients admitted to one of the EDs during the August 2003 heat wave in Paris and having a core temperature >38.5°C. Baseline clinical and biological data in ED, patient’s course and 1-year survival rate were recorded. Potential prognostic factors associated with death were assessed by Cox proportional-hazards analysis.
Results
A total of 1,456 patients were included. Mean age was 79 ± 19 years. Critically ill conditions were noted in 391 patients (27%), but only 72 (5%) were admitted into an intensive care unit. The survival rate was 57% at 1 year as compared to an expected 90% (P < 0.001). Nine independent prognostic factors were identified: previous treatment with diuretics, living in an institution, age >80 years, cardiac disease, cancer, core temperature >40°C, systolic arterial pressure <100 mmHg, Glasgow coma scale <12 and transportation to hospital by ambulance. We defined three risk groups: low, intermediate and high risk, with a 1-year survival rate of 85, 61 and 18%, respectively.
Conclusions
We observed a low survival rate and developed a risk score based on easily obtained variables that may be useful to clinicians managing casualties from future heat waves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In contrast to exertional heatstroke related to a high production of heat during strenuous exercise, non-exertional heatstroke results from prolonged exposure to high temperature [1]. Non-exertional heatstroke is encountered in tropical areas, but exceptional heat waves have been increasingly reported in temperate countries [2–4] and are possibly related to climate change [5]. The health consequences of these heat waves can be catastrophic, leading to overcrowding of health facilities [6], considerable excess mortality [7] and poor long-term outcome in surviving patients [8, 9].
Most previous studies have assessed the climate risk factors for heat wave [10, 11] or the individual risk factors for developing heatstroke [3]. A recent meta-analysis identified several prognostic factors associated with a high risk (being confined to bed, not leaving home daily, being unable to care for oneself, and pre-existing psychiatric, cardiovascular or pulmonary illnesses) or a low risk (having home air-conditioning, visiting cool environments and increasing social contacts) of death during heat waves [12]. Identifying these prognostic factors could help to detect those individuals who are at risk during heat waves and who may benefit from risk-reducing interventions. Very few studies have assessed the prognostic factors in patients having heat-related illness during a heat wave. What studies have taken place have included relatively few patients, mostly those having suffered severe heatstroke and particularly those admitted to the intensive care unit (ICU) [8, 13, 14]. Knowledge of these risk factors is important since a heat wave is a catastrophic event leading to considerable overload in emergency departments (ED) [6]. Therefore, determining the therapeutic priorities, including access to the ICU, appears essential. A further important hypothesis is that, during a heat wave, extended criteria of elevated core temperature should be used because of the considerable excess mortality encountered in an elderly population [6, 7].
For these reasons, immediately after the French heat wave that occurred in 2003, we initiated an observational cohort study in the Paris urban area to identify the prognostic factors associated with mortality. We included all patients with a core temperature >38.5°C.
Methods
Patients
We performed a multi-center observational cohort-study of febrile patients admitted to 16 EDs belonging to the teaching hospital network of the Paris area during the heat wave of 2003. The criteria for inclusion in the study were: (1) emergency admission in the adult ED of one of the participating centers between 5 and 14 August 2003; (2) core temperature ≥ 38.5°C. The study period covered the core period of the heat wave and of the excess short-term mortality rate recorded during this heat wave [6, 8]. There were no exclusion criteria, except age <16 years.
An electronic clinical record form was used to collect data (Télémédecine Technologies, Boulogne, France). Data were verified by on-site clinical monitoring. Inconsistencies among data were systematically checked and solved. The complete chart was examined by an expert panel who decided if the primary diagnosis was heatstroke or not, and if the patient had critically ill conditions that might have required admission to the ICU. To assess the effect on morbidity, the activities of daily living (ADL, from 0: worse to 6: best, autonomy free) scale was recorded at baseline and at 1-year follow-up [15].
Patients were followed until death or until 1 year after admission to the ED. This long-term follow-up was preferred to short-term outcome since, during non-exertional heatstroke, an old and frail population is concerned, and geriatricians know that any health impact in this population is responsible for early deaths as well as late deaths related to decreased autonomy and frailty [16]. Surviving patients or their family were contacted and interviewed by telephone. If contact could not be made, tracking was attempted through health care providers, general practitioners or any acquaintances identified in the medical record. When patients were lost to follow-up, an inquiry was sent to the French National Registry of Death (Institut National de la Statistique et des Etudes Economiques, Paris, France).
Statistical analysis
Data are expressed as mean ± SD. Survival was estimated by the Kaplan–Meier method, and differences in survival were assessed by the log–rank test. Univariate and multivariate Cox proportional-hazards models were used to determine the contribution of variables, expressed as hazard ratio and its 95% confidence interval (CI). To avoid overfitting, we used a conservative approach and included only the significant variables in the univariate analysis (P value of entry ≤0.10), except for some variables that were thought to be prognostic or had been demonstrated to be prognostic in previous studies. If the Pearson correlation coefficient between variables was 0.60 or more, only the variable judged to be clinically more relevant was entered. Continuous variables were transformed in dichotomous variables using receiver-operating characteristic (ROC) curves, the threshold being that which minimized the distance to the ideal point [17]. Inclusion in the final model was determined by a backward stepwise process. The discrimination of the model was assessed using the C statistic and its calibration using the Hosmer–Lemeshow statistic. To internally validate this model, we performed a ten-fold cross validation [18].
To develop a prognostic score, we assigned the prognostics factors identified by multivariate analysis using only variables available at admission and using weighted points proportional to the β regression coefficient values and rounded to the nearest integer. The population was divided into three categories: low, intermediate and high risk for death. The uncertainty surrounding the survival rate was evaluated using the standard bootstrap technique, and we calculated the mean and 95% CI of the survival rate at 1 year in the three risk groups [19].
To determine the naturally expected survival rate in this population, age and sex-specific mortality rate ratios were calculated with the use of life-expectancy values derived from life tables of the French national database (Institut National de la Statistique et des Etudes Economiques, Paris, France). We also performed a multiple backward logistic regression to assess variables associated with loss on follow-up.
All P values were two-tailed, and a P value < 0.05 was considered significant. Statistical analysis was performed using NCSS 2001 software (Statistical Solutions Ltd., Cork, Ireland).
Results
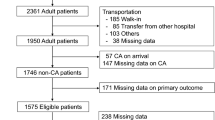
We included 1,461 patients, 5 of whom were excluded for missing important variables or young age. Thus, 1,456 patients constituted the study sample. Figure 1 shows the number of patients included at each date of the study period and the corresponding excess deaths and mean outside temperatures observed in France. The mean age was 79 ± 19 years; 689 patients (47%) had a core temperature >40°C, and 391 patients (27%) had critically ill conditions, but only 72 (5%) were admitted into an ICU (Fig. 2). According to the expert panel, the main diagnosis was heat-related illness in 88% of cases. Infection was diagnosed in 515 patients (36%), mainly pulmonary (45% of infected patients).
Number of patients included (c) and comparison with the number of excess death (b) and mean maximum and minimum outside temperatures (a) recorded in France during August 2003. Data in a and b were those published by Fouillet et al. [8]. The vertical dotted lines indicate the inclusion period of the study
A cooling procedure was applied in 927 patients (64%), 15% during the prehospital phase and 59% in the ED. The methods of cooling were application of ice packs (66% of cooled patients) or wet sheets (84%), air ventilation (27%), spraying with aerosolized water (19%), putting the patient in an air-conditioned room (9%) and intravenous injection of cooled saline (5%). Antipyretic drugs (mainly acetaminophen) were administered in 932 patients (64%) and corticoids in 16 (1.1%).
During up to 1 year of follow-up, 160 patients (11%) entering the study were lost to follow-up. Four variables were significantly associated with a loss to follow-up: social precariousness (odds ratio 4.22, 95% CI 2.14–8.31, P < 0.001), lack of critically ill conditions (odds ratio 1.75, 95% CI 1.00–3.12, P = 0.05), a Glasgow coma score of 15 (odds ratio 1.92, 95% CI 1.22–3.03, P = 0.005) and lack of any pre-existing disease (odds ratio 1.75, 95% CI 1.47–3.33, P < 0.001).
The survival rate at 1 year was 57% (95% CI 54–59) and was significantly lower than that expected (90%, 95% CI 88–91) (Fig. 3). The hazard rate of the cohort rapidly decreased during the first 3 months (from 15.9 to 0.7%/week), then slowly decreased to 0.4 at 1 year and was close to that expected (0.2%/week) (Fig. 3).
The ADL scale at baseline was determined in 1,031 patients (71%). Among them, 41% died. It should be pointed out that 35% of the non-survivors were autonomy free (ADL = 6). The ADL scale at baseline and 1 year later was available in 607 surviving patients, and an impairment in ADL scale was noted in 18% (Fig. 4).
Table 1 shows that several variables were associated with a higher risk of death. Missing data from one or more variables restricted the analysis to 1,032 patients (71%). After exclusion of variables with a low prevalence or exhibiting multicolinearity, 26 variables were found to be of prognostic significance in the univariate analysis, and 11 variables maintained their prognostic significance after multivariate analysis (Table 1): age >80 years, cancer, cardiac disease, use of diuretics, living in an institution, transportation to hospital by an ambulance, white blood cell count >14,000/mm3, a systolic arterial pressure <100 mmHg, a Glasgow coma scale <12, a core temperature >40°C, and the occurrence of pulmonary and/or bloodstream infection. Using the ten-fold cross validation technique, the area under the ROC curve [0.77 (95% CI 0.74–0.80)] was not significantly different from that of the model [0.78 (95% CI 0.76–0.81)].
To calculate a risk score, we used only variables available at the admission (Table 2). Missing data from one or more variables restricted the analysis to 1,198 patients (82%). Survival estimates for each value of the score was used to define three groups: a low risk group (score: 0–6), an intermediate-risk group (score: 7–12) and a high-risk group (score: 13–22) (Fig. 5). The survival rates at 1 year for these three groups were 85% (95% CI 81–89), 61% (95% CI 57–65) and 18% (95% CI 13–23). The bootstrap analysis provided a survival rate at 1 year of 87% (95% CI 83–90), 63% (95% CI 59–66) and 19% (95% CI 14–24). In comparison, the expected survival at 1 year in these three groups was 92% (95% CI 89–94), 88% (95% CI 85–91) and 87% (95% CI 83–91).
Kaplan–Meier survival curves according to the prognostic classification. Because data for some variables were missing for some patients, the prognostic classification was based on a total of 1,181 patients rather than 1,456. Risk categories were determined by adding up the points for each prognostic factor (see Table 2). The number of patients at risk at each time is shown above the figure
Discussion
In this large cohort we observed a low survival rate, although entry to the study was on the basis of a definition of hyperthermia of core temperature >38.5°C, and a very low proportion of these patients was admitted into an ICU. We developed a risk score based on easily obtained variables that accurately predict mortality in these patients. In addition, the study has shown that 18% of surviving patients displayed impairment in their autonomy 1 year later.
The inclusion criteria used in our study require some comments. In contrast to previous studies [1, 9, 13], we included patients with a core temperature >38.5°C rather than >40°C or even 40.6°C. Our main argument was that considerable excess deaths were observed, even in patients without very high core temperatures, mainly because the population involved was aged and frail. The 1-year survival rate we observed was low (57%) and very close to that observed previously in patients with a higher core temperature [8]. Also because cooling was applied before reaching the hospital in at least 15% of these patients, the maximum temperature recorded may not reflect the maximum core temperature reached. Thus, our results suggest that the usual criteria for heatstroke (core temperature above 40°C and central nervous system involvement)[1], which remain valid in exertional heatstroke occurring in young individuals, should no longer be retained during non-exertional heatstroke occurring during a heat wave, which represents a catastrophic event with major overcrowding of all health facilities. We also did not attempt to exclude infectious processes in our study for the following reasons: (1) many heatstroke were complicated by an infectious process, as previously reported [12, 13, 20]; (2) having an infectious process may facilitate the occurrence of a heatstroke. It should also be noted that since febrile patients represent less than 1–2% of patients usually seen in an ED [21], the proportion of patients having mainly an infectious process would have been limited in our study.
The survival rate was low, as previously reported [1, 9], but cannot be assessed without a comparison with expected survival rate since the population was old. This comparison shows that the hazard ratio (observed/expected) was close to 1 at 3 months after presentation (Fig. 3b), suggesting that most of the deleterious effects of heat-related illness can be observed during the first 3 months of the condition. Therefore, the number of excess deaths in France that was calculated during only August 2003 has probably been largely underestimated, as previously reported [22]. In surviving patients, heatstroke has been reported to be associated with important neuropsychological sequelae that may be important in elderly patients [7, 9]. Our study suggests that 18% of surviving patients experienced impairment in their autonomy (Fig. 5). Moreover, 35% of non-survivors were completely autonomous at baseline. This finding is at odds with the belief that almost all elderly patients who die during a heat wave are already severely disabled, indicating that more efforts should be attempted to provide appropriate prevention and care.
Our study demonstrates that the risk of death in patients attending EDs for heat-related illnesses during heat wave exposure could be predicted by the presence of 11 variables easily available at admission. These variables integrate an overview of parameters involved in heat-related illnesses severity: some of them indicate a greater susceptibility to heatstroke (age, pre-existing disease such as cancer, cardiac disease or chronic medication with diuretics), other variables more specifically reflect the severity of heatstroke’s consequences itself (core temperature, systolic arterial pressure, consciousness and leucocyte count) [12, 23], both (transportation to hospital by ambulance), or finally heatstroke complications (pulmonary or bloodstream infection). Our risk score (Table 2) accurately classified patients into subgroups at low, intermediate and high risk for death (Fig. 5). The survival rate of the the low-risk group was close to that expected using the life expectancy tables. The present study enables a clearer understanding of prognostic factors for heatstroke during a heat wave and those that favored death when heatstroke occurred. For example, psychiatric disorders and/or use of psychotropic drugs have been constantly reported as a risk factor for heatstroke exposure [2, 12], but appear to not be very important when heatstroke occurs. This was also the case for social precariousness.
The health consequences of a heat wave may be catastrophic [2–4, 8–13]. For this reason, considerable efforts have been made to identify climatic factors that predict the occurrence of heat waves, individual factors that favor the occurrence of heatstroke during a heat wave, or even factors that may alert the ED to prehospital heat-related excess mortality [24]. Our risk score provides a useful tool for the emergency team, allowing better allocation of therapeutic options, including access to the ICU. Although many of the patients in this study were probably not good candidates for admission to the ICU either because of old age and/or comorbidities or reduced autonomy, we think that the very low proportion of patients finally admitted (5%) indicates considerable overwhelming of health capacities as well as an underestimation of the severity of the disease, mainly in patients without a very high core temperature. It is noteworthy that there is convincing evidence in the literature that age, by itself, is not an appropriate means of selection of patients admitted to the ICU [25].
This study has several limitations. First, we have probably overestimated the expected survival in our cohort since the life expectancy tables used refer to the whole French population, and it is likely that patients presenting at an ED have a lower survival rate because of more frequent associated diseases. This overestimation is probably of minor magnitude since the survival rate in the low-risk group was not markedly different from that expected. Second, some important prognostic variables may have been missed because they were recorded in only a few patients (arterial pH, troponin) or because of the lack of power in the case of low prevalence. We also excluded variables not available at admission in our risk score, although some of them provided useful prognostic information (leucocytes, infectious complication). Third, the multivariate model identifies an association between prognostic factors and death and does not indicate causality. Nevertheless, there is strong suspicion of a causal link among the identified prognostic factors because they refer either to the severity of heatstroke (core temperature, systolic arterial pressure, consciousness), to a greater susceptibility to heatstroke (age, preexisting disease) or to a complication of heatstroke (severe infection). Finally, the multivariate model did not identify cooling as an independent protective factor (see Table 1), even when this variable was forced into the model (data not shown). This could be explained either by a severity bias (the most severe patients having a higher probability of death and a higher probability to receive cooling procedures) or by the discrepancy between time of measurement of temperature and time of cooling since some patients received prehospital cooling.
In conclusion, in a large cohort of patients with non-exertional heat-related illness, we observed a low 1-year survival rate (56%), although our inclusion criterion for considering heatstroke was a body temperature as low as 38.5°C. This low survival rate should be compared with the relatively low proportion of cooled patients (64%), the high proportion of pulmonary and/or bloodstream infection (35%) and the low proportion of patients admitted to the ICU (5%). We developed a risk score based on easily obtained variables that accurately predict mortality in these patients. These findings may be useful to clinicians in order to allocate therapeutic priorities during a heat wave catastrophic event, which markedly enhances the usual overcrowding of ED. The classical definition of heatstroke (core temperature >40°C) should probably be modified (core temperature >38.5°C) during a heat wave.
References
Bouchama A, Knochel JP. (2002) Heatstroke. N Engl J Med 346:1978–1988
Jones TS, Liang AP, Kilbourne EM, Griffin MR, Patriarca PA, Wassilak SG, Mullan RJ, Herrick RF, Donnell HD Jr, Choi K, Thacker SB (1982) Morbidity and mortality associated with the July 1980 heat wave in St Louis and Kansas City, MO. JAMA 247:3327–3331
Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL (1996) Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335:84–90
Rooney C, Mc Michael AJ, Kovats RS, Coleman P (1998) Excess mortality in England and Wales and in Greater London, during the 1996 heatwave. J Epidemiol Community Health 52:482–486
Patz JA, Campbell-Lendrum D, Holloway T, Foley JA (2005) Impact of regional climate change on human health. Nature 438:310–317
Dhainaut JF, Claessens YE, Ginsburg C, Riou B (2004) Unprecedented heat-related deaths during the 2003 heat wave in Paris: consequences on emergency departments. Crit Care 8:1–2
Al Bubrek D, Bakon S, Moran DS, Faibel M, Epstein Y (1997) Heat stroke-induced cerebellar atrophy: clinical course, CT and MRI findings. Neuradiology 39:195–197
Fouillet A, Rey G, Laurent F, Pavillon G, Bellec S, Guihenneuc-Jouyaux C, Clavel J, Jougla E, Hémon D (2006) Excess mortality related to the August 2003 heat wave in France. Int Arch Occup Environ Health 80:16–24
Argaud L, Ferry T, Le QH, Marfisi A, Ciorba D, Achache P, Ducluzeau R, Robert D (2007) Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med 167:2177–2183
O’Neill MS, Zanobetti A, Schwartz J (2003) Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol 157:1074–1082
Baccini M, Biggeri A, Accetta G, Kosatsky T, Katsouyanni K, Analitis A, Anderson HR, Bisanti L, D’Ippoliti D, Danova J, Forsberg B, Medina S, Paldy A, Rabczenko D, Schindler C, Michelozzi P (2008) Heat effects on mortality in 15 European cities. Epidemiology 19:711–719
Bouchama A, Dehbi M, Mohamed G, Matthies F, Shoukri M, Menne B (2007) Prognostic factors in heat wave-related deaths. A meta-analysis. Arch Intern Med 167:2170–2176
Misset B, de Jonghe B, Bastuji-Garin S, Gattolliat O, Boughrara E, Annane D, Hausfater P, Garrouste-Orgeas M, Carlet J (2006) Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: a National-multicenter risk-factor study. Crit Care Med 34:1087–1092
Pease S, Bouadma L, Kermarrec N, Schortgen N, Régnier B, Wolff M (2009) Early organ dysfunction course, cooling time and outcome in classic heatstroke. Intensive Care Med 35:1454–1458
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 185:914–919
Rivera R, Antognini JF (2009) Perioperative drug therapy in elderly patients. Anesthesiology 110:1176–1181
Fellahi JL, Parienti JJ, Hanouz JL, Plaud B, Riou B, Ouattara A (2008) Perioperative use of dobutamine in cardiac surgery and adverse cardiac outcome: propensity-adjusted analyses. Anesthesiology 108:979–987
Molinaro AM, Simon R, Pfeiffer RM (2005) Prediction error estimation: a comparison of resampling method. Bioinformatics 21:3301–3307
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman & Hall, New York
Dematte JE, O’Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, Adiga RB, Ndukwu IM (1998) Near-fatal Heat stroke during the 1995 heat wave in Chicago. Ann Intern Med 129:173–181
Hausfater P, Juillien G, Madonna-Py B, Haroche J, Bernard M, Riou B (2007) Serum procalcitonin measurements as diagnostic and prognostic marker in febrile adult patients presenting to the emergency department. Crit Care 11:R60
Hausfater P, Riou B (2007) Coup de chaleur Encycl Med Chir Urgences 24-116-A20. Elsevier, Paris
Huisse MG, Pease S, Hurtado-Nedelec M, Arnaud B, Malaquin C, Wolff M, Gougerot-Pocidalo MA, Kermarrec N, Bezeaud A, Guillin MC, Paoletti X, Chollet-Martin S (2008) Leukocyte activation: the link between inflammation and coagulation during heatstroke. A study of patients during the 2003 heat wave in Paris. Crit Care Med 36:2455–2456
Claessens YE, Taupin P, Kierzek G, Pourriat JL, Baud M, Ginsburg C, Jais JP, Jougla E, Riou B, Dhainaut JF, Landais P (2006) How emergency departments might alert for prehospital heat-related excess mortality? Crit Care 10:R156
Kaarlola A, Tallgren M, Pettila V (2006) Long-term survival, quality of life, and quality-adjusted life-years among critically ill elderly patients. Crit Care Med 34:2120–2126
Acknowledgments
We are indebted to Emmanuelle de Magondeau and Christine Lanau for their excellent data monitoring and management. We thank Dr. David J. Baker, DM, FRCA (Department of Anesthesiology, CHU Necker-Enfants Malades, Paris, France) for reviewing the manuscript and Prof. Paul Landais, MD, PhD (Department of Biostasistics, CHU Necker-Enfants Malades, France) and Yannick Le Manach (Department of Anesthesiology and Critical Care, CHU Pitié-Salpêtrière, Paris, France) for statistical advice. The study was supported by the Direction Régionale de la Recherche Clinique d’Ile de France (Paris, France), grant no. CRC 03-150.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The other investigators in the study are listed in the Appendix.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00134-009-1728-5
Appendix
Appendix
The other investigators were (in alphabetical order): Joëlle Benkel (CHU Jean Verdier, Bondy), Dominique Brun-Ney, MD (CHU Ambroise Paré, Boulogne, currently the Direction de la Politique Médicale, Assistance Publique-Hôpitaux de Paris), Enrique Casalino, MD, PhD (CHU Bicêtre, Le Kremlin-Bicêtre, currently CHU Bichat and Université Denis Diderot-Paris 7), Alain Davido (Hôpital Européen Georges Pompidou, Paris), MD, Jean-François Dhainaut, MD (CHU Cochin-St Vincent de Paul and Université René Descartes-Paris 5, currently the Agence de l’Evaluation de la Recherche et de l’Enseignement Supérieur, Paris, France), David Elkharrat, MD (CHU Lariboisière, Paris, currently CHU Ambroise Paré, Boulogne, and Université Paris Ouest), Anika Fichelle (CHU Bichat Claude-Bernard, Paris), M.D., Bertrand Galichon MD (CHU Lariboisière, Paris), Christine Ginsburg, MD, (CHU Cochin-St Vincent de Paul, Paris), Philippe Héricord MD (CHU Saint Antoine, Paris), Philippe Hoang, MD (deceased) (CHU Avicenne, Bobigny), Côme Légaut, MD, (CHU Antoine Béclère, Clamart), Virginie Lemiale, MD (CHU Henri Mondor, Créteil), Jafar Manamani, MD (CHU Saint Louis, Paris, currently CHU Saint Antoine, Paris), Alice Marichez, MD, and Dominique Meyniel, MD (CHU Tenon, Paris), Dominique Pateron, MD, PhD (CHU Jean Verdier, Bondy, currently CHU Saint-Antoine and Université Pierre et Marie Curie-Paris 6), Florence Péviriéri, MD (CHU Jean Verdier, Bondy), Jean-Louis Pourriat, MD (CHU Hôtel Dieu, Paris and Université René Descartes-Paris 5), Bertrand Renaud, MD (CHU Henri Mondor, Créteil), Pierre Taboulet, MD (CHU Saint Louis, Paris), Stéphane Wadjou, MD, (CHU Pitié-Salpêtrière, Paris), Patrick Werner MD (CHU Beaujon, Clichy); all in emergency departments of Assistance Publique-Hôpitaux de Paris, Paris, France.
Rights and permissions
About this article
Cite this article
Hausfater, P., Megarbane, B., Dautheville, S. et al. Prognostic factors in non-exertional heatstroke. Intensive Care Med 36, 272–280 (2010). https://doi.org/10.1007/s00134-009-1694-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1694-y