Abstract
This study was conducted at ambient (398 ± 10 µmol mol−1), elevated (450 ± 10 µmol mol−1) and elevated (550 ± 10 µmol mol−1) atmospheric CO2 under three moisture regime and also three level of temperature (4, 25, and 40°C) to assess the degradation of pretilachlor and butachlor. Under dry condition at 398 ± 10 µmol mol−1, T1/2 was 28.5 and 59.4 days for pretilachlor and butachlor, respectively; slowly decreased to 18.2 and 44.5 days at 550 ± 10 µmol mol−1 indicated that elevated condition enhanced degradation than ambient condition. Under field capacity with increasing CO2 levels from ambient to elevated, T1/2 decreased from 18.9 to 11.6 days and 39.4 to 16.2 days for of pretilachlor and butachlor, respectively. Similarly, under submerged conditions with increasing CO2 levels T1/2 decreased 14.7–7.1 and 26.3–11.8 days for pretilachlor and butachlor, respectively. Study also revealed that both pretilachlor and butachlor dissipated faster at 40°C (T1/2, 9.7 and 19.4 days) than 25°C (T1/2, 16.2 and 36.7 days). Slower dissipation was recorded at 4°C (T1/2, 87.6 and 182.4 days).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Globally averaged combined land and ocean surface temperature data shows a warming of 0.85 [0.65–1.06]°C over the period 1880–2012 (IPCC 2014). The CO2 concentration in the atmosphere has increased by nearly 37%, since the dawn of industrial revolution. Besides it is likely to increase upto 570 ppm by the middle of the current century with 2.0–4.5°C warmer of earth surface. Elevated atmospheric CO2 concentration may have a profound effect on dissipation pattern of soil applied different pesticides. It has well proven that changes in soil temperature and atmospheric CO2 due to global warming affect the fate of different xenobiotics (Das 2013). The persistence of pesticides in soil depends on intrinsic chemical properties and extrinsic environmental factors, mostly soil properties and climatic conditions (Das et al. 2017). Intrinsic pesticide properties, such as vapour pressure, water solubility, biological activity and resistance to chemical changes, indicate the tendencies of pesticide fate, while the extrinsic factors, such as soil microbial activity, soil organic carbon content, soil temperature, solar radiation and rainfall can greatly modify the pesticide fate (Rao and Davidson 1980). Soil temperature influence degradation rate of different xenobiotics as most reaction catalyzed by enzymes tends to double for every 10°C increase in temperature (Mukherjee et al. 2016). Temperatures 40°C, or higher is desirable for the faster degradation rate of different organochlorine pesticide like DDT. The half-life of pesticides in soils might decrease by 60% with 10°C increase in temperature (Bloomfield et al. 2006; Das 2014a, b, c). Degradation rate of pesticide in soil depends on various factors like soil texture, soil moisture (Das et al. 2015), composition of organic matter and available nitrogen, soil acid/base, number of strong and weak bond and also mode of action (Das 2014a, b, c). Increased CO2 may not affect pesticide degradation directly but may be indirectly (by altering soil microbial community) has reported by Mukherjee et al. (2016). Butachlor and pretilachlor belongs to the chloroacetanilide group of herbicides that are one of the most consumed herbicides in agriculture worldwide (Zheng et al. 2012). They are mainly applied on rice, tea, wheat, corn, soybean and beans (Dwivedi et al. 2012) and commonly used to control broad leaf weeds and annual grass (Wang et al. 2016). Both butachlor and pretilachlor inhibit in lipid, fatty acids, isoprenoids, flavonoids and proteins biosynthesis process. These herbicides are often detected in the ground water and are highly persistent in the water bodies (Samuel et al. 2015). The half-life of butachlor is 1.65–2.48 days in field water and 2.67–5.33 days in soil (Huarong et al. 2010). Pretilachlor dissipate readily in rice soil and does not affect soil properties (Adachi et al. 2007). There was no evidence of residue buildup due to continuous use of pretilachlor herbicide and dissipation of butachlor and pretilachlor in two soil types under laboratory and field conditions was very rapid. Absolutely no real-time information/study is available on the degradation of pretilachlor and butachlor under three different level of CO2 and temperature condition. Thus the investigation was carried out to study the effect of three different level of atmospheric CO2 (398 ± 10, 450 ± 10, and 550 ± 10 µmol mol−1) and temperature (4, 25, and 40°C) on degradation pattern pretilachlor and butachlor in subtropical soil under National Phytotron Facility at IARI, New Delhi, India.
Materials and Methods
Soil was collected from the experimental farm of IARI, New Delhi, India (28°38′23″N and 77°09′27″E; 228.61 m above mean sea level; subtropical India) at a depth of 0–15 cm. Physico-chemical parameters of soil were analysed with standard method (Jackson 1967). Certified Reference Material of pretilachlor (98.7%) and butachlor (97.4%) were purchased from Rallis India Ltd., Bangalore, India. All glassware washed with H2CrO4 solution and then thorough washing with H2O, rinsed with C3H6O and dried at 115°C for 4 h prior to use. Extraction solvent was purchased from Merck Specialties Private Ltd, New Delhi for residue analysis. Chemical structure of butachlor and pretilachlor has shown in Fig. 1. Degradation study was carried out in a CRD experiment with three treatments and three replication in the National Phytotron Facility, IARI, New Delhi, India in a circular shaped UV shielded chamber (4.93 × 2.87 m). The treatment were 398 ± 10, 450 ± 10, and 550 ± 10 µmol mol−1 level CO2. At three condition viz. chamber maintained at ambient (398 ± 10 µmol mol−1) and elevated CO2 level (450 ± 10 and 550 ± 10 µmol mol−1) effect of CO2 on pretilachlor and butachlor degradation in soil was studied at 10.0 µg g−1 fortification level at three moisture regimes viz., dry, field capacity and submerged condition. For treatment under field capacity moisture regime, the calculated amount of water was added to bring the soil to field capacity moisture level (19.9%), while in case of submerged condition enough water was added to raise the level of water to about 3 cm above the soil surface. No water was added in dry treatments (Mukherjee et al. 2016; Das 2014a, b, c). The treated and control soil samples were transferred to 250 mL beakers and with aluminium foil beaker were closed. Two set of beaker were transferred to chamber having elevated CO2 concentration (450 ± 10 and 550 ± 10 µmol mol−1) while another set to chamber at ambient CO2 concentration (398 ± 10 µmol mol−1) at three moisture levels. Constant weight was maintained throughout the experiment by adding required quantity of water every alternate day. All the beakers in triplicate were withdrawn at 0, 3, 5, 7, 10, 15, 30, 50 and 60 days interval along with control sample for all moisture regime. For submerged and field capacity moisture regime residues in soil were extracted with ethyl acetate (50 mL) in 150 mL separatory funnel by intermittent shaking followed by filter with Whatman Filter Paper No. 1 and again extracted with fresh 50 + 30 mL ethyl acetate. Ethyl acetate extracts pooled, concentrated and redissolved in 10 mL of acetone and were quantified for pretilachlor and butachlor residues using gass liquid chromatography (GLC). Samples kept under air dry condition were mixed with 0.2 mL of NH3 solution followed by activated Florisil (0.5 g) thoroughly mixed. The mixture was dried in a glass column containing a layer (2 cm) of anhydrous sodium sulfate at the bottom and eluted with about 125 mL of a mixture of ethyl acetate + hexane (5 + 95 v/v). The extract concentrated, redissolved in 5 mL of acetone and were quantified. Degradation behavior of pretilachlor and butachlor in soil was also studied at 10 µg mL−1 fortification level under three different temperatures regimes (4, 25, and 40°C) at field capacity moisture level only. The experiment was conducted in a complete randomized design (CRD) under laboratory condition. Samples were fortified at 10 µg mL−1 level and after fortification the beakers were divided into three sets. First set was kept in refrigerator maintained at 4 ± 1°C, second set was kept at 40 ± 1°C in incubating oven and third set placed in BOD incubator at 25 ± 1°C. Samples in triplicate were withdrawn at 0, 3, 5, 7, 10, 15, 30, 50 and 60 days interval along with control. Residues in soil were extracted with ethyl acetate (50 mL) in 150 mL separatory funnel by intermittent shaking followed by filter through Whatman Filter Paper No. 1. Then again extracted with fresh 50 + 30 mL ethyl acetate. All extracts pooled, concentrated and redissolved in 5 mL of acetone and finally quantified for pretilachlor and butachlor residues using GLC. Residues of pretilachlor and butachlor were estimated by GLC system (Hewlett-Packard 5890 Series II) electron capture detector (63Ni). Flow rate through column (CP-SIL 5CB; 30 × 0.25 mm i.d., × 0.25 µ film thickness) was 2 mL min−1 and make up flow was 27 mL min−1 having injection volume 2 µL. Pretilachlor eluted at retention time 5.19 min and butachlor at 7.96 min under these conditions. GLC chromatogram of pretilachlor and butachlor are presented in Fig. 2. Half-life (T1/2) value was calculated using first-order dissipation kinetics.
Results and Discussion
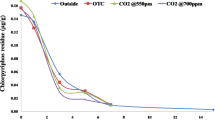
Half-life values (T1/2) for pretilachlor and butachlor under different CO2 level and moisture regime at 10.0 µg g−1 are presented in Table 1 and Fig. 3. From the first order dissipation kinetics calculated half-life for pretilachlor under ambient CO2 condition was 28.5, 18.9, and 14.7 days (Table 1; Fig. 3). Calculated half-life for butachlor under ambient CO2 condition were 59.4, 39.4 and 26.3 days (Table 1; Fig. 3). Dissipation of pretilachlor and butachlor in soil at different moisture regime at 398 ± 10 µmol mol−1 CO2 level has been presented in Fig. 4. Half-life values for butachlor under ambient CO2 condition were 52.2, 28.4 and 19.4 days (Table 1 and Fig. 3). Dissipation of pretilachlor and butachlor in soil at different moisture regime at 450 ± 10 µmol mol−1CO2 level has been presented in Fig. 5. Calculated half-life for butachlor under ambient CO2 condition was 44.5, 16.2 and 11.8 days (Table 1; Fig. 3). Dissipation of pretilachlor and butachlor in soil at different moisture regime at 550 ± 10 µmol mol−1 CO2 level has been presented in Fig. 6. Considering ambient condition (398 ± 10 µmol mol−1) the pattern for degradation followed BD < BFC < PD < BSM < PFC < PSM (where, PD = pretilachlor in dry soil, PFC = pretilachlor in field capacity, PSM = pretilachlor in submerged; BD = Butachlor in dry soil, BFC = Butachlor in field capacity, BSM = Butachlor in submerged soil). Considering elevated CO2 (450 ± 10 µmol mol−1) the pattern for degradation followed BD < BFC < PD < BSM < PFC < PSM. Similarly, considering elevated CO2 (550 ± 10 µmol mol−1) the pattern for degradation follow BD < PD < BFC < BSM < PFC < PSM. In case of pretilachlor, its persistency was longer under dry ambient CO2 level (T1/2 28.5) and lowest persistency was observed in submerged elevated CO2 (550 ± 10 µmol mol−1) level (T1/2 7.1). Similarly, in case of butachlor, its persistency was longer under dry ambient CO2 level (T1/2 59.4) and lowest persistency was observed in submerged elevated CO2 (550 ± 10 µmol mol−1) level (T1/2 11.8). Among all the treatment combination, longer persistence was found under ambient condition for pretilachlor in dry moisture regime (T1/2 59.4) and faster degradation was found under elevated condition (550 ± 10 µmol mol−1) for pretilachlor in submerged moisture regime (T1/2 7.1). Comparing the degradation between both the herbicide, degradation was faster in pretilachlor than butachlor though both of them are in same chemical group. The half-life of pretilachlor in flood water ranged from 3.0 to 3.6 days. Scarponi et al. (2003) reported that half-life value of pretilachlor is 6 days in rice soil. Adak et al. (2016) reported that an increased CO2 concentration led to increase in temperature (1.2–1.8°C) that played a critical role in chlorpyriphos persistence. But Manna et al. (2013) reported elevated CO2 did not have any significant effect on the persistence of azoxystrobin in rice-planted soil. The herbicides viz., pretilachlor and butachlor were found to persist longer under ambient condition (398 ± 10 µmol mol−1) followed by two elevated condition (450 ± 10 and 550 ± 10 µmol mol−1). It is well established that increased in CO2 concentration led to increase in temperature (1.2–1.8°C) that played a critical role in degradation. At elevated condition higher temperature may results in increased thermolysis and causes volatilization losses which hasten faster degradation of pretilachlor and butachlor. Chatterjee et al. (2013) showed similar type of result in metaflumizone degradation. On the other hand Mukherjee et al. (2016) reported that flubendiamide was found to persist longer under outdoor condition than ambient and elevated condition at 1 and 10 µg g−1 fortification level. He also reported that increased CO2 levels and temperature following global warming adversely affect flubendiamide degradation in soil. It seems that at elevated CO2 level slightly higher temperature maintained in chamber is responsible for the faster pretilachlor and butachlor degradation. Considering moisture regimes, pretilachlor and butachlor were found to persist longer under dry condition followed by field capacity and submerged moisture regime. Slower dissipation and hence longer persistence under dry condition could be attributed to very negligible soil microbiological activity. Besides soil under this study has very low organic carbon content (0.31%) which disfavor soil microbes in the entire three moisture regime. Slower dissipation in dry condition due to lower microbial activity already been reported by Mukherjee et al. (2012). They also reported that faster dissipation under submerged conditions could be attributed to partial anaerobic conditions (redox condition) and anaerobic microbes are more capable in degrading pesticides than aerobic microbes. Similar results have been reported in cyfluthrin which is dissipated readily under anaerobic soil conditions. Also Kumar et al. (2017) reported that tricyclazole dissipated at a faster rate under elevated CO2 condition than under ambient CO2 condition. Faster dissipation under submerged moisture regime has also been reported for pendimethalin. Khandelwal et al. (2014) have reported the same for kresoxim-methyl and also reported that increase in the CO2 level from 300 to 660 g g−1 slightly increases atrazine loses (Figs. 7, 8).
Dissipation of pretilachlor and butachlor in soil at different moisture regime at 398 ± 10 μmol mol−1 CO2 level at 10.0 μg g−1 level. PD pretilachlor in dry soil, PFC pretilachlor in field capacity, PSM pretilachlor in submerged; BD butachlor in dry soil, BFC butachlor in field capacity, BSM butachlor in submerged soil
Regression equation and half-life of pretilachlor and butachlor in soil under different temperature condition viz., 4, 25, and 40°C at field capacity moisture regime has been presented in Table 2. Results revealed that residues dissipated faster at higher temperature 40°C (98.4%) followed by 25°C (93.6%) and 4°C (29.8%) after 60 days. The half-life (T1/2) value for pretilachlor was 9.7, 16.2 and 87.6 at 40, 25, and 4°C, respectively. Faster dissipation of pretilachlor at high temperature could be due to the breakdown of weak bond present in the molecule. The half-life (T1/2) value for butachlor was 19.4, 36.7 and 182.4 at 40, 25, and 4°C, respectively. Significant effect observed at 25°C level on dissipation of pretilachlor as compared to butachlor. The faster degradation of these herbicides under 40°C could be attributed to thermolysis process that breakdown the amide linkage present in the molecules. The above findings were in close conformity with the findings of several other workers (Sultana et al. 2005; Chatterjee et al. 2013). Sondhia and Dubey (2006) reported that butachlor degrade at faster rate at 40°C at pH 7.0 and also rapidly lost by photo decomposition and volatilization. Lower temperature delayed the degradation process for both the pesticides. But at higher temperature soil thermophilic microbes may be in active form and may cause faster degradation. Higher temperature enhanced degradation of various pesticides molecules has been reported by Starner et al. (1999). Xinfeng and Hanwen (2016) found that degradation rate of atrazine increased from 5% to 20% when treating soils at 25°C. Faster dissipation at higher temperature has also been reported for endosulfan (Kaur et al. 1998). Das et al. (2016) reported that flubendiamide dissipated faster at 40°C than 25°C and least at 4°C. Thus increased temperature following global warming might adversely affect the degradation of both the herbicides in soil. The study indicating that elevated condition enhanced degradation than ambient condition for both pretilachlor and butachlor. In this experiment it is clear that butachlor degraded slower than pretilachlor under all the treatment. Persistency was higher under dry condition followed by field capacity and submerged condition for all the three level of CO2. Study also revealed that both pretilachlor and butachlor dissipated faster at 40°C followed by 25 and 4°C.
References
Adachi A, Komura T, Andoh A, Okano T (2007) Effect of spherosomes on degradation of pretilachlor and esprocarp in soil. J Health Sci 53:600–603
Adak T, Munda S, Kumar U, Berliner J, Pokhare SS, Jambhulkar NN (2016) Effect of elevated CO2 on chlorpyriphos degradation and soil microbial activities in tropical rice soil. Environ Monit Assess 188(2):105. https://doi.org/10.1007/s10661-016-5119-4
Bloomfield JP, Williams RJ, Gooddy DC, Cape JN, Guha P (2006) Impact of climate change on the fate and behavior of pesticides in surface and ground water—an UK perspective. Sci Total Environ 369:163–177
Chatterjee NS, Gupta S, Varghese E (2013) Degradation of metaflumizone in soil: impact of varying moisture, light, temperature, atmospheric CO2 level, soil type and soil sterilization. Chemosphere 90:729–736
Das SK (2013) Mode of action of pesticides and the novel trends—a critical review. Int Res J 3:393–401
Das SK (2014a) Scope and relevance of using pesticide mixtures in crop protection: a critical review. Int J Environ Sci Toxicol 2:119–123
Das SK (2014b) Recent developments in clean up techniques of pesticide residue analysis for toxicology study: a critical review. Univers J Agric Res 2:198–202
Das SK (2014c) Recent development and future of botanical pesticides in India. Popul Kheti 2:93–99
Das SK, Mukherjee I, Das SK (2017) Metsulfuron-methyl herbicide on dehydrogenase and acid phosphatase enzyme activity on three different soils. Int J Bio-Resour Stress Manag 8:236–241
Das SK, Mukherjee I, Kumar A (2015) Effect of soil type and organic manure on adsorption–desorption of flubendiamide. Environ Monit Assess 187:403. https://doi.org/10.1007/s10661-015-4623-2
Das SK, Mukherjee I, Roy A (2016) Alachlor and metribuzin herbicide on N2-fixing bacteria in a sandy loam soil. Int J Bio-Res Stress Manage 7:334–338
Dwivedi S, Saquib Q, Al-Khedhairy AA, Musarrat J (2012) Butachlor induced dissipation of mitochondrial membrane potential, oxidative DNA damage and necrosis in human peripheral blood mononuclear cells. Toxicology 302:77–87. https://doi.org/10.1016/j.tox.2012.07.014
Huarong GUO, Licheng YIN, Zhang S, Feng W (2010) The toxic mechanism of high lethality of herbicide butachlor in marine flatfish flounder, Paralichthys olivaceus. J Ocean Univ China 9:257–264
IPCC (2014) Climate change 2007: the physical sciences basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 847–940
Jackson ML (1967) Soil chemical analysis. Prentice Hall, New Delhi
Kaur I, Mathur RP, Tandon SN, Dureja P (1998) Persistence of endosulfan (technical) in water and soil. Environ Technol 19:115–119
Khandelwal A, Gupta S, Gajbhiye VT, Varghese E (2014) Degradation ofkresoxim-methyl in soil: impact of varying moisture, organic matter, soilsterilization, soil type light and atmospheric CO2 level. Chemosphere 111:209–217
Kumar N, Mukherjee I, Sarkara B, Paul RK (2017) Degradation of tricyclazole: effect of moisture, soil type, elevated carbon dioxide and Blue Green Algae (BGA). J Hazard Mater 321:517–527
Manna S, Singh N, Singh VP (2013) Effect of elevated CO2 on degradation of azoxystrobin and soil microbial activity in rice soil. Environ Monit Assess 185:2951–2960
Mukherjee I, Das SK, Kumar A (2012) A fast method for determination of flubendiamide in vegetables by liquid chromatography. Pestic Res J 24:159–162
Mukherjee I, Das SK, Kumar A (2016) Degradation of flubendiamide as affected by elevated CO2, temperature, and carbon mineralization rate in soil. Environ Sci Pollut Res 23:19931–19939
Rao PSC, Davidson JM (1980) Estimation of pesticide retention and transformation parameters required in nonpoint source pollution models. In: Overcash MR, Davidson JM (eds) Environmental impact of nonpoint source pollution. Ann Arbor Science Publications, Ann Arbor, pp 23–67
Samuel TH, Morrison NI, Walker AS, Marubbi T, Yao J, Collins HL, Gorman K, Davies E, Nina A, Simon W, Anthony MS, Luke A (2015) Pest control and resistance management through release of insects carrying a male-selecting transgene. BMC Biol 13:49
Scarponi L, Buona DB, Vischetti C (2003) Persistence and detoxification of pretilachlor and fenclorim in rice. Agronomie 23:147–151
Sondhia S, Dubey RP (2006) Determination of terminal residues of butachlor and pendimethalin in onion. Pestic Res J 18:85–86
Starner K, Kuivila KM, Jennings B, Moon GE (1999). Degradation rates of six pesticides in water from the sacramento river, California, U.S. geographical survey toxic substances hydrology program: proceeding of the technical meeting, Charleston, South Carolina. http://ca.water.usgs.gov/archive/reports/wrir994018/CA-0216.pdf
Sultana P, Kotiguchi T, Shimazawa H, Nakagoshi N (2005) Measuring of some selected herbicides in paddy surface water in the sojo basin, Western Japan. J Agron Sustain Dev 25:55–61
Wang Y, Cang T, Yu R, Wu S, Liu X, Chen C, Wang Q, Cai L (2016) Joint acute toxicity of the herbicide butachlor and three insecticides to the terrestrial earthworm, Eisenia fetida. Environ Sci Pollut Res Int 23:11766–11776
Xinfeng D, Hanwen S (2016) Effect of temperature and moisture on degradation of herbicide atrazine in agricultural soil. Int J Environ Agric Res 2:150–157
Zheng J, Li R, Zhu J, Zhang J, He J, Li S, Jiang J (2012) Degradation of the chloroacetamide herbicide butachlor by Catellibacterium caeni sp. nov DCA-1T. Int Biodeterior Biodegradation 73:16–22
Acknowledgements
This study was funded by Indian Council of Agricultural Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, I., Das, S.K. & Kumar, A. Atmospheric CO2 Level and Temperature Affect Degradation of Pretilachlor and Butachlor in Indian Soil. Bull Environ Contam Toxicol 100, 856–861 (2018). https://doi.org/10.1007/s00128-018-2340-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2340-6