Abstract
Impact of elevated CO2 on chlorpyriphos degradation, microbial biomass carbon, and enzymatic activities in rice soil was investigated. Rice (variety Naveen, Indica type) was grown under four conditions, namely, chambered control, elevated CO2 (550 ppm), elevated CO2 (700 ppm) in open-top chambers and open field. Chlorpyriphos was sprayed at 500 g a.i. ha−1 at maximum tillering stage. Chlorpyriphos degraded rapidly from rice soils, and 88.4 % of initially applied chlorpyriphos was lost from the rice soil maintained under elevated CO2 (700 ppm) by day 5 of spray, whereas the loss was 80.7 % from open field rice soil. Half-life values of chlorpyriphos under different conditions ranged from 2.4 to 1.7 days with minimum half-life recorded with two elevated CO2 treatments. Increased CO2 concentration led to increase in temperature (1.2 to 1.8 °C) that played a critical role in chlorpyriphos persistence. Microbial biomass carbon and soil enzymatic activities specifically, dehydrogenase, fluorescien diacetate hydrolase, urease, acid phosphatase, and alkaline phosphatase responded positively to elevated CO2 concentrations. Generally, the enzyme activities were highly correlated with each other. Irrespective of the level of CO2, short-term negative influence of chlorpyriphos was observed on soil enzymes till day 7 of spray. Knowledge obtained from this study highlights that the elevated CO2 may negatively influence persistence of pesticide but will have positive effects on soil enzyme activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rising levels of anthropogenic greenhouse gases (particularly carbon dioxide) led to unwanted consequences in agroecosystems. The combined land and ocean surface temperature increased 0.65 to 1.06 °C for the period of 1880 to 2012 as per the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC 2014). Increases in temperature and atmospheric CO2 alter the relationship between plants and herbivores through complex mechanisms (Harrington et al. 1999). Managing these herbivores will be a great challenge under changing climatic situations. Persistence of pesticides may change, and it can cause a setback in pest management. Researchers predicted climate change variables mainly temperature, precipitation, and CO2 will influence on the rate of degradation of pesticides in soil and in plant. For example, soil and aquatic concentrations of pesticides might reduce due to a combination of increased volatilization and degradation (Bailey 2004; Benitez et al. 2006). The half-life of pesticides in soils might decrease by 60 % with 10 °C increase in temperature (Bloomfield et al. 2006). Increased CO2 concentration will have influence on soil rhizospheric region by changing crop-soil-microbial interaction. Increased CO2 might not directly influence pesticide degradation as reported by Manna et al. (2013) for azoxystrobin, but might play a major role in microbial degradation of pesticides.
Chlorpyriphos [O,O-diethyl-O-(3,5,6-trichloro-2-pyridinyl) phosphorothionate] is a broad spectrum organophosphorus insecticide, registered for application to more than 40 different food commodities worldwide. Hua et al. (2009) reported that chlorpyriphos residues in soil had a temporary or short-term inhibitory effect on soil microbes. Singh et al. (2002) reported that the effects from chlorpyriphos on the soil microbial characteristics were either very small or insignificant. However, little information is available to confirm these assumptions on chlorpyriphos. On the other hand, there may be few soil microbial parameters which may accelerate the degradation of chlorpyriphos. Sikora et al. (1990) reported a correlation between soil phosphatase activity and degradation of organophosphorus insecticides.
Soil enzyme activities are “sensors” of soil microbial functioning, play important role in the soil fertility, and are the indicators of soil quality. Earlier researchers reported that elevated CO2 had positive effect on soil enzyme activities like FDA and dehydrogenase activity (DHA) (Bhattacharyya et al. 2013; Li et al. 2010). Few other researchers reported minimum or no effect of CO2 on soil enzymatic activities (Bazot et al. 2006; Lesaulnier et al. 2008). However, studies on the effect of elevated CO2 in rice crop had suggested that microbial biomass carbon (MBC) was significantly higher in rice soils grown under elevated CO2 environment than the plant maintained at ambient CO2 (Inubushi et al. 2011; Bhattacharyya et al. 2013). Manna et al. (2013) reported elevated CO2 did not affect dehydrogenase, fluorescein diacetate, and acid phosphatase activity in rice soil, whereas Bhattacharyya et al. (2014) reported that acid and alkaline phosphatase activity was significantly higher under elevated CO2 compared to the open field rice soil. Increased available C input into the soil under elevated CO2 stimulates soil microbes in tropical rice soil, which leads to increased soil enzymatic activities (Bhattacharyya et al. 2014).
The major objective of our research was to know the influence of elevated CO2 on soil enzymatic activities on chlorpyriphos degradation. Simultaneously, we studied the effect of chlorpyriphos on MBC and soil enzymes.
Materials and methods
Reagents and solvents
Chlorpyriphos standard was purchased from Sigma-Aldrich, India. Analytical grade chemicals and solvents were used for routine work, and for gas liquid chromatography (GLC) analysis, analysis grade solvents from Merck, India, were used.
Experimental site
The study site was situated at the experimental farm of the National Rice Research Institute, Cuttack (20° 27′ 10″ N, 85° 56′ 9″ E; 24 m above mean sea level), in the eastern part of India. The soil was an Aeric Endoaquept with sandy clay loam texture (25.9 % clay, 21.6 % silt, 52.5 % sand), bulk density 1.40 Mg m−3, cation exchange capacity 15.3 cmol(+)kg−1, total C 0.79 %, and total N 0.077 %.
Experimental design
A pot experiment in complete randomized design was set up with four treatments, namely, (i) unchambered control, i.e., open field (outside; 394 ± 10 mol mol−1 CO2); (ii) chambered control (OTC; 394 ± 10 mol mol−1 CO2); (iii) elevated CO2 (CO2 at 550 ppm, 550 ± 30 mol mol−1 CO2); and (iv) elevated CO2 (CO2 at 700 ppm, 700 ± 30 mol mol−1 CO2). The experiment was conducted in circular shaped, UV-shielded open-top chambers (OTCs; diameter 4 m and height 3 m) (M/S Neogenesis Engineering, Thane, Maharashtra, India). The elevated CO2 concentrations were maintained daytime throughout the crop growing period in rice-rice ecosystem. Atmospheric temperature was measured automatically through a sensor.
Rice (var. Naveen, Indica type) seeds were sown in soils of respective treatments in December, 2012. Twenty-five-day-old seedlings were transplanted in plastic pots (20 cm × 20 cm). Soils for the experiment were obtained from the respective OTC chambers. Two seedlings were planted in each pot containing 4 kg soil, and a total of 204 pots were maintained as three chambered and one unchambered treatments. Chlorpyriphos was applied at maximum tillering stage to each pot at recommended dose (500 g a.i. ha−1) that corresponded to 0.9 mg per pot. Chlorpyriphos was mixed with minimum quantity of acetone followed by mixing in water to apply on crops using a manual hand sprayer. Among each set, 24 pots were treated with chlorpyriphos while 27 other pots were maintained as untreated control. Pots were maintained with 4–5 cm standing water, and water loss was supplemented daily. Recommended agronomic package of practices were followed to raise the crops. Pots were removed at regular intervals on 0 (before spray), 1, 3, 5, 7, 15, 21, 31, and 40 days after pesticide spray for extraction of chlorpyriphos residues from soil and to determine its effect on different soil enzyme activities.
Chlorpyriphos extraction and analysis
Three pots from each group of pesticide-treated and untreated were removed for analysis on each sampling day. Soil sample was collected from the rhizospheric region and were mixed thoroughly. Chlorpyriphos was extracted from fifty grams soil (oven dry basis) using acetonitrile (50 + 30 + 20 mL) by dipping and shaking method. Acetonitrile fractions were pooled and clean up was done by adding a pinch of activated charcoal followed by drying over anhydrous Na2SO4 and concentrated for further analysis.
Chlorpyriphos was analyzed in Agilent 6820 gas chromatograph (GLC) equipped with capillary column, HP-I (15 m × 0.53 mm × 0.5 μm) and electron capture detector (ECD). The operating parameters of the instrument were as follows: oven temperatures 150 °C (2 min) → 5 °C min−1 → 200 °C (3 min) → 10 °C min−1 → 250 °C (2 min), injection port at 200 °C and detector at 300 °C. Flow rate of nitrogen (carrier gas) was 30 mL min−1, but through column, it was 1 mL min−1 and injection was done in splitless mode. Under these operating conditions, the retention time of chlorpyriphos was found to be 14.45 min. Limit of detection of the method was ≤0.0025 μg g−1 soil (S/N, 3). The recovery of the chlorpyriphos from soil was more than 87 %.
Microbial biomass carbon and soil enzyme activities
Soil MBC was measured by modified chloroform fumigation extraction method (Vance et al. 1987). A set of fresh soil samples was fumigated with chloroform for overnight under dark condition. Without the addition of chloroform, another set of soil samples was kept under dark condition. MBC was extracted using 0.5 M K2SO4 from both fumigated and nonfumigated soil and followed by UV-visible spectrophotometric determination at 280 nm (Paul et al. 2009). DHA was determined by reduction of triphenyl tetrazolium chloride (TTC) (Casida et al. 1964). Soil sample was treated with CaCO3 and TTC and incubated for 24 h at 37 °C. The triphenyl formazan (TPF) was extracted from the reaction mixture with methanol and assayed at 485 nm. FDA hydrolase activity was measured by the potassium phosphate buffer method followed by extraction with chloroform/methanol (2:1 v/v) as described by Adam and Duncan (2001). Soil samples were treated with potassium phosphate buffer (pH 7.6) and FDA. FDA hydrolase activity was assayed at 490 nm. Urease activity was determined by the nonbuffer method of Zantua and Bremner (1977). The amount of residual urea was extracted using 2 M KCl-PMA solution. Coloring agent comprising of acidified diacetylmonoxime and thiosemicarbazide was added to the extract. Urease activity was assayed at 527 nm. Alkaline and acid phosphatase activity was assayed by treating 1 g of soil sample with 0.2 mL of toluene, 4 mL of modified universal buffer (pH 6.5 for acid phosphatase and pH 11 for alkaline phosphatase) and 1 mL of p-nitrophenyl phosphate solution (Eivazi and Tabatabai 1977; Juma and Tabatabai 1977). After 1 h of incubation at 37 °C, 0.5 M CaCl2 and 0.5 M NaOH solution was added. The suspension was filtered, and the color intensities of filtrates were measured at 420 nm.
Statistical analysis
The degradation rate of chlorpyriphos in soil was fitted to a first-order kinetic model. The rate constant (k) was determined using the algorithm C t /C 0 = e −kt, where C 0 is the amount of chlorpyriphos in the soil at time zero and C t is the amount of chlorpyriphos in the soil at time t. Linear regression (ln (C t /C 0) of the chemical data and time) was used to calculate the time in which the chlorpyriphos concentration in the soil was reduced by 50 % (Hoskin 1961).
Data were analyzed following analysis of variance (SAS Software packages, SAS EG 4.3), and means of treatments were compared based on Tukey’s minimum significant difference test (HSD) at 0.05 probability level. Correlation coefficients among traits were determined using SAS software packages, SAS EG 4.3. Two-way ANOVA was performed using SAS 9.3 to know the effects of pesticide, time, and their interaction on soil microbial activities.
Results and discussion
Dissipation of chlorpyriphos
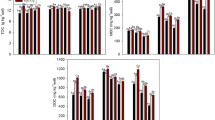
Dissipation of chlorpyriphos in rice soil is represented under four different treatments as Fig. 1. We could not observe difference among treatments in dissipation pattern of chlorpyriphos from the rice-planted soils. However, the rate of dissipation was slower in open field treatment. Chlorpyriphos recovered from rice soil after 2 h of spray was in the tune of 0.146–0.168 μg g−1. By day 5, 88.4 % of initially applied chlorpyriphos was dissipated from the soil maintained under elevated CO2 at 700 ppm, whereas the amount of chlorpyriphos dissipated from the treatments under elevated CO2 at 550 ppm, ambient CO2 in OTC and open field soil were 83, 80.4, and 80.7 %, respectively. After 15 days of spray, chlorpyriphos was not detected from rice grown soil under both elevated CO2 concentrations and ambient CO2 in OTC.
Dissipation data from all of the four treatments fitted well in first order kinetics. The coefficient of determination value ranged from 0.94 to 0.99. Half-life values determined from first-order kinetics found to be 2.4 to 1.7 days. In open field soil, chlorpyriphos had better half-life of 2.4 days as compared with soils under chambered OTC, elevated CO2 at 550 ppm and elevated CO2 at 700 ppm which registered 1.8, 1.7, and 1.7 days, respectively. Dissipation pattern of chlorpyriphos from the rice-planted soils under different conditions were similar except the higher rate of degradation for elevated CO2-treated soils. The experiment was conducted with the hypothesis that elevated CO2 will increase the atmospheric temperature which will lead to increased volatilization loss of pesticides. In addition to this, better microbial growth under elevated CO2 will have influence in microbial degradation of pesticides. We observed an increase of 1.2–1.8 °C temperature in elevated CO2 chambers. The role of increased temperature in pesticide degradation has already been ascertained earlier. Zhang et al. (2012) reported the half-life of 1.35 days for the dissipation of chlorpyriphos from soil under paddy field conditions. Bloomfield et al. (2006) used a simulation model and predicted that for every 10 °C increase in temperature, the half-life of pesticides in soil might decrease by 60 %. In another study, Bailey (2004) examined that increased degradation of isoproturon in warmer soils. Additionally, increased water temperature was also found to increase the degradation rate of several phenyl-urea pesticides (Benitez et al. 2006). Direct role of elevated CO2 on pesticide degradation cannot be proved here. Increased temperature in elevated CO2 chambers compared to unchambered control helped in chlorpyriphos degradation. Manna et al. (2013) reported that elevated CO2 did not have any significant effect on the persistence of azoxystrobin in rice-planted soil, but they reported that elevated CO2-treated soil had less half-life of azoxystrobin compared to unchambered control.
Microbial biomass carbon and soil enzyme activities
MBC in chlorpyriphos-treated and chlorpyriphos-nontreated soils under lowland rice ecosystem were investigated over a time. It has been found that irrespective of pesticide spray, MBC content was higher in both the elevated CO2 treatments compared to control chambered and nonchambered rice soils throughout the investigation. MBC content was recorded 161–296 μg g−1 in chlorpyriphos-nontreated soil, whereas it was 146–297 μg g−1 in chlorpyriphos-treated soil under elevated CO2 at 700 ppm condition (Table 1). Under elevated CO2 at 550 ppm, MBC content was recorded 145–260 μg g−1 in chlorpyriphos-nontreated soil, whereas it was 121–260 μg g−1 in chlorpyriphos-treated soil. MBC content in soils under ambient OTC and unchambered soil recorded maximum of 201 and 180 μg g−1 of soil, respectively. MBC content in chlorpyriphos-treated soil did not differ from the untreated soils at the end of the experiment. But, it has been observed that, up to sixth days of spray, MBC content was less in treated soil than the nontreated soil. Beyond that, negligible effect of chlorpyriphos was ascertained. However, time, i.e., crop growth stages have influence on MBC content in soil (p < 0.0001). Interaction between time and pesticides had influence on MBC content in soil in all the four treatment conditions (Table 2).
Among the different microbial parameters, soil microbial biomass (MBC) is considered to be responsible for regulating nutrient cycling (Singh et al. 1989) and is closely linked to the primary productivity of an ecosystem (Marcel et al. 2008) and soil health (Sparling 1997). Impacts on MBC are of great importance to understand the below ground processes in soils exposed to high CO2 (Drigo et al. 2008). This increase could be attributed to more soil exudates in rice grown under elevated CO2 environment (Hill et al. 2007). Inubushi et al. (2011) reported that microbial biomass carbon in rice grown in elevated CO2 environment was significantly higher than rice soil maintained at ambient CO2. In our study, we observed more number of tiller (data not shown) in CO2 elevated treatments compared to both the controls. This may lead to increased secretion of root exudates, thereby MBC content in soil.
DHA in rice soils maintained under different CO2 environments is presented in Fig. 2. DHA varied according to the different CO2 environments in chlorpyriphos-nontreated soils. In case of chlorpyriphos-treated soils, DHA did not vary among the treatments during the experiment time. Highest DHA activities recorded in both CO2-enriched soils compared to both control soils irrespective of pesticide treatment. Crop duration had an effect on DHA activities (p ≤ 0.0004). It was found that DHA activities were maximum after 6–14 days of spray, which coincide with panicle initiation, irrespective of different CO2 environments. In our present study, increased in the DHA activity under elevated CO2 conditions suggest impact of climate change drivers on the soil microbial activity. The activity of dehydrogenase is exclusively intracellular and can function only within viable cells. It is considered as an indicator of the oxidative metabolism in soils (Wlodarczyk et al. 2002). Earlier, Inubushi et al. (2010) and Manna et al. (2013) studied dehydrogenase activity in rice soils under elevated CO2 and reported that there was no significant difference in the dehydrogenase activity of these soils. However, Das et al. (2011) reported increased dehydrogenase activity in rice soils incubated at elevated CO2 in a laboratory incubation study. Increased DHA activities may be due to higher deposition of carbon material around the root zone.
No significant difference in FDA activities was observed among the different CO2 treatments in pesticide-treated soils over time (Fig. 3). After 14 days of spray, untreated soil recorded maximum FDA activity of 13.12 μg g−1 soil h−1 in elevated CO2 at 700 ppm. Chlorpyriphos did not affect the FDA activity in pesticide-treated soils under different CO2 treatments. But, among the different CO2 treatments, there were significant differences till 21 days of spray in pesticide-nontreated soil. We observed no significant differences in the interaction between pesticide and duration of application. Das et al. (2011) reported that FDA hydrolysis activity increased significantly under elevated CO2.
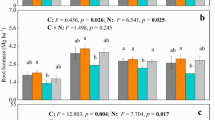
Positive effects of both the elevated CO2 treatments were observed in soil urease activity (Table 3). Urease activity in untreated soil under CO2 at 700 ppm treatment was maximum (407 μg g−1 soil h−1) after 14 days of application. There was significant difference among the four treatments over the period of experimentation. Chlorpyriphos has played a role in soil urease activity in all the CO2 treatments. Role of phosphatases in transformation of organic and inorganic phosphorous compounds in soil are already established in literature, and these activities are important factors in maintaining and controlling the rate of P cycling through soils. Acid and alkaline phosphatase activities of the soils are presented in Figs. 4 and 5. Both the elevated CO2 treatments had effect on both acidic and alkaline phosphatise activity compare to both control. Highest acid phosphatase activity was observed in pesticide-nontreated soil under elevated CO2 at 550 ppm in the tune of 44.75 μg g−1 soil h−1, and minimum was observed in pesticide-treated soil under outside control in the tune of 20.51 μg g−1 soil h−1. In case of alkaline phosphatase activity, highest activity was observed in pesticide-nontreated soil under elevated CO2 at 700 ppm condition in the tune of 44.99 μg g−1 soil h−1 and minimum was observed in pesticide-treated soil under ambient OTC in the tune of 25.19 μg g−1 soil h−1. Chlorpyriphos did not have any significant effect on acid phosphatase, but it has certain role in alkaline phosphatase activity in soils maintained under ambient OTC condition, elevated CO2 at 550 and 700 ppm. There was significant interaction present between pesticide and time of application for treatments maintained under both elevated CO2 treatments for alkaline phosphatase activity. Earlier research has indicated the possible inhibitory effect of the metabolites of chlorpyriphos, i.e., 3,5,6-trichloro-2-methoxy pyridine and 3,5,6-trichloropyridinol (TCP) on di-nitrogen-fixing bacteria and phosphate-solubilizing microorganism leading to short-term negative effect on urease and both phosphatase activities (Sardar and Kole 2005). Degradation of chlorpyriphos by an alkaline phosphatase obtained from Spirulina platensis was reported by Thengodkar and Sivakami (2010). Increased phosphatase activities and urease activities under elevated CO2 treatments supported by earlier reports (Kang et al. 2005; Das et al. 2011; Bhattacharyya et al. 2014). Phosphatase activities in wetlands under elevated CO2 increased considerably as microbes in the soil might be stimulated to obtain more phosphate, resulting in higher phosphatase activity (Kang et al. 2005). In general, our experiment has observed better soil enzymatic properties under elevated CO2 irrespective of pesticide treatments. It may be due to rhizospheric deposition of carbon materials led to better microbial activities.
Correlation among different enzymatic parameters was determined after 7 days of pesticide spray (table not included). It was found that except FDA, all other parameters were highly correlated and statistically significant. MBC was positively correlated with DHA (0.80), ALP (0.68), ACP (0.67), and urease (0.81). The correlation value between MBC and FDA was 0.25. Alkaline and acid phosphatases were highly correlated to each other with correlation value of 0.75. Both the phosphatase activities were similarly correlated with other soil microbial properties, namely, MBC, FDA, DHA, and urease.
Quantity of chlorpyriphos present on day 1 of spray did not show any negative effect on soil enzymatic activities (table not included). We found nonsignificant but positive correlation between chlorpyriphos present on day 1 of spray and on soil enzymatic properties like DHA, FDA, ACP, and urease. However, ALP was negatively correlated (−0.26) with quantity of pesticide present on day 1 of spray. After 7 days of spray, we observed nonsignificant but negative correlation between quantity of chlorpyriphos present with soil enzyme activities like DHA, ALP, ACP, and urease. Microbial biomass carbon was negatively correlated with quantity of pesticide present on 7 days of spray. It means chlorpyriphos has some effect on soil enzyme activities for initial days of spray. However, there was no residual effect of pesticides on soil microbial activities in later stage. Effect of chlorpyriphos on MBC was negligible in all the four treatments. Thus, when chlorpyriphos is used at recommended dose, it did not affect MBC in long run. Dutta et al. (2010) reported that application of chlorpyriphos at recommended field dose to agricultural soil is not likely to be detrimental to soil microbial activity. Repeated application of chlorpyriphos to the soil did not result in the development of a microbial population with the enhanced ability to degrade the pesticide (Singh et al. 2002). Earlier, Kumar (2011) reported that effect of chlorpyriphos on DHA depends on dose. Higher doses of chlorpyriphos will have prolonged negative effects on dehydogenase activity, whereas Menon et al. (2005) reported that chlorpyriphos and its metabolite had stimulatory effect on DHA. FDA was considered as a suitable tool for measuring the early detrimental effect of pesticides on soil microbial biomass (Nayak et al. 2007). Dutta et al. (2010) reported that chlorpyriphos at field rate did not influence FDA activity.
Conclusions
Increase in CO2 concentration led to increase in atmospheric temperature which was responsible for reduced chlorpyriphos persistence. This may contribute toward environmental safety although it may lead to frequent use of pesticides to combat evolving pest scenario. The increase in root exudates under elevated CO2 conditions improved the soil enzymatic activities and microbial biomass carbon. Chlorpyriphos had shown a transient negative effect on soil enzymes. This short-term study was for generating initial knowledge to assess the impact of elevated CO2 on chlorpyriphos degradation, microbial biomass carbon, and enzymatic activities in rice soil. However, to have a realistic assessment of global warming on fate of pesticide, long-term studies are required.
References
Adam, G., & Duncan, H. (2001). Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biology and Biochemistry, 33, 943–951.
Bailey, S. W. (2004). Climate change and decreasing herbicide persistence. Pest Management Science, 60(2), 158–62.
Bazot, S., Ulff, L., Blum, H., Nguyen, C., & Robin, C. (2006). Effects of elevated CO2 concentration on rhizodeposition from Lolium perrene grown on soil exposed to 9 years of CO2 enrichment. Soil Biology and Biochemistry, 38, 729–736.
Benitez, F. J., Real, F. J., Acero, J. L., & Garcia, C. (2006). Photochemical oxidation processes for the elimination of phenyl-urea herbicides in waters. Journal of Hazardous Materials, 138(2), 278–87.
Bhattacharyya, P., Roy, K. S., Dash, P. K., Neogi, S., Shahid, M., Nayak, A. K., Raja, R., Karthikeyan, S., Balachandar, D., & Rao, K. S. (2014). Effect of elevated carbon dioxide and temperature on phosphorus uptake in tropical flooded rice (Oryza sativa L.). European Journal of Agronomy, 53, 28–37.
Bhattacharyya, P., Roy, K. S., Neogi, S., Manna, M. C., Adhya, T. K., Rao, K. S., & Nayak, A. K. (2013). Influence of elevated carbon dioxide and temperature on belowground carbon allocation and enzyme activities in tropical flooded soil planted with rice. Environmental Monitoring and Assessment, 185, 8659–8671.
Bloomfield, J. P., Williams, R. J., Gooddy, D. C., Cape, J. N., & Guha, P. (2006). Impacts of climate change on the fate and behaviour of pesticides in surface and groundwater—a UK perspective. Science of the Total Environment, 369(1–3), 163–77.
Casida, L. E., Klein, D. A., & Santoro, T. (1964). Soil dehydrogenase activity. Soil Science, 98, 371–376.
Das, S., Bhattacharyya, P., & Adhya, T. K. (2011). Interaction effects of elevated CO2 and temperature on microbial biomass and enzyme activities in tropical rice soils. Environmental Monitoring and Assessment, 182, 555–569.
Drigo, B., Kowalchuk, G. A., & van Veen, J. A. (2008). Climate change goes underground: Effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biology and Fertility of Soils, 44, 667–679.
Dutta, M., Sardar, D., Pal, R., & Kole, R. K. (2010). Effect of chlorpyrifos on microbial biomass and activities in tropical clay loam soil. Environmental Monitoring and Assessment, 160, 385–391.
Eivazi, F., & Tabatabai, M. A. (1977). Phosphates in soils. Soil Biology and Biochemistry, 9, 167–172.
Harrington, R., Woiwod, I., & Sparks, T. (1999). Climate change and trophic interactions. Trends in Ecology & Evolution, 14, 146–150.
Hill, P. W., Marshall, C., Williams, G. G., Blum, H., Harmens, H., & Jones, D. L. (2007). The fate of photosynthetically fixed carbon in Lolium perenne grassland as modified by elevated CO2 and sward management. New Phytologist, 173, 766–777.
Hoskin, M. L. (1961). Mathematical treatment of loss of pesticide residues. F.A.O. Plant Protection Bulletin, 9, 163–169.
Hua, F., Yunlong, Y., Xiaoqiang, C., Xiuguo, W., Xiaoe, Y., & Jingquan, Y. (2009). Degradation of chlorpyriphos in laboratory soil and its impact on soil microbial functional diversity. Journal of Environmental Sciences, 21(3), 380–6.
Inubushi, K., Cheng, W., Mizuno, T., Lou, Y., Hasegawa, T., & Sakai, H. (2011). Microbial biomass carbon and methane oxidation influenced by rice cultivars and elevated CO2 in a Japanese paddy soil. European Journal of Soil Science, 62, 69–73.
Inubushi, K., Mizuno, T., Lou, Y., Hasegawa, T., Lin, Y., Cheng, W., Kobayashi, K. & Okada, M. (2010). Microbial biomass and activities in a Japanese paddy soil with differences in atmospheric CO2 enrichment, soil/water warming and rice cultivars. 19th World Congress of Soil Science, Soil Solutions for a Changing World, 1–6 August 2010, Brisbane, Australia
IPCC. (2014). In Core Writing Team, R. K. Pachauri, & L. A. Meyer (Eds.), Climate Change 2014: Synthesis Report (Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, p. 151). Geneva: IPCC.
Juma, N. G., & Tabatabai, M. A. (1977). Effects of trace elements on phosphatase activity in soils. Soil Science Society of America Journal, 41, 343–346.
Kang, H., Kim, S. W., Fenner, N., & Freeman, C. (2005). Shifts of soil enzyme activities in wetlands exposed to elevated CO2. Science of the Total Environment, 337, 207–212.
Kumar, S. (2011). Fluctuation of soil bacterial dehydrogenase activity in response to the application of endosulfan and chlorpyrifos. Journal of Cell & Tissue Research, 11(2), 2847–2851.
Lesaulnier, C., Papamichail, D., McCorkle, S., Ollivier, B., Skiena, S., & Taghavi, S. (2008). Elevated atmospheric affects soil microbial diversity associated with trembling aspen. Environmental Microbiology, 10, 926–941.
Li, X., Han, S., Guo, Z., Shao, D., & Xin, L. (2010). Changes in soil microbial biomass carbon and enzyme activities under elevated CO2 affect fine root decomposition processes in a Mongolian oak ecosystem. Soil Biology and Biochemistry, 42, 1101–1107.
Manna, S., Singh, N., & Singh, V. P. (2013). Effect of elevated CO2 on degradation of azoxystrobin and soil microbial activity in rice soil. Environmental Monitoring and Assessment, 185, 2951–2960.
Marcel, G. A., Heijden, V. D., Bardgett, R. D., & van Straalen, N. M. (2008). The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecological Letters, 11, 296–310.
Menon, P., Gopal, M., & Parsad, R. (2005). Effects of chlorpyrifos and quinalphos on dehydrogenase activities and reduction of Fe3+ in the soils of two semi-arid fields of tropical India. Agriculture Ecosystem and Environment, 108, 73–83.
Nayak, D., Jagadeesh Babu, Y., & Adhya, T. K. (2007). Long-term application of compost influences microbial biomass and enzyme activities in a tropical Aeric Endoaquept planted to rice under flooded condition. Soil Biology and Biochemistry, 39, 1897–1906.
Paul, S., Prasanna, R., Lata & Dhar, D. W. (2009). Microbiotech-A manual for agricultural microbiologists, Division of Microbiology and CCUBGA, IARI, New Delhi, Pp-26-27.
Sardar, D., & Kole, R. K. (2005). Metabolism of chlorpyriphos in relation to its effect on the availability of some plant nutrients in soil. Chemosphere, 61, 1273–1280.
Sikora, L. J., Kaufman, D. D., & Horng, L. C. (1990). Enzyme activity in soils showing enhanced degradation of organophosphate insecticides. Biology and Fertility of Soils, 9, 14–18.
Singh, B. K., Walker, A., & Wright, D. J. (2002). Degradation of chlorpyriphos, fenamiphos, and chlorothalonil alone and in combination and their effects on soil microbial activity. Environmental Toxicology and Chemistry, 21(12), 2600–2605.
Singh, J. S., Raghubanshi, A. S., Singh, R. S., & Srivastava, S. C. (1989). Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature, 338, 499–500.
Sparling, G. P. (1997). Soil microbial biomass, activity and nutrient cycling as indicators of soil health. In C. Pankhurst, B. M. Doube, & V. V. S. R. Gupta (Eds.), Biological indicators of soil health (pp. 97–119). Wallingford: CAB International.
Thengodkar, R. R., & Sivakami, S. (2010). Degradation of Chlorpyrifos by an alkaline phosphatase from the cyanobacterium Spirulina platensis. Biodegradation, 21(4), 637–44.
Vance, E. D., Broke, P. C., & Jenkinson, D. S. (1987). Microbial biomass measurements in forest soils: the use of chloroform fumigation–incubation method in strongly acidic soils. Soil Biology and Biochemistry, 19, 697–702.
Wlodarczyk, T., Stepniewski, W., & Brzezinska, M. (2002). Dehydrogenase activity, redox potential, and emissions of carbon dioxide and nitrous oxide from Cambisols under flooding conditions. Biology and Fertility of Soils, 36, 200–206.
Zantua, M. T., & Bremner, J. M. (1977). Stability of urease in soils. Soil Biology and Biochemistry, 9, 135–140.
Zhang, X., Shen, Y., Yu, X., & Liu, X. (2012). Dissipation of chlorpyriphos and residue analysis in rice, soil and water under paddy field conditions. Ecotoxicology and environmental safety, 78, 276–280.
Acknowledgments
The authors sincerely thank Dr. Pratap Bhattacharyya, Principal Scientist, Division of Crop Production, for his constructive suggestions in the preparation of the manuscript. Authors duly acknowledge the technical and financial support provided by the Director, ICAR-National Rice Research Institute, Cuttack.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adak, T., Munda, S., Kumar, U. et al. Effect of elevated CO2 on chlorpyriphos degradation and soil microbial activities in tropical rice soil. Environ Monit Assess 188, 105 (2016). https://doi.org/10.1007/s10661-016-5119-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5119-4