Abstract
Objectives
To determine the incidence and outcomes of intensive care unit-acquired neuromyopathy and to investigate the role of methylprednisolone in survivors of persistent acute lung injury.
Design
Secondary analysis of completed randomized placebo-controlled trial.
Setting
Twenty-five hospitals in the NHLBI ARDS Network.
Patients and participants
Patients enrolled in the ARDS Network study of methylprednisolone versus placebo for persistent ARDS who survived 60 days or to hospital discharge.
Measurements and results
One hundred and twenty-eight study patients survived 60 days. Forty-three (34%) of these patients had evidence by chart review of ICU-acquired neuromyopathy, which was associated with prolonged mechanical ventilation, return to mechanical ventilation, and delayed return to home after critical illness. Treatment with methylprednisolone was not significantly associated with an increase in risk of neuromyopathy (OR 1.5; 95% CI 0.7–3.2).
Conclusions
ICU-acquired-neuromyopathy is common among survivors of persistent ARDS and is associated with poorer clinical outcomes. We did not find a significant association between methylprednisolone treatment and neuromyopathy. Limitations of this study preclude definitive conclusions about the causal relationship between corticosteroids and ICU-acquired neuromuscular dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired neuromuscular function is a common and important problem for critically ill patients. Muscle weakness has been reported in 25–33% of general intensive care unit (ICU) patients receiving 7 or more days of mechanical ventilation [1, 2]. Electrophysiologic testing reveals neuromuscular dysfunction in 47–60% of ICU patients, with higher incidence in severe sepsis and acute respiratory distress syndrome (ARDS) [3–5]. Longitudinal follow-up studies of ARDS survivors show weakness, muscle atrophy, fatigue [6] and associated impairments in health-related quality of life [6–9] that persist for months to years after recovery from critical illness. ARDS appears to be an independent risk factor for poor physical function in survivors, compared with matched survivors of critical illness [10].
Many questions about ICU-acquired neuromuscular dysfunction remain unanswered, particularly in patients with ARDS. Specifically, previous studies of the association between corticosteroids and ICU-acquired neuromuscular dysfunction have been observational and yielded disparate results [1, 2, 5, 11–17], despite evidence of direct myotoxicity in animal studies [18–21]. The clinical importance of corticosteroids in the development and outcomes of ICU-acquired neuromuscular dysfunction remains unclear.
The National Heart Lung and Blood Institute’s ARDS Network recently completed a randomized, placebo-controlled trial of moderate-dose methylprednisolone for the treatment of persistent ARDS [22] that included a chart review for evidence of ICU-acquired neuromuscular dysfunction. This study presented conflicting results about the relationship between methylprednisolone and neuromyopathy: serious adverse events of weakness, myopathy and neuropathy were strongly associated with methylprednisolone, but overall there were similar numbers of neuromyopathy cases in the methylprednisolone and placebo groups [22]. We analyzed data from that trial to determine the incidence and outcomes of neuromyopathy, and to investigate the role of methylprednisolone. Preliminary results from this study were presented at the American Thoracic Society meeting in San Diego, May 2006 [23].
Materials and methods
Study design
This study is a secondary analysis of the ARDS Network-randomized controlled trial of methylprednisolone versus placebo for persistent ARDS. Study methods and results have been previously described [22].
Definition of neuromyopathy
All subjects were monitored for evidence of neuromuscular dysfunction during the parent study via chart review in two phases: retrospectively (added after 89 patients had been enrolled), and prospectively for the remaining subjects. Neuromyopathy was defined by the presence of the terms “myopathy,” “neuropathy,” “myositis,” “paralysis” or “unexplained muscle weakness” anywhere in the medical record after ICU admission. Charts of patients with evidence of neuromyopathy were abstracted for severity [24], duration of abnormality and confirmatory tests (including specialist consultation, electrophysiologic testing and muscle biopsy). Prospective chart review was added to the protocol for the remainder of the study, using the same terms to define neuromyopathy. Limited data were abstracted if patients met neuromyopathy criteria. Patients’ charts were monitored for these conditions for at least 60 days or until hospital discharge.
We investigated a more specific definition of neuromyopathy, “early neuromyopathy,” that was limited to detection of neuromuscular dysfunction (using the same terms in chart abstraction) in the first 28 days of the study. This definition was chosen to select subjects who were recognized with weakness during or soon after the time of administration of study drug (methylprednisolone or placebo).
Eligibility
Eligibility for the current study required survival after study enrollment to 60 days or to hospital discharge. This criterion minimizes selective misclassification of the sickest subjects with unrecognized neuromuscular dysfunction as noncases. To be included in methylprednisolone analyses, subjects could not have evidence of ICU-acquired neuromyopathy before study entry.
Data collection and analysis
All data for the present investigation were collected in the parent study. Details of electrophysiologic testing and severity were only collected during the retrospective chart review. We used Student’s t test, Chi-square and Fisher’s exact test, and Wilcoxon rank sum testing for bivariate analyses of parametric, binomial, and nonparametric data, respectively. We used logistic regression to study the association of methylprednisolone with neuromyopathy. We tested exposure to methylprednisolone by randomization group (intention to treat). Exploratory analyses included logistic regression to study the association of methylprednisolone with early neuromyopathy and with clinical diagnosis of myopathy. We performed additional analyses limited to the retrospective neuromyopathy cases to explore characteristics of those identified as serious adverse events.
Results
Incidence and outcomes
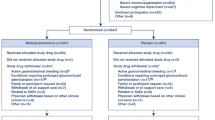
There were 128 survivors to 60 days or hospital discharge, 65 randomized to the placebo group and 63 to methylprednisolone. All had charts reviewed for evidence of neuromyopathy. There was evidence of neuromyopathy in 43 of these patients (34%). There were no significant demographic differences between patients with and without neuromyopathy (Table 1). Baseline physiology was also similar, except for a trend toward poorer oxygenation and significantly higher serum glucose in patients with neuromyopathy.
Neuromyopathy was associated with prolonged mechanical ventilation (Table 2). Return to mechanical ventilation was more common among patients with neuromyopathy. Patients with neuromyopathy took a much longer time to return to home. Follow-up of neuromuscular dysfunction was not systematically performed.
Methylprednisolone and neuromyopathy
To investigate the association of randomization to methylprednisolone and development of neuromyopathy, we excluded the six patients with documented evidence of ICU-acquired neuromyopathy at study entry. Five of these six were randomized to the methylprednisolone group. There was no statistically significant association between randomization to methylprednisolone and neuromyopathy, with an odds ratio of 1.5 (95% CI 0.7–3.2) for methylprednisolone. Patients in the methylprednisolone group were more likely than placebo patients to have documented neuromyopathy in the first 28 days of the study, with an odds ratio of 4.8 (95% CI 1.5–15.5), and were more likely to be described with “myopathy,” with an odds ratio of 4.5 (95% CI 1.1–17.9).
Clinical features and timing of neuromyopathy
Of the 26 cases of neuromyopathy detected by retrospective review, 11 had electrophysiologic testing performed. Testing consisted of sensory and motor nerve conduction, concentric needle electromyography and repetitive nerve stimulation studies assessing at least one upper and one lower extremity. Three patients had evidence of localized neuropathies. Nine of the 11 patients studied had evidence of critical illness myopathy, with findings of decreased compound motor action potential amplitudes in the presence of normal sensory nerve action potentials, as well as positive sharp waves and fibrillation potentials. Eight of the patients who underwent electrophysiologic testing were in the methylprednisolone group.
Timing of documentation of neuromyopathy was variable. Six patients had chart review evidence of weakness or neuropathy acquired after ICU admission but before study entry. Twenty-six patients had a documented onset day of neuromyopathy, with median onset study day 14 (interquartile range study days 4–19).
There were eight serious adverse events (SAEs) of neuromyopathy among survivors. Compared with neuromyopathy cases that were not recognized as SAEs, these events were more likely to be described as myopathy (100 vs. 29%, P ≤ 0.001) and identified within the first 28 days of the study (88 vs. 49%, P = 0.05). Among the 27 neuromyopathy cases detected by retrospective chart review, the six cases submitted as SAEs were more likely to be graded as severe [67 vs. 7%, P = 0.003 (severity data missing in six non-SAEs)], evaluated with electrophysiologic testing (100 vs. 25%, P ≤ 0.001), and consistent with critical illness myopathy (100 vs. 60%, P = 0.08).
Discussion
This study has two main contributions. First, we are adding to the growing body of evidence that ICU-acquired neuromuscular dysfunction is a common and important issue for critically ill patients [19, 25]. With 128 survivors of ARDS, this is the largest report of ICU-acquired neuromuscular dysfunction in patients with acute lung injury. We found that neuromyopathy was identified in more that one-third of survivors, and was associated with poorer clinical outcomes including return to mechanical ventilation, delays in liberation from mechanical ventilation and in return to home. Second, this is the first analysis of ICU-acquired neuromuscular dysfunction in a randomized controlled trial of corticosteroids. We found that randomization to moderate doses of methylprednisolone was not significantly associated with an increased incidence of ICU-acquired neuromyopathy.
Incidence and outcomes of neuromyopathy in ARDS
We found neuromyopathy in 34% of survivors of persistent ARDS—a group with many risk factors for ICU-acquired neuromuscular dysfunction. This incidence is similar to several previous studies of critically ill patients in which neuromyopathy was defined by clinically recognizable weakness [1, 26], but considerably less than the incidence of 60% described in patients with ARDS [5]. We suspect that our finding is an underestimate of the true incidence of ICU-acquired neuromuscular dysfunction in ARDS, since we included only survivors, did not systematically evaluate patients and did not include electrophysiologic assessment of nerve and muscle function [4, 25, 26].
In agreement with previous studies, we found that total duration of assisted breathing is greater for patients with neuromyopathy [1, 19, 27–30]. We also found that neuromyopathy was associated with an increased return to assisted breathing, as previously shown in a cohort of patients with sepsis [27]. Weakness is often not recognized before patients return to assisted breathing [31]; perhaps improving strength assessment would enable clinicians to anticipate and potentially prevent recurrent respiratory failure in this high-risk population.
Previous studies have demonstrated associations of ICU-acquired neuromuscular dysfunction with hospital length of stay [19]; it appears to be associated with destination as well as timing of hospital discharge. A recent study of 136 mechanically ventilated medical ICU patients reported that 83% of patients with ICU-acquired weakness were discharged from the hospital to locations other than home, compared with 52% of patients who were not weak [30]. We found that return to home was delayed for neuromyopathy patients due to prolonged stays in acute and subacute care, likely representing continued care needs and functional impairment beyond hospital discharge.
Corticosteroids and neuromyopathy
Why did we not find an association between corticosteroids and neuromyopathy? It is possible that corticosteroids truly increase the incidence or severity of ICU-acquired weakness but that this effect was not detected in the present investigation. It may be that the effect is true but small; with only 122 survivors in this analysis, we were underpowered to detect an absolute difference less than 35%. Alternatively, it may be that our ability to identify the effect of corticosteroids was limited by a case definition that was both insensitive (patients may have had unrecognized pathology) and imprecise (combining all types of ICU-acquired neuromuscular dysfunction, including neuropathies, rather than focusing on myopathy).
Serious adverse events of neuromyopathy were increased by corticosteroids, but overall cases were not [22]. Neuromyopathy serious adverse events were all described as myopathy, were recognized early and were graded of higher severity than neuromyopathy cases that were not recognized as serious adverse events—features associated with corticosteroids in our analyses. It is possible that corticosteroids lead to myopathy that contributes to prolonged hospitalization and disability (criteria of serious adverse events), but not necessarily to a sizeable increase in all causes of ICU-acquired neuromuscular dysfunction. Additionally, as we have previously postulated, corticosteroids may both diminish risk of ICU-acquired neuromuscular dysfunction in some patients by decreasing the duration of shock and mechanical ventilation and increase risk of myopathy [22].
It is possible that we did not detect an association between neuromyopathy and corticosteroids because there was no effect in this study. Perhaps, higher doses of corticosteroids are needed to significantly influence neuromyopathy, such as the case in animal models of critical illness myopathy [32]. It may even be the case that corticosteroids are not causal in the development of critical illness myopathy in particular or ICU-acquired weakness in general. In fact, a recent study has suggested that in the setting of intensive insulin therapy, corticosteroids may be protective of neuromyopathy [33].
Limitations
The most significant limitation to this study is the use of chart review to diagnose neuromyopathy. Assessment of neuromyopathy was most problematic in subjects who died before weakness might have been detected; we addressed this by excluding nonsurvivors, but recognize that our incidence is likely an underestimate. Our population is not generalizable to all patients with acute lung injury or critical illness, and may not generalize to current practices of critical care. We did not perform systematic follow-up; so, this study does not contribute to understanding recovery from neuromyopathy or its relationship with long-term outcomes. Despite these limitations, we believe that this study is an important contribution to the epidemiology of neuromyopathy and its relationship to corticosteroids in critically ill patients, specifically, those with acute lung injury.
Conclusions
Neuromyopathy is common in patients with ARDS and is associated with poorer clinical outcomes. While we did not find a significant association between randomization to corticosteroids and increased risk of neuromyopathy, the relationship between corticosteroid treatment, ICU-acquired neuromuscular dysfunction and short- and long-term outcomes after ARDS remains unclear and further research is needed.
References
De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphael JC, Outin H, Bastuji-Garin S (2002) Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 288:2859–2867
de Letter MA, Schmitz PI, Visser LH, Verheul FA, Schellens RL, Op de Coul DA, van der Meche FG (2001) Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med 29:2281–2286
Leijten FS, De Weerd AW, Poortvliet DC, De Ridder VA, Ulrich C, Harink-De Weerd JE (1996) Critical illness polyneuropathy in multiple organ dysfunction syndrome and weaning from the ventilator. Intensive Care Med 22:856–861
Berek K, Margreiter J, Willeit J, Berek A, Schmutzhard E, Mutz NJ (1996) Polyneuropathies in critically ill patients: a prospective evaluation. Intensive Care Med 22:849–855
Bercker S, Weber-Carstens S, Deja M, Grimm C, Wolf S, Behse F, Busch T, Falke KJ, Kaisers U (2005) Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit Care Med 33:711–715
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348:683–693
McHugh LG, Milberg JA, Whitcomb ME, Schoene RB, Maunder RJ, Hudson LD (1994) Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med 150:90–94
Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, Pinsky MR (2001) Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1389–1394
Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA (1997) Health-related quality of life after acute lung injury. Am J Respir Crit Care Med 156:1120–1128
Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP (1999) Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA 281:354–360
Amaya-Villar R, Garnacho-Montero J, Garcia-Garmendia JL, Madrazo-Osuna J, Garnacho-Montero MC, Luque R, Ortiz-Leyba C (2005) Steroid-induced myopathy in patients intubated due to exacerbation of chronic obstructive pulmonary disease. Intensive Care Med 31:157–161
Campellone JV, Lacomis D, Kramer DJ, Van Cott AC, Giuliani MJ (1998) Acute myopathy after liver transplantation. Neurology 50:46–53
Coakley JH, Nagendran K, Yarwood GD, Honavar M, Hinds CJ (1998) Patterns of neurophysiological abnormality in prolonged critical illness. Intensive Care Med 24:801–807
Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar A, Garnacho-Montero MC, Moyano-Del-Estad MR (2001) Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med 27:1288–1296
Lefaucheur JP, Nordine T, Rodriguez P, Brochard L (2006) Origin of ICU acquired paresis determined by direct muscle stimulation. J Neurol Neurosurg Psychiatry 77:500–506
Rudis MI, Guslits BJ, Peterson EL, Hathaway SJ, Angus E, Beis S, Zarowitz BJ (1996) Economic impact of prolonged motor weakness complicating neuromuscular blockade in the intensive care unit. Crit Care Med 24:1749–1756
Thiele RI, Jakob H, Hund E, Tantzky S, Keller S, Kamler M, Herold U, Hagl S (2000) Sepsis and catecholamine support are the major risk factors for critical illness polyneuropathy after open heart surgery. Thorac Cardiovasc Surg 48:145–150
Friedrich O (2006) Critical illness myopathy: what is happening? Curr Opin Clin Nutr Metab Care 9:403–409
Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM (2007) Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 33:1876–1891
Horinouchi H, Kumamoto T, Kimura N, Ueyama H, Tsuda T (2005) Myosin loss in denervated rat soleus muscle after dexamethasone treatment. Pathobiology 72:108–116
Massa R, Carpenter S, Holland P, Karpati G (1992) Loss and renewal of thick myofilaments in glucocorticoid-treated rat soleus after denervation and reinnervation. Muscle Nerve 15:1290–1298
Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354:1671–1684
Hough CL, Steinberg KP, Goodman RB, Thompson BT, Hudson LD (2006) Incidence, risk factors, serum markers, and outcomes of neuromyopathy in persistent ARDS. Am J Respir Crit Care Med 3:A570
NCI (1999) Common toxicity criteria. http://ctepcancergov/forms/CTCv20_4-30-992pdf
Hough CL (2006) Neuromuscular sequelae in survivors of acute lung injury. Clin Chest Med 27:691–703
Witt NJ, Zochodne DW, Bolton CF, Grand’Maison F, Wells G, Young GB, Sibbald WJ (1991) Peripheral nerve function in sepsis and multiple organ failure. Chest 99:176–184
Garnacho-Montero J, Amaya-Villar R, Garcia-Garmendia JL, Madrazo-Osuna J, Ortiz-Leyba C (2005) Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit Care Med 33:349–354
De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L (2004) Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med 30:1117–1121
Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ (2005) Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 64:1348–1353
Ali NA, O’Brien JM Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, Connors AF Jr, Marsh CB (2008) Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 178:261–268
Latronico N, Guarneri B, Alongi S, Bussi G, Candiani A (1999) Acute neuromuscular respiratory failure after ICU discharge. Report of five patients. Intensive Care Med 25:1302–1306
Rich MM, Pinter MJ, Kraner SD, Barchi RL (1998) Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann Neurol 43:171–179
Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, Bruyninckx F, Van den Berghe G (2007) Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med 175:480–489
Acknowledgments
Support for this work was provided by the Francis Family Foundation and the National Heart Lung and Blood Institute through the ARDS Network and Mentored Career Development Award (K23HL74294).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-008-1305-3.
Rights and permissions
About this article
Cite this article
Hough, C.L., Steinberg, K.P., Taylor Thompson, B. et al. Intensive care unit-acquired neuromyopathy and corticosteroids in survivors of persistent ARDS. Intensive Care Med 35, 63–68 (2009). https://doi.org/10.1007/s00134-008-1304-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1304-4