Abstract
The use of chemical pesticides has increased environmental pollution and affected ichthyofauna as non target organisms. In the present study, the histopathological alterations in the larvae and fingerlings of the Caspian kutum, Rutilus frisii kutum, were used as a model to investigate the toxic effects of triazine herbicide, atrazine. To investigate toxic effects of atrazine, fish were exposed to sublethal concentration of ½ LC50 for 96 h. Histologically, the most significant alterations in kidney tissues were hyperplasia, necrosis, vacuolation, swelling, hypertrophy, aggregation of hyaline droplets, and disruption of the haematopitic tissue of the head kidney. The damage was more severe in larvae than the fingerlings. Results showed that alterations in kidney tissue caused by atrazine were not specific but it could be concluded that atrazine is excessively toxic for Caspian kutum even at sublethal concentration and acute exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Atrazine (2-chloro-4-(ethylamino)-6-(isopropylamino)-S-triazine) is a widely used herbicide both on agricultural crops and in urban settings. It reaches aquatic environments in run off from treated rice farms and agricultural land due to its high use and its relatively high mobility in soils, atrazine is frequently detected in surface and ground waters and affects the non-target aquatic organisms (Kiely et al. 2004). The effects of herbicides on fish as well as other non-target aquatic organisms are not as well documented. Atrazine belongs to the s-triazine family of herbicides, which are some of the most significant water pollutants in rain, fresh, marine and ground waters all over the world (Felding 1992; Tasli et al. 1996). Atrazine persists particularly in anaerobic or denitrified soils (Tasli et al. 1996) and in some aquatic systems at biologically effective levels for several weeks (Pratt et al. 1997). It has been shown that atrazine alters the hydromineral balance and gill function in crabs (Prasad et al. 1995) as well as alters the haematology (Prasad et al. 1991; Hussein et al. 1996), hormonal system (Spanò et al. 2004; Cericato et al. 2008), metabolism (McCarthy and Fuiman 2008) and growth (Fortin et al. 2008) of fish. Triazine-based herbicides (e.g. atrazine) are practically unaffected by microbial or hydrolytic degradation processes and the average half-life of atrazine in soil ranges from 13 to 261 days (Gamble et al. 1983; US EPA 2003). Thus any data concerning its toxic effects on aquatic organisms are very useful for assessing its potential hazard to aquatic systems. Early developmental stages are considered to be one of the most sensitive stages in the fish life cycle to the toxic effects of chemical contaminants (Weis and Weis 1987). Short-term sublethal effects on growth, behavior or osmotic control may affect the survival of these critical stages and impact recruitment (Houde 1987, 1989; Sclafani et al. 1997; Alvarez and Fuiman 2005). It has been previously demonstrated that the main osmoregulatory organs of most teleost fish are gill, kidney, digestive system and the integument (Varsamos et al. 2005) and any intervention with their physiological process could damages the fish osmoregulation and thus other surviving abilities. For example, loss of osmotic control altering water content may influence larval density and buoyancy. The vertical position of larvae in the water column affects their patterns of drift and their interactions with prey or predators. Thus, a temporary loss of osmotic control in fish larvae may increase their susceptibility to predation or impair their feeding ability (Sclafani et al. 1997). Caspian kutum, Rutilus frisii kutum (Kamensky 1901), is a native, and commercially important fish species of the Caspian Sea. This anadromous species spawns from March to April in rivers and lagoons, favoring aquatic weeds, gravel and sandy substrates (Heidari et al. 2009). The species has a great importance in commercial market of the region and it is proliferate annually by the Iran’s government for stock recruitment in the Caspian Sea ecosystem, due to its endangered statues caused by over fishing, urban and agricultural pollution, destroy of natural spawning rivers and etc. A detailed description of the toxic effects of various contaminants on different tissue of fish has been extensively studied. However, compared to the wealth of information regarding the toxic effects of various contaminants on different tissues, much fewer data are available on the toxic effects of atrazine on kidney. Due to the mentioned above, and the fact that the early life stages are the most sensitive stages in response to the environmental factors, it was felt necessary to investigate the kidney histopathology that may occur in R. frisii kutum following exposure to sublethal concentrations of atrazine at the larval and fingerling stages. Based on the lack of information on environmental realistic concentration of atrazine in the study area, the sublethal concentration of atrazine was chosen as the experimental concentration following the LC50 test for assessing the toxic effects of atrazine on the test species. The information obtained may be useful for management and monitoring of atrazine contamination in the environment.
Methods and Materials
Caspian Kutum, R. frisii kutum, larvae (newly hatched) and fingerlings (60 days average age) were obtained from Shahid Ansari Fish Proliferation and Culture Center, Rasht, Iran, in April–July 2011. Following determination of 96 h-LC50 of atrazine for the larvae and fingerlings separately (Khoshnood et al. 2014), two sublethal concentrations was determined as ½ LC50 as 9.25 and 12.47 ppm for larvae (~0.048 g/L biomass) and fingerlings (~0.43 g/L biomass), respectively. Fish were exposed to this sublethal concentration for 96 h in triplicate group of 30 fish each, in the laboratory conditions. One triplicate group of the larvae and fingerlings was held in clean water as the control group. No mortality was observed during the experiments in all experimental groups. The water parameters monitored daily through the experiment for all experimental groups using Eutech instruments, pcd650 and the values were as follow: temperature, 14.5 ± 0.5°C, pH 7.6 ± 0.1, dissolved oxygen 8.5 ± 0.5 mg/L. Water quality conditions (pH, temperature and O2) did not differ among treatments.
For histological studies, ten fish from each developmental stage were euthanized in 100 mg/L of MS222 and 100 mg/L of sodium bicarbonate and immediately immersed into Bouin’s fixative for 24 h, washed and dehydrated in an ascending series of ethanol for embedding in Paraffin (Merck). Following embedment in Paraffin, transversal and longitudinal sections of 6 µm were cut on a Leica RM2255 microtome and collected on glass slides and stained with Haematoxylin and Eosin (Khoshnood et al. 2010). Histopathological alterations detected in kidney of larvae and fingerling were recorded as present or absent and expressed as a percentage of fish affected (prevalence) per experimental group (ten fish each). Histopathological alterations quantified in kidney of larvae and fingerling were analyzed by means of ANOVA in SPSS (16.0) software.
Atrazine was analyzed in the following exposure solutions: atrazine nominal concentration of 0 ppm on day 0 for control group, two atrazine nominal concentrations (9.25 and 12.47 ppm) at the beginning (t-0) and end (t-24) of each 24-h exposure period in atrazine exposed groups of larvae and fingerling. These measurements were used to assess the potential contamination of the controls, variability associated with preparation of the solution and/or potential degradation of atrazine during the 24 h period of exposure. Chemical analyses at the end (t-24) of each 24-h exposure period of the 96-h assay were performed on pooled water samples of all three replicates of each experimental group. Various volumes of exposure solutions were collected, depending on the nominal atrazine concentration, and transferred into a clean glass bottle with 10 ng of the labeled surrogate compound 13C12 PCB-101 to assess the extraction efficiency. Extraction was performed three times with a volume of dichloromethane corresponding to approximately 25 % of the collected solution. Combined extracts were then reduced to about 50 µL and completed with 50 µL of tris (4-chlorophenyl) methane (TCPMe, 100 pg/µL) as internal standard. Analysis was conducted on a gas chromatograph (GC) equipped with a DB-5MS capillary column coupled to a Varian Saturn 2000 ion trap mass spectrometer (MS) by a transfer line kept at 300°C. The injector was operated in splitless mode. Helium was used as the carrier gas (flow rate, 1.0 mL/min). The ionization was performed by electron impact at 70 eV and the ion trap was operated in MS–MS mode. Atrazine concentrations were calculated on the basis of their response relative to the one of 13C12 PCB-101 in the same sample. Relative response factor was determined on the basis of a four point calibration curve for atrazine, while 13C12 PCB-101 and TCPMe were kept at constant concentration (100 pg/µL). Atrazine concentrations were corrected on the basis of the recovery of the surrogate compound. Limit of quantification was 0.003 ng/L for atrazine and analytical precision was 6 %.
Results and Discussion
Measured atrazine concentrations at t-0 (Table 1) were within 85 %–105 % of the nominal concentrations. Fish were exposed to nearly constant atrazine concentrations over the bioassay period. Variations of atrazine concentration within each 24-h period were of the same amplitude as the day to day variation at t-0. In one replicate of control group, atrazine was occasionally detected in trace amounts (<0.01 µg/L) which could have agricultural source, but based on the results of the effects of atrazine concentrations on kidney tissue, it has been concluded that this trace amounts could not interfere with the control results due to the significant difference with the sublethal concentrations.
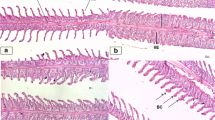
Kidneys were observed right behind the branchial chamber at the primary part of the trunk, and they divided into three distinct part: the head kidney, which contained pronephrus tubules and heamtopoitic tissue, the trunk kidney which composed of bowman’s capsule, glomerulus, proximal I and II, distal and collective tubule (Figs. 1, 2), and at the ending part, the tail kidney, which composed of two elongated ureters ended up to a short bladder (not shown).
Normal histological structure (a, e, j) and histopathological alterations (b–d, f–i, k–l) in kidney of R. frisii kutum larvae after 96 h of exposure to atrazine. Head kidney is composed of haematopoitic tissue and kidney tubules (a). After the Bowman’s capsule the tubular system of the nephron is composed of short neck tubule (a), proximal tubule (j) which has cuboidal epithelial cells with apical brush border, distal tubule (e) which its epithelial cells have no brush border, collective tubule (j). Bowman’s capsule is the first part of the nephron surrounding the capillary network named the glumerulus (a). Tissue alterations after exposure to atrazine were as follow: reduction of the glomerulus and increasing of the Bowman’s space (b); necrosis of the glomerulus (c); necrosis and disruption of the haematopitic tissue of the head kidney (d); swelling, hypertrophy, vacuolation and aggregation of hyaline droplets in renal tubular epithelial cells (f–h, k); hyperplasia of the renal tubular epithelial cells (g); necrosis of the renal tubular epithelial cells (h); decreasing in lumen space and complete congestion of lumen in renal tubules (i, k); hyperemia (l). BS Bowman’s space, G Glumerulus, HT haematopoitic tissue, N neck, DT distal tubule, PT proximal tubule, CT collective tubule, (→): Tissue alterations
Normal histological structure (a, e, h) and Histopathological alterations (b–d, f–g, i–j) in kidney of R. frisii kutum fingerling after 96 h of exposure to atrazine. Bowman’s capsule is the first part of the nephron surrounding the capillary network named the glumerulus (a), Head kidney is composed of haematopoitic tissue and kidney tubules (e), After the bowman’s capsule the tubular system of the nephron is composed of short neck tubule (a), proximal tubule (a, e) which has cuboidal epithelial cells with apical brush border, distal tubule (h) which its epithelial cells have no brush border, collective tubule (h). Tissue alterations after exposure to atrazine were as follow: hyperplasia of proximal tubule epithelial cells (c, d) and detachment of epithelial cells from the basement membrane (c, d), Congestion of tubular lumen (f, g, j); necrosis and disruption of haematopoitic tissue in head kidney (f, b); necrosis, swelling and disorientation of ureter epithelial cells (i). G glumerulus, HT haematopoitic tissue, N neck, DT distal tubule, PT proximal tubule, CT collective tubule, (→): Tissue alterations
After 96 h of exposure to sublethal concentration of atrazine, significant changes were observed in the kidney of larvae and fingerlings of the R. frisii kutum. These changes were including hyperplasia of the renal tubular epithelial cells (Figs. 1, 2) swelling, hypertrophy and aggregation of hyaline droplets in renal tubular epithelial cells (Figs. 1, 2) decreasing in lumen space and complete congestion of lumen in renal tubules due to severe hypertrophy of renal tubular epithelial cells (Figs. 1, 2), reduction of the glomerulus and increasing of the bowman’s space (Figs. 1, 2), necrosis of the glomerulus (Figs. 1, 2), necrosis of the renal tubular epithelial cells (Figs. 1, 2), hyperemia (Figs. 1, 2), necrosis and disruption of the haematopitic tissue of the head kidney (Figs. 1, 2), and finally vacuolation and massive hypertrophy of the renal tubular epithelial cells at the final hours of the exposure (Figs. 1, 2). In addition to observed alterations, disorientation of the ureter epithelial cells (Figs. 1, 2), hyperplasia of the proximal tubule epithelial cells and detaching of these cells from the basement membrane were excessive changes observed specially in kidneys of the fingerlings exposed to atrazine. In the present study, kidney of the fish often showed swelling in tubule cells. This alteration can be identified by the hypertrophy of the cells and the presence of small granules in the cytoplasm. This initial stage in the degeneration process can progress to hyaline degeneration, characterized by the presence of large eosinophilic granules inside the cells. These granules may be formed inside the cells or by the reabsorption of plasma proteins lost in the urine, indicating damage in the corpuscle (Hinton and Laurén 1990; Takashima and Hibya 1995). In more severe cases, the degenerative process can lead to tissue necrosis (Takashima and Hibya 1995). The presence of tubule degeneration, coupled with the necrosis in the kidney in the present study indicates that the kidney suffered damage after exposure to the atrazine, even at the short period of exposure. There are few reports on the effects of atrazine on kidney in fish, for example, exposure of Gobiocypris rarus to atrazine caused glomerulus shrinkage and expansion of Bowman’s space, necrosis of renal tubular epithelium and dilution of the tubules (Yang et al. 2010). In addition, Oulmi et al. (1995) showed that atrazine at concentration of 10–80 µg/L can cause necrosis in kidney proximal tubule of the rainbow trout, which lead to the renal loss of ions and proteins.
Results of the quantitative observation of the histopathological alterations in kidney tissue of larvae and fingerling showed that generally the alterations in larvae were more severe than the fingerling, in addition the most significant alterations in larvae were hyperplasia, decreasing of lumen space and vacuolation; and the most significant alterations in fingerling were hyperplasia and decreasing of lumen space (Fig. 3).
Prevalence (%) of kidney histopathological alterations in R. frisii kutum larvae and fingerling. Histopathological alterations of the kidney tissue in larvae (except for congestion of lumen) were more severe than fingerling. Alterations marked with (asterisk) were significantly different in two experimental groups (p < 0.05). Values are expressed as mean (±) SE. HP hyperplasia, HT hypertrophy, AHD aggregation of hyaline droplets, DLS decreasing of lumen space, CL congestion of lumen, RG reduction of the glomerulus, IBS increasing of the Bowman’s space, NG necrosis of the glomerulus, NREC necrosis of the renal tubular epithelial cells, H hyperemia, NHT necrosis of haematopitic tissue, V vacuolation, DBM detaching of cells from the basement membrane
The teleostean kidney is one of the first organs to be affected by contaminants in the water (Thophon et al. 2003). Most common alterations found in the kidney of fishes exposed to water contamination are tubular degeneration (swelling and hyaline droplets) and changes in the corpuscle, such as dilation of capillaries in the glomerulus and reduction of Bowman’s space (Takashima and Hibya 1995). Exposure to metals frequently causes alterations in the tubules and glomerulus, such as was described by Thophon et al. (2003) for the perch (Lates calcarifer) exposed to cadmium; Handy and Penrice (1993) found swollen Bowman´s capsule cells and melanomacrophages in the kidney of trout (Salmo trutta) and tilapia (Oreochromis mossambicus) exposed to mercuric chloride. Similar alterations were found in fishes exposed to organic contaminants (Veiga et al. 2002) and mixed environmental contaminants (Schwaiger et al. 1997; Pacheco and Santos 2002). These reports suggest that the histopathological changes in the kidney could not be considered specific to the stressors. Results of the present study showed that atrazine at sublethal concentration and short term (acute) exposure can cause severe damages in kidney tissue of the larvae and fingerlings of R. frisii kutum. It has been demonstrated that any damage to the renal tissue could lead to imbalances in ion and water regulation that could affect survival capabilities (Yang et al. 2010).
References
Alvarez MDC, Fuiman LA (2005) Environmental levels of atrazine and its degradation products impair survival skills and growth of red drum larvae. Aqua Toxicol 74:229–241
Cericato L, Neto JGM, Fagundes M, Kreutz LC, Quevedo RM, Finco J, da Rosa JGS, Koakoski G, Centenaro L, Pottker E, Anziliero D, Gil Barcellos LJ (2008) Cortisol response to acute stress in jundiá Rhamdia quelen acutely exposed to sub-lethal concentrations of agrichemicals. Com Biochem Physiol (C) 148:281–286
Felding G (1992) Leaching of atrazine into groundwater. Pestic Sci 35:39–41
Fortin MG, Couillard CM, Pellerin J, Lebeuf M (2008) Effects of salinity on sublethal toxicity of atrazine to mummichog (Fundulus heteroclitus) larvae. Mar Environ Res 65:158–170
Gamble DS, Khan SU, Tee QS (1983) Alrazine hydrolysis: part I. Proton catalysis at 25°C. Pestic Sci 14:537–545
Handy RD, Penrice WS (1993) The influence of high oral doses of mercuric chloride on organ toxicant concentrations and histopathology in rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol (C) 106:717–724
Heidari B, Shabanipour N, Savari A, Yavari V, Hosseini N (2009) The oocyte development of Kutum, Rutilus frisii kutum, K. with special emphasis on the zona radiata structure. Anim Reprod 6(3):465–472
Hinton DE, Laurén DJ (1990) Liver structural alterations accompanying chronic toxicity in fishes: potentioal biomarkers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, pp 51–65
Houde ED (1987) Fish early life dynamics and recruitment variability. Am Fish Soc Symp 2:17–29
Hussein SY, El-Nasser MA, Ahmed SM (1996) Comparative studies on the effects of herbicide atrazine on freshwater fish Oreochromis niloticus and Chrysichteres auratus at Assiut, Egypt. Bull Environ Contam Toxicol 57:503–510
Khoshnood Z, Mokhlesi A, Khoshnood R (2010) Bioaccumulation of some heavy metals and histopathological alterations in liver of Euryglossa orientalis and Psettodes erumei along North Coast of the Persian Gulf. Af J Biotech 9(41):6966–6972
Khoshnood Z, Jamili S, Khodabandeh S, Mashinchian Moradi A, Mottalebi A (2014) Histopathological effects and Toxicity of Atrazine Herbicide in Caspian Kutum, Rutilus frisii kutum, Fry. Ir J Fish Sci. In press
Kiely T, Donaldson D, Grube A (2004) Pesticide industry sales and usage, 2000 and 2001 Market Estimates. U.S. Environ. Prot. Agency, U.S. Dept. Agric. Publ. No. 733-R04-001
McCarthy ID, Fuiman LA (2008) Growth and protein metabolism in red drum (Sciaenops ocellatus) larvae exposed to environmental levels of atrazine and malathion. Aquat Toxicol 88:220–229
Oulmi Y, Negele RD, Braunbeck T (1995) Cyptopathology of liver and kidney in rainbow trout Oncorhynchus mykiss after long-term exposure to sublethal concentrations of linuron. Dis Aquat Org 21:35–52
Pacheco M, Santos MA (2002) Biotransformation, genotoxic and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol Environ Saf 53:331–347
Prasad TAV, Srinivas T, Rafi GM, Reddy DC (1991) Effect in vivo of atrazine on haematology and O2 consumption in fish Tilapia mossambica. Biochem Int 23:157–161
Prasad TAV, Srinivas T, Reddy JS, Reddy DC (1995) Atrazine toxicity on transport properties of hemocyanin in the crab (Ozioteiphusa senex). Ecotoxicol Environ Saf 30:124–126
Pratt JR, Melendez AE, Barreiro R, Bowers NJ (1997) Predicting the ecological effects of herbicides. Ecol Appl 7:1117–1124
Schwaiger JR, Wanke S, Adam M, Pawert W, Honnen A, Triebskorn R (1997) The use of histopatological indicators to evaluate contaminant-related stress in fish. J Aquat Eco Stres Rec 6:75–86
Sclafani M, Stirling G, Leggett WC (1997) Osmoregulation, nutritional effects and buoyancy of marine larval fish: a bioassay for assessing density changes during the earliest life-history stages. Mar Biol 129:1–9
Spanò L, Tyler CR, van Aerle R, Devos P, Mandiki SNM, Silvestre F, Thomé JP, Kestemont P (2004) Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus). Aquat Toxicol 66:369–379
Takashima F, Hibya T (1995) An atlas of fish histology: normal and pathological features, 2nd edn. Kodansha, Tokyo
Tasli S, Patty L, Boetti H, Ravanel P, Vachaud G, Scharif C et al (1996) Persistence and leaching of atrazine in corn culture in the experimental site of La Cote Saint Andre´ (Ise`re, France). Arch Environ Contam Toxicol 30:203–212
Thophon S, Kruatrachue M, Upathan ES, Pokethitiyook P, Sahaphong S, Jarikhuan S (2003) Histopathological alterations of white seabass, Lates calcarifer in acute and subchronic cadmium exposure. Environ Pol 121:307–320
United States Environmental Protection Agency (2003) Ambient aquatic lifewater quality criteria for atrazine. EPA-822-R-03-023, 178 pp. Revised Draft
Varsamos S, Nebel C, Charmantier G (2005) Ontogeny of osmoregulation in postembryonic fish: a review. Comp Biochem Physiol A 141:401–429
Veiga ML, Rodrigues EL, Pacheco FJ, Ranzani-Paiva MJT (2002) Histopathologic changes in the kidney tissue of Prochilodus lineatus, 1836 (Characiformes, Prochilodontidae) induced by sublethal concentration of Trichlorfon exposure. Braz Arch Biol Tech 45:171–175
Weis JS, Weis P (1987) Pollutants as developmental toxicants in aquatic organisms. Environ Health Perspect 71:77–85
Yang L, Zha J, Li W, Li Z, Wang Z (2010) Atrazine affects kidney and adrenal hormones (AHs) related genes expressions of rare minnow (Gobiocypris rarus). Aqua Toxicol 97:204–211
Conflict of interest
The author declares that there is no conflict of interest.
Ethical standards
The experiments of present study comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khoshnood, Z. Histopathological Alterations in the Kidney of Caspian Kutum, Rutilus frisii kutum, Larvae and Fingerlings Exposed to Sublethal Concentration of Atrazine. Bull Environ Contam Toxicol 94, 158–163 (2015). https://doi.org/10.1007/s00128-014-1431-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1431-2