Abstract
An experiment was conducted under three levels of atmospheric CO2 [ambient (398 ± 10 μmol mol−1), elevated (570 ± 10 μmol mol−1) and open condition], three levels of temperature (4, 25, and 40 °C) to study the degradation pattern of flubendiamide in soil and also carbon mineralization in soil. Results of this study revealed that flubendiamide was found to persist longer under outdoor condition (T1/2, 177.0 and 181.1 days) than ambient (T1/2, 168.4 and 172.3 days) and elevated condition (T1/2, 159.3 and 155.3 days) at 1 and 10 μg g−1 fortification level, respectively. Results also revealed that flubendiamide dissipated faster at 40 °C (T1/2, 189.4 days) than 25 °C (T1/2, 225.3 days). Slower dissipation was recorded at 4 °C (T1/2, 326.3 days). Thus, increased CO2 levels and temperature following global warming might adversely affect flubendiamide degradation in soil. Laboratory study on microbial biomass carbon (MBC) and carbon mineralization (Cmin) in soil revealed that in des-iodo flubendiamide-treated soils, MBC significantly increased up to 45 days and then decreased. Flubendiamide-treated soil showed a non-significantly decreasing trend of soil MBC with time up to the 15th day of incubation and after 15 days significantly decreased up to 90 days of incubation. In des-iodo flubendiamide-treated soil, the evolution of CO2 decreased up to 45 days, which was increased after 45 days up to 90 days. In flubendiamide-treated soil, CO2 evolution decreased up to 30 days and after 45 days, it increased up to 90 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concentration of CO2 in atmosphere has risen from pre-industrial times (∼280 μmol mol−1) to a current global mean (∼398 μmol mol−1). Climate scientists are predicting that CO2 will exceed 500 μmol mol−1 during the later half of this century. Using IPCC report on climate model projections it indicated that during the twenty-first century, atmospheric CO2 concentration is likely to be doubled along with global average temperature probably rise further by 1.1–6.4 °C (IPCC 2007). For the development of sustainable plant protection practices, monitoring of pesticide residues in environmental body is very critical. Persistence of agrochemicals in soil and water body has social, environmental, as well as economic significance, which are often used for the environmental impacts and monitoring of pesticide use. The change in atmospheric CO2, as well as changes in temperature, may have effects on the fate of xenobiotics that end up in environment when sprayed (Das and Mukherjee 2011; Das and Mukherjee 2012a; Das and Avasthe 2015). The temperature of the soil influences degradation rates of pesticide; the rate of most reactions catalyzed by enzymes tends to double for every 10 °C increase in temperature [between 10 and 45 °C]. An increase in soil temperature will thus lead to an increase in degradation rate. The grade of the degradation is increased with the rise of the temperature, as proved in some studies completed under tropical circumstances. Some studies suggest a temperature of 40 °C, or higher, as the desirable temperature for the best degradation rate of DDT (Guerin 1999). No more literature is available on the effect of CO2 and temperature on pesticide fate in our environment. Most of the reports are based on modeling of the available data and suggested that increase in temperature has resulted in decrease in pesticides persistence over the last few decades (Williams et al. 1992; Bailey 2003; Bloomfield et al. 2006). Rate of pesticide degradation in soil depends on different physico-chemical properties like soil texture (Choi et al. 1988), composition of organic matter (Xing and Pignatello 1997), soil pH (Walker et al. 2001), mode of action of pesticide, chemical structure, etc. Microbial biomass carbon (MBC) in soil acts as a strong predictor of the pesticide degradation capacity of a particular soil (Voos and Groffman 1997) and is considered as an important attribute of soil quality (Doran and Parkin 1994) and carbon mineralization (Cmin) is an age-old reliable method for studying microbial activities in soil. Changes in Cmin have also been used as criteria for pesticide toxicity (Torstenssen and Stenstorm 1986). Drigo et al. (2008), who reviewed effect of elevated CO2 on soil microbial parameters and activities in rhizosphere of upland crops, showed mixed response on MBC; some suggested increase in MBC (De Graaff et al. 2006; Carney et al. 2007; Li et al. 2010), while others suggested no effect (Janus et al. 2005; Bazot et al. 2006; Lesaulnier et al. 2008).
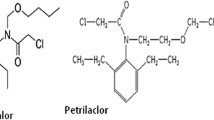
Flubendiamide, N 2-[1,1-dimethyl-2-(methylsulfonyl)ethyl]-3-iodo-N 1-[2-methyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl]-1,2-benzene dicarboxamide, which belongs to phthalic acid diamide group (Fig. 1) is mainly effective for controlling lepidopteron pests in rice, cotton, other fruits, and vegetables like chilli, cabbage, etc. (Tohnishi et al. 2005). It affects Ca+2 ion balance irrespective of Na+ or K+ ion balance, which is responsible for contraction of insect skeletal muscle (Masaki et al. 2006). In insects, it activates ryanodine sensitive intracellular calcium release channels (Das et al. 2015). Literature showed that flubendiamide persisted more in dry soil (T1/2, 206.6–215.0 days) as compare to field capacity (T1/2, 177.0–181.1 days) and submerged (T1/2, 150.5–158.4 days) condition (Das et al. 2012). Under sterile and nonsterile conditions, DT50 of flubendiamide in red soil were found to be 140.3 and 93.7 days, respectively, and in alluvial soil it was 181.1 and 158.4 days, respectively (Das and Mukherjee 2014). Flubendiamide dissipated in okra fruits with T1/2 ranged from 4.7 to 5.1 days (Das and Mukherjee 2012b). Absolutely no real-time information/study is available on the fate of flubendiamide under elevated CO2 and temperature and also its effect on Cmin. Owing to the ever increasing use of flubendiamide in the agricultural land, the investigation was carried out to study the effect of elevated atmospheric CO2 (570 ± 10 μmol mol−1) and temperature (4, 25, and 40 °C) on degradation pattern flubendiamide in soil. Keeping in mind the toxicological importance of photo metabolite, in the present study, investigation also finds the effect of flubendiamide and its photo metabolite des-iodo flubendiamide on MBC and Cmin.
Materials and methods
Soil sampling and chemicals
A sandy loam soil used in the present study was collected from the experimental farm of IARI, New Delhi with no history of pesticide application. Different physico-chemical properties of the soil were analyzed with standard method (Jackson 1967). Sand, silt, and clay contents were measured by using Bouyoucos hygrometer (Bouyoucos and Cook 1967). Exchangeable Ca+2, Mg+2, K+, and Na+, along with acid oxalate-extractable Fe+2 and Al+3, were determined by Blakemore et al. (1987) method. Soil pH (1:2.0 H2O), Olsen P (0.5 M NaHCO3 extraction), CEC [1-M NH4OAc (pH 7.0) extraction], and SO4 −2 [0.04-M Ca(H2PO4)2 extraction] were measured by Black et al. 1965 method. By using the Walkley and Black (1965) method organic carbon content. Electrical conductivity of soil was measured with conductivity meter. Analyzed soil properties are given in Table 1. Analytical grade flubendiamide (99.5 %) and des-iodo flubendiamide (99.2 %) were purchased from M/s Bayer Crop Science Limited, Mumbai, India. All the glassware were washed with chromic acid solution and then thoroughly washed with water, rinsed with acetone, and then dried at 115 °C for 4 h prior to use. The solvents and other chemicals were purchased from Merck Specialties Private Ltd, New Delhi, India. High-performance liquid chromatography (HPLC) solvents were doubled filtered and de-gassed prior to use.
Degradation study under different CO2 level
Effect of elevated CO2 study was carried out in a completely randomized design experiment in the National Phytotron Facility, IARI, New Delhi, India in an open top chamber (1.85 and 1.62 m). Continuous injection of pure CO2 into the open top chamber was carried out to maintain elevated levels of CO2 in open top chamber (OTC) at 570 ± 10 μmol mol−1. Before entering into the chamber, it was mixed with air from the air compressor. From the middle of the chamber, the air sample was drawn periodically into a CO2 sensor. Thereafter, in the chamber set, level of CO2 was properly maintained. CO2 data logging, control, and operation were performed through digital input and output module using a computer on a real-time basis. At three conditions, viz. open top chamber maintained at ambient (398 ± 10 μmol mol−1), elevated CO2 level (570 ± 10 μmol mol−1), and open condition effect of CO2 on flubendiamide degradation in soil was studied with two concentration level viz., 1.0 and 10.0 μg g−1. It was considered that in phytotron growth chamber, the narrow range of fluctuations of CO2 concentration was ±10 μmol mol−1 which has been reported elsewhere (Sujatha et al. 2008). This study was carried out at 1.0 and 10.0 μg g−1. Standard solution (1000 μg g−1) of flubendiamide was added (20 mL) in 200-g air dry soil to fortify at the level of 100 μg g−1. To obtain 10.0 and 1.0 μg g−1 levels, this fortified soil sample was serially diluted with the untreated soil. Air dry soil was brought to field capacity moisture level (20.1 %) by adding required amount of water as per the methodology of Das and Mukherjee 2014. The treated and control soil samples were transferred to 250-mL beakers, and with aluminium foil, all beakers were closed. Constant weight was maintained throughout the experiment by adding required quantity of water every alternate day. One set of beaker was transferred to OTC having elevated CO2 concentration (570 ± 10 μmol mol−1), one set to OTC at ambient CO2 concentration (398 ± 10 μmol mol−1), while the third set was kept in the open condition. Beakers in triplicate were withdrawn at 0, 3, 5, 7, 10, 15, 30, 50, 60, and 90-day interval along with control sample. Residues in soil were extracted with acetone (50 mL) in 150-mL separatory funnel by intermittent shaking. The contents in the flask were collected through filtered paper (Whatman filter paper no. 1). Soil was then transferred back to beaker and again extracted with fresh 50-mL acetone (two times). All acetone extracts were pooled and concentrated by rotary evaporator. Two percent of saturated NaCl solution (100 mL) was mixed with concentrated extract, transferred to separatory funnel, and exchanged into DCM solvent (3 × 30 mL). Combined DCM extract was collected in a round bottle flask passed through anhydrous NaSO4. Extract was evaporated to dryness and dissolved in HPLC grade acetonitrile for residue analysis.
Degradation study under different temperature regimes
Degradation behavior of flubendiamide in soil was studied at 1-μg mL−1 fortification level under three different temperature regimes (4, 25, and 40 °C). The experiment was conducted in a completely randomized design under laboratory condition. In this study, three levels of temperature as mentioned above were carefully chosen to simulate the current and future levels of temperature, which is expected to prevail over the growing regions of Indian subtropical condition in the later half of this century. Three temperature levels were selected based on the prevailing temperature regime in Indian subtropical condition to make it India-specific. Samples were fortified at 1-μg mL−1 level. After fortification, the beakers were divided into three sets. First set was kept in refrigerator maintained at 4 ± 1 °C; second set was kept at 40 ± 1 °C in incubating oven; and the third set placed in BOD incubator at 25 ± 1 °C. The beakers were closed with aluminium foil, and constant weight of the beakers was maintained throughout the experiment by replenishing the lost water every alternate day. Samples in triplicate, i.e., three beakers per fortification level per moisture regime were withdrawn at 0, 3, 5, 7, 10, 15, 30, 50, 60, and 90-day interval along with control. Triplicate samples removed for analysis by HPLC.
Residue analysis and calculation
Final extracts were dissolved in double-filtered HPLC grade acetonitrile for quantification. Residues of flubendiamide were estimated by HPLC system (Merck-Hitachi). The mobile phase was a mixture of acetonitrile-water (70: 30 v/v) and flow rate 0.5 mL min−1 having injection volume of 10 μL. Flubendiamide was eluted at retention time (10.1 min) under these conditions at 210 nm (λmax). HPLC chromatogram of flubendiamide and des-iodo flubendiamide is presented in Fig. 2. The limit of detection (LOD) was 0.01 μg g−1. Obtained residue data were undergone for regression analysis. Half-life (T1/2) value was calculated using first-order dissipation kinetics.
Carbon mineralization and microbial biomass carbon study
This study was carried out in laboratory conducted at room temperature. At first, air dry soil was brought to field capacity moisture level (Das and Mukherjee 2014). In dark condition, field capacity soil was then acclimatized at room temperature for about 7 days. Fifty-gram soil in individual beaker was taken. To simulate field application rate, flubendiamide at 24.0 g a.i. ha−1 and its metabolite des-iodo flubendiamide at 24 g a.i. ha−1 was applied in solution form. Both flubendiamide and des-iodo flubendiamide-treated soils were incubated at 25 °C, along with control with three replication. The entire beaker contained 0.1-N NaOH in a vial, which help to trap the evolved CO2 the. The beakers were withdrawn at 0, 1, 3, 5, 7, 10, 15, 30, 45, 60, and 90 days of incubation for estimation of Cmin. For microbial biomass carbon experiment, three sets of soil samples (20 g) were taken. One set fumigated with ethanol-free chloroform (25 mL) placed in a vacuum desiccator and then chloroform was allowed to boil for 2 min under reduced pressure. They were then incubated at 25 °C for 1 day (Joergensen 1996). Unfumigated second set was kept under similar conditions in the desiccators. For moisture estimation, third set was kept in open condition. Fumigated and unfumigated soil sample were extracted with 50 mL 0.5 (M) K2SO4 by an oscillating shaker at 500 rpm for 20 min. Estimation of extracted carbon and MBC calculation was done by the method of Vance et al. (1987). Evolved CO2–C from soil was measured by back titrating the unused alkali verses hydrochloric acid (Zibilski 1994). This was used for estimating potential carbon mineralization. Data analyzed by SAS software packages 9.3 was subjected to Duncan’s multiple range test. The details of the insecticides used for the experiment are presented in Table 2.
Results and discussion
Effect of elevated CO2 on degradation of flubendiamide
Flubendiamide degradation at 1.0 and 10.0 μg g−1 levels under three different CO2 conditions is shown in Figs. 3 and 4, respectively. Under outdoor condition, mean-starting residue deposits were 0.98 and 9.91 μg g−1 at 1.0 and 10 μg g−1 fortifications. With time lapse, flubendiamide residues go down slowly and found that at 10 μg g−1 fortification, residues were 9.38, 8.75, 7.89, and 7.01 μg g−1 during 10, 30, 60, and 90 days, respectively. These losses amount from 5.3 to 29.2 % at 10 μg g−1 level (Table 3). In the untreated control samples, no interfering peaks/residues were observed. From the first-order dissipation kinetics, calculated half-life values were 177.0 and 181.1 days at 1 and 10 μg g−1 fortification levels (Table 4).
Under ambient CO2 condition, mean-starting deposits calculated were 0.98 and 9.91 μg g−1 at 1.0- and 10-μg g−1 fortification. With time lapse, flubendiamide residues go down slowly and found that at 10 μg g−1 fortification, residues were 9.34, 8.47, 7.30, and 6.60 μg g−1 during 10, 30, 60, and 90 days, respectively. These losses amount from 5.7 to 33.4 % (Table 3). At 1.0-μg g−1 fortification level, 0.87, 0.81, 0.71, and 0.64 μg g−1 residues were detected during 10, 30, 60 and 90 days, respectively. These losses amount from 11.2 to 34.6 % (Table 3). In the untreated control samples, no interfering peaks/residues were observed. From the first-order dissipation kinetics, calculated half-life values were 168.4 and 172.3 days at 1 and 10 μg g−1 fortification levels (Table 4). Das et al. (2012) also showed that flubendiamide persisted in field capacity with a T1/2 of 177.0–181.1 days.
Under elevated CO2 condition, mean initial deposits calculated were 0.97 and 9.95 μg g−1 at 1.0- and 10-μg g−1 fortification, respectively. With time lapse, flubendiamide residues go down slowly and found that at 10-μg g−1 fortification, residues were 9.44, 8.57, 7.01, and 6.12 μg g−1 during 10, 30, 60, and 90 days, respectively. These losses amount from 5.1 to 38.1 % at 10-μg g−1 level (Table 3). At 1.0-μg g−1 level, 0.88, 0.82, 0.68, and 0.60 μg g−1 residues were detected during 10, 30, 60, and 90 days, respectively. These amount from 9.2 to 38.1 % (Table 3). Calculated half-life values from first-order dissipation kinetics were 159.3 and 155.3 days at 1.0 and 10 μg g−1 fortifications (Table 4). Flubendiamide was found to persist longer under outdoor condition than ambient condition followed by elevated condition. Higher temperature in OTC may result in increased volatilization losses which hasten faster degradation of flubendiamide. Similar results have been shown by Chatterjee et al. 2013 in metaflumizone. It seems that at elevated CO2 level, slightly higher temperature maintained in open top chamber (OPT) is responsible for the faster flubendiamide degradation. Das et al. (2012) reported similar degradation pattern under submerged soil with T1/2 of 150.5–158.4 days.
Effect of temperature on degradation of flubendiamide
At different temperatures, the residue data at different time intervals are presented in Table 5. The calculated mean initial deposits at 4, 25, and 40 °C were found to be 0.97, 0.965, and 0. 97 μg mL−1, respectively (Fig. 5). On the 15th day, the percent dissipation recorded was 4.53–6.52, while during the 60th day it was 12.25–21.60 % and 13.28–26.64 % on the 90th day. Results revealed that residues dissipated faster at higher temperature 40 °C (26.64 %), followed by 25 °C (22.17 %), and 4 °C (13.28 %) after 90 days. At the initial stage, i.e., after 15 days, residues of flubendiamide dissipated faster at 25 °C (6.52), followed by 40 °C (5.14), and 4 °C (4.53). The calculated half-life values along with correlation coefficient are demonstrated in Table 6. Correlation coefficients in all the cases were found to be >0.95. Dissipation of the pesticide followed mono-phasic first-order kinetics. Calculated T1/2 values using first-order kinetics varied from 189.4 to 326.3 days (Table 6). Results revealed that flubendiamide dissipated faster at 40 °C (T1/2, 189.4 days) than 25 °C (T1/2, 225.3 days). The faster dissipation of flubendiamide in soil under 40 °C could be due to the breakdown of the two amide linkage present in the molecule. Besides at higher temperature soil thermophilic microbes may play an important role for degradation. But slowest dissipation was recorded at 4 °C (T1/2, 326.3 days). In the untreated control samples, no interfering peaks/residues were observed. Faster dissipation of flubendiamide at high temperature could be due to the breakdown of amide linkage present in the molecule. Higher temperature-enhanced degradation of various pesticides molecules has been reported by Starner et al. (1999), Bobe et al. (1998), and Morishima and Iigaya (1995). Faster dissipation at higher temperature has also been reported for synthetic pyrethroids (Watters et al. 1983) and endosulfan (Kaur et al. 1998).
Effect flubendiamide and des-iodo flubendiamide on MBC and Cmin in Soil
Effects of flubendiamide and des-iodo flubendiamide addition on soil microbial biomass carbon are shown in Table 7. In des-iodo flubendiamide-treated soils, results showed that MBC significantly increased up to 45 days and then decreased spontaneously with time up to 90 days. The MBC was statistically higher in des-iodo flubendiamide-treated soil, compared to the flubendiamide-treated soil and control throughout the experiment. Flubendiamide-treated soil showed a decreasing (non-significant) trend of soil MBC, with time up to the 15th day of incubation and after 15 days, decreased (significant) up to 90 days of incubation. But comparing with control, it was significantly lower from day 15 onward throughout the incubation period. Handa et al. (1999) reported that during incubation period, an increase in soil microbial biomass might be due to increased availability of nutrient and energy source with the mineralization of insecticides. During the first 45 days, higher MBC in des-iodo flubendiamide-treated soil and control soil might be due to a proliferation of microbial populations. The toxic effect of flubendiamide decreased with increase in time; thereby, it resulted in increase in microbial population after a certain point of time.
The Cmin data has been represented in Table 8. In des-iodo flubendiamide-treated soil, the evolution of CO2 decreased up to 45 days. But it increased after 45 days up to 90 days. Similar trend was also shown by the control-treated soil. In flubendiamide-treated soil, CO2 evolution decreased up to 30 days and after 45 days, it increased up to 90 days. But CO2 evolution remained steady between 30 and 45 days in flubendiamide-treated soil. Interestingly, during the incubation period among the control, des-iodo flubendiamide and flubendiamide-treated soil, there were no significant differences in Cmin. This showed that applied doses of flubendiamide and its photo metabolite des-iodo flubendiamide in soil are not high enough to suppress microbial population. Till date, no study has carried out in flubendiamide for Cmin study. But, Tu (1992), in his experiment, reported that the Cmin increased significantly after 96 h of incubation with atrazine. Also, Zelles et al. (1985) reported a very little effect of lindane, atrazine, and captan on the Cmin pattern. In des-iodo flubendiamide-treated soil, the decline in Cmin up to 45 days, soils could be due to utilization of nutrients and energy by soil microorganism and the establishment of a suitable environment (Sengupta et al. 2009). Thus, flubendiamide at recommended field doses (24 g a.i.) and des-iodo flubendiamide, which is produced by photolysis of flubendiamide in presence of light, have only marginal effects on MBC and Cmin and that effect may be recovered by microbial population rapidly. But, laboratory and field studies may differ considerably due to presence of various biotic and abiotic factors. HPLC-MS chromatogram for mineralization pattern of flubendiamide and its degraded product is shown in Fig. 6. HPLC-mass spectrometry analysis indicated that flubendiamide showed molecular ion peak at m/z 680.8 (M+) and after mineralization the molecular ion peak observed at m/z 274.8 (M+).
References
Bailey SW (2003) Climate change and decreasing herbicide persistence. Pest Manag Sci 60:158–162
Bazot S, Ulff L, Blum H, Nguyen C, Robin C (2006) Effects of elevated CO2 concentration on rhizodeposition from Lolium perenne grown on soil exposed to 9 years of CO2 enrichment. Soil Biol Biochem 38:729–736
Black CA, Evans DD, White JL, Ensminger LE, Clark FE (eds) (1965) Methods of soil analysis, 2nd edn. In Agronomy monograph 9. Madison: Agronomy Society of America and Soil Science Society of America.
Blakemore LC, Searle PL, Daly BK (1987) Methods for chemical analysis of soils. NZ Soil Bureau Scientific Report, series 80. NZ DSIR, New Zealand, p 103
Bloomfield JP, Williams RJ, Gooddy DC, Cape JN, Guha P (2006) Impact of climate change on the fate and behavior of pesticides in surface and ground water—a UK perspective. Sci Total Environ 369:163–177
Bobe A, Cooper JF, Coste CM, Muller MA (1998) Behavior of fipronil in soil under Sahelian plain field conditions. Pestic Sci 52:275–281
Bouyoucos GJ, Cook RL (1967) Measuring the relative humidity of soils at different moisture contents by the gray hydrocal hygrometer. Soil Sci 104:297–305
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc Natl Acad Sci U S A 104:4990–4995
Chatterjee NS, Gupta S, Varghese E (2013) Degradation of metaflumizone in soil: impact of varying moisture, light, temperature, atmospheric CO2 level, soil type and soil sterilization. Chemosphere 90:729–736
Choi JS, Fermanian TW, Wehner DJ, Spomer LA (1988) Effect of temperature, moisture, and soil texture on DCPA degradation. Agrono J 80:108–113
Das SK, Avasthe RK (2015) Biochar as carbon negative in carbon credit under changing climate. Curr Sci 109:1223
Das SK, Mukherjee I (2011) Effect of light and pH on persistence of flubendiamide. Bull Environ Contam Toxicol 87:292–296
Das SK, Mukherjee I (2012a) Effect of moisture and organic manure on persistence of flubendiamide in soil. Bull Environ Contam Toxicol 88:515–520
Das SK, Mukherjee I (2012b) Flubendiamide transport through packed soil columns. Bull Environ Contam Toxicol 88:229–233
Das SK, Mukherjee I (2014) Influence of microbial community on degradation of flubendiamide in two Indian soils. Environ Monit Assess 186:3213–3219
Das SK, Mukherjee I, Das SK (2012) Dissipation of flubendiamide in/on okra [Abelmoschus esculenta (L.) Moench] fruits. Bull Environ Contam Toxicol 88:381–384
Das SK, Mukherjee I, Kumar A (2015) Effect of soil type and organic manure on adsorption–desorption of flubendiamide. Environ Monit Assess 187:403. doi:10.1007/s10661-015-4623-2
De Graaff M, Van Groenigen K, Six J (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biol 12:2077–2091
Doran JW, Parkin TB (1994) Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for sustainable environment, vol Special pub 35. Soil Sci Soc Am, Inc., Madison
Drigo B, Kowalchuk GA, van Veen JA (2008) Climate change goes underground: effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biol Fertil Soils 44:667–679
Guerin T (1999) Natural attenuation of a low mobility chlorinated insecticide in low-level and high-level contaminated soil: a feasibility study. Remediation J 9:51–63
Handa SK, Agnihotri NP, Kulshreshtha G (1999) Pesticide residues: significance, management and analysis. Research Periodicals and Book Publishing House, Texas
IPCC (2007) Climate Change 2007: The Physical Sciences Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, pp. 847–940. Cambridge University Press, Cambridge, UK.
Jackson ML (1967) Soil chemical analysis. Prentice Hall, New Delhi
Janus L, Angeloni N, McCormack J, Rier S, Tuchman N, Kelly J (2005) Elevated atmospheric CO2 alters soil microbial communities associated with trembling aspen (Populus tremuloides) roots. Microbial Ecology 50:102–109
Joergensen RG (1996) The fumigation–extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31
Kaur I, Mathur RP, Tandon SN, Dureja P (1998) Persistence of endosulfan (technical) in water and soil. Environ Technol 19:115–119
Lesaulnier C, Papamichail D, McCorkle S, Ollivier B, Skiena S, Taghavi S (2008) Elevated atmospheric affects soil microbial diversity associated with trembling aspen. Environ Microb 10:926–941
Li X, Han S, Guo Z, Shao D, Xin L (2010) Changes in soil microbial biomass carbon and enzyme activities under elevated CO2 affect fine root decomposition processes in a Mongolian oak ecosystem. Soil Biol Biochem 42:1101–1107
Masaki T, Yasokawa N, Tohnishi M, Motoba K, Hirooka T (2006) Flubendiamide, a novel Ca2+ channel modulator, reveals evidence for functional cooperation between Ca2+ pumps and Ca2+ release. Mol Pharmacol 69:1733–1739
Morishima N, Iigaya M (1995) Photo degradation of (Phenyl-UL-14C) KTU 3616 in water. Interner Bericht der Nihon Bayer Agrochem K K, Yuki, Japan
Sengupta D, Aktar W, Purkait S, Ganguly M (2009) Impact of quinalphos on microbial biomass and activities in tropical clay loam soil. Electron J Environ Agric Food Chem 8(11):1127–1135
Starner K, Kuivila KM, Jennings B, Moon GE (1999) Degradation rates of six pesticides in water from the Sacramento River, California, U.S. geographical survey toxic substances hydrology program: proceeding of the technical meeting, Charleston, South Carolina. http://ca.water.usgs.gov/archive/reports/wrir994018/CA-0216.pdf. Accessed on 20th May 2015.
Sujatha KB, Uprety DC, Rao DN, Rao PN, Dwivedi N (2008) Up-regulation of photosynthesis and sucrose-P synthase in rice under elevated carbon dioxide and temperature conditions. Plant Soil Environ 54:155–162
Tohnishi M, Nakao H, Furuya T, Fujioka S, Kodama H, Hirooka T, Nishimatsu T (2005) Flubendiamide—a novel insecticide highly active against Lepidopteron insect pests. J Pestic Sci 30:354–360
Torstenssen L, Stenstorm J (1986) Basic respiration rate as a tool for prediction of pesticide persistence in soil. Toxic Assess 1:57–72
Tu CM (1992) Effect of some herbicides on activities of microorganisms and enzymes in soil. J Environ Sci Heal B 27:695–709
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass. Soil Biol Biochem 19:703–707
Voos G, Groffman PM (1997) Relationship between microbial biomass and dissipation of 2, 4-D and dicamba in soil. Biol Fertil Soils 24:106–110
Walker A, Jurado-Exposito M, Bending GD, Smith VJR (2001) Spatial variability in the degradation rate of isoproturon in soil. Environ Pollut 111:407–415
Walkley, Black CA (1965) Organic carbon: methods of soil analysis (parts 1 and 2). Agronomy 9:1367–1378, American. Society of Agronomy, Inc., Madison, WI
Watters FL, White NDG, Cote D (1983) Effect of temperature on toxicity and persistence of three pyrethroids insecticides applied to fir plywood for the control of the red flour beetle (Coleopteran: Tenebrionidae). J Eco Entomol 76:11–16
Williams JR, Richardson CW, Griggs RH (1992) The weather factor: incorporating weather variance into computer simulation. Weed Technol 6:731–735
Xing B, Pignatello JJ (1997) Dual-mode sorption of low-polarity compounds in glassy poly (vinyl chloride) and soil organic matter. Environ Sci Technol 31:792–799
Zelles L, Scheunert I, Korte F (1985) Side effects of some pesticides on non-target soil microorganisms. J Environ Sci Heal B 20:457–488
Zibilski LM (1994) Carbon mineralization. In: Bingham JM, Mickelson SH (eds) ‘Methods of soil analysis’, Part 2. Microbiological and biochemical properties. SSSA, Book Series No. 5. ASA, SSSA, Madison.
Acknowledgments
The first author thanks Director, IARI, New Delhi, and Head, Division of Agricultural Chemicals, IARI, New Delhi, for providing support and facilities for the research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Mukherjee, I., Das, S.K. & Kumar, A. Degradation of flubendiamide as affected by elevated CO2, temperature, and carbon mineralization rate in soil. Environ Sci Pollut Res 23, 19931–19939 (2016). https://doi.org/10.1007/s11356-016-7145-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7145-8