Abstract
This study continued our previous work (Sai et al. in Bull Environ Contam Toxicol 95:157–163, 2015a) by analysing the effects of simazine on the liver histological structure and metamorphosis in the developing Xenopus laevis. Tadpoles (Nieuwkoop-Faber stage 46) were exposed to simazine at 0.1, 1.2, 11.0 and 100.9 μg/L for 100 days. When tadpoles were exposed to simazine at 11.0 and 100.9 µg/L, an increased mortality and damaged liver tissues were observed together with significant inhibition of percent of X. laevis completing metamorphosis on days 80 and 90 and prolonged time of completing metamorphosis. On the other hand, we found that simazine has no significant effects on liver weight and altered hepatosomatic index. Results of this study may be considered to inform risk assessment of the effects of simazine on the development of X. laevis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Simazine belongs to the group of environmental endocrine disrupting chemicals (EDCs) (US EPA 2009). Simazine is one of the most commonly detected pesticide in surface and ground waters in the US, Europe, and Australia and China, presumably due to relatively high persistence in soil and water (Velisek et al. 2012; Ma et al. 2006). To date, studies have shown that in vivo simazine produces adverse effects on the growth, development, immune and endocrine systems in a wide range of species (Kim et al. 2003; Ren et al. 2009; Turner 2003), in especial it was found to affect the reproductive system in mammals and amphibians species (Li et al. 2008; Zorrilla et al. 2010) which also have been shown in in our previous study (Sai et al. 2015a, b).
Amphibians are ideal model organisms for testing EDCs exposure and resultant effects due to its complex life cycle and permeable skin that can be exposed to environmental contaminants through multiple routes on land and in the water (Storrs and Kiesecker 2004). Particularly, Xenopus laevis is considered to be an appropriate species for the assessment of EDCs in phaseIItesting due to its ease of culture, prolific egg production with high fertilization and hatching rates and lifetime living in the water (Bantle et al. 1994).
For assessing the reproductive toxicology of simazine on X. laevis, our recently published study only demonstrated the effects of simazine on the gonadal systems in X. laevis (Sai et al. 2015a). In this paper, we continued to demonstrated another range of biological responses such as liver histological alternation and metamorphosis of X. laevis treated with simazine at various concentrations (0.1, 1.2, 11.0 and 100.9 μg/L).
Materials and Methods
Reagents, experimental animals, exposure conditions and actual concentrations were described in our previous work (Sai et al. 2015a).
Larvae were observed daily for monitoring their morphological changes and health status. Dead or moribund animals were removed and recorded.
After treatment, the frogs were euthanized by immersion in para aminobenzoic acid solution. Liver tissues were weighted and tissue collected. The hepatosomatic index (HSI) (Van der Oost et al. 2003) was calculated as HSI = (liver weight/body weight) × 100.
The liver tissues of ten males and ten females from each group were isolated and fixed in 10 % formalin solution and paraffin-embedded. The sections were cut at 5 µm serially and stained with hematoxylin and eosin (HE). The specimens were examined microscopically to assess the liver development.
One-way ANOVA was used to analyze the mortality of tadpoles, the body and liver weights, the days of the all animals completing metamorphosis. Independent-samples T test was used to analyze HSI of animals. The percentage of animals completing metamorphosis at different time in each group was calculated. These data were analyzed for evaluating simazine-related effects using the repeated-measures ANOVA. All data are presented as mean ± SD. All statistical analyses were performed using STATA 10.0 (http://www.stata.com/) software package. Group differences were evaluated by Fisher’s Least Significant Difference (LSD) test. Statistical probability of p < 0.05 was considered significant.
Results and Discussion
In our previous studies, simazine at high concentrations (11.0 and 100.9 μg/L) increased significantly the mortality of X. laevis compared to the control group. In that study, we had also shown that simazine did not produce obviously phenotype effects on X. laevis and liver weight and HSI did not show significant changes after treatments (p > 0.05, Table 1, Sai et al. 2015a). In this study, HSI and histopathological lesions were examined to evaluate the general status of the liver tissue. HSI has been used as a potential physiological biomarker for fish to reflect responses to environmental toxicant exposure through providing information on energy reserves and general health status of the organism (Van der Oost et al. 2003). However, the specificity of the biomarkers may depend on the dosage level of exposure or species. For example, another triazine herbicides atrazine was reported to cause significant increases of HSI in rare minnow as well as liver damage (Yang et al. 2010). However, we did not observe significant changes of HSI in frogs exposed to simazine. Thus our results suggest that HSI may not be a suitable biomarker for simazine exposure in amphibians.
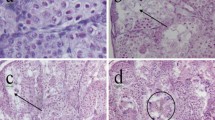
Livers of frogs from the control group showed regular array of hepatocytes and well-defined nuclei as shown in Fig. 1a, b. In the two simazine-treated groups (11.0 and 100.9 µg/L), all animals had irregular array, necrosis, atrophy and vacuolization of hepatocytes in both male and female X. laevis as shown (Fig. 1c, d). Particularly, more severe lesions were observed in livers of frogs from the 100.9 µg/L simazine treatment group.
Histological examinations in livers of X. laevis from simazine-treated and control groups. a Liver from a female control, HE 200×, b Liver from a male control, HE 200×, c Liver from a female X. laevis exposed to 100.9 μg/L simazine, HE 200×, d Liver from a male X. laevis exposed to 11.0 μg/L simazine, HE 200×
In all simazine-treated animals (11.0 and 100.9 µg/L), varying degrees of necrosis, atrophy and vacuolization of hepatocytes were observed in both male and female X. laevis (Fig. 1c, d) particularly, in livers of frogs exposed to simazine at 100.9 µg/L (Fig. 1c). A study using carp (Cyprinus carpio L.) (Stara et al. 2012), has shown that chronic exposure of simazine to carp (Cyprinus carpio L.) increased the production of reactive oxygen species (ROS) leading to oxidative damage to lipids, proteins and inhibition of antioxidant capacity, and that the activity of the antioxidant enzymes in the liver decreased after 60 days of exposure as compared with the control group.
The consequence of oxidative stress can lead to loss of lipids or induction of nuclear and cytoplasmic inclusions resulting in pathological lesions (Hinton and Lauren 1990) as observed in our present study. Moreover, there was no significant difference in liver damages between females and males which suggests that there was no sex difference in hepatotoxicity of X. laevis exposed to simazine. Therefore, liver lesions observed in the present study may have been due to simazine causing oxidative damage to lipids and proteins through increased ROS production and inhibiting antioxidant capacity.
Figure 2 showed the percentages of X. laevis from both control and simazine treatment groups completing metamorphosis on days 60, 70, 80 and 90, respectively. The percentages of tadpoles completing metamorphosis from 11.0 µg/L simazine group on days 80 and 90 were significant lower than the control group (8.1 % lower, p < 0.05 and 22.2 % lower, p < 0.01, respectively). Moreover, the percentages of tadpoles completing metamorphosis from the 100.9 µg/L simazine group were significant lower than the control group on days 80 and 90 (13.5 % lower and 21.8 % lower, p < 0.01, respectively). Figure 3 showed the days of all tadpoles completing metamorphosis. Higher concentrations of simazine caused significantly the delay of time completing metamorphosis compared to that of control group (prolonged 6.3 % and 8.7 %, p < 0.05). The metamorphosis of X. laevis depends on thyroxine (TH) including triiodothyronine and tetraiodothyronine. Being regulated by other hormone, TH is the necessary for the metamorphosis of amphibians. From NF stage 54, the secretion of TH increases gradually as the metamorphosis process until the tail of X. laevis is absorbed (Shi 2000). One study has shown that the inhibited metamorphosis of X. laevis by estradiol (Hogan et al. 2008). The reduced percentage of X. laevis completing metamorphosis at different time by simazine at high concentrations (11.0 and 100.9 µg/L) may be due to the affected of thyroid hormone and sonsequent disturbed metamorphosis of X. laevis, and the degree of interference aggravated gradually as the development of X. laevis.
In conclusion, we previously reported that simazine at high levels (11.0 and 100.9 µg/L) has significant effect on the mortality of X. laevis. Moreover, simazine was found to induce significantly abnormalities on metamorphosis which may be a consequence of inhibition in the metamorphosis of X. laevis through targeting the pituitary hormone secretion. In the present study our results have demonstrated that simazine can cause impairment on the histologic structure of the liver of X. laevis by inducing necrosis, atrophy and vacuolization of hepatocytes. Results of the present study, combined with our previous results, provide an important data set for assessing potential risks of simazine to amphibians.
References
Bantle JA, Burton TD, Dawson DA et al (1994) Fetax interlaboratory validation study: phaseIItesting. Environ Toxicol Chem 13:1629–1637
Hinton DE, Lauren DL (1990) Liver structural alterations accompanying chronic toxicity in fishes: potential biomarkers of exposure. In: McCarthy J, Shugart LR (eds) Biological markers of environmental contamination. Lewis Publishing Inc., Chelsea, pp 17–57
Hogan NS, Duarte P, Wade MG, Lean DRS, Trudeau VL (2008) Estrogenic exposure affects metamorphosis and alters sex ratios in the northern leopard frog (Rana pipiens): identifying critically vulnerable periods of development. Gen Comp Endocrinol 156:515–523
Kim KR, Son EW, Hee-Um S, Kim BO, Rhee DK, Pyo S (2003) Immune alterations in mice exposed to the herbicide simazine. J Toxicol Environ Health A 66:1159–1173
Li H, Qin Z, Qin X, Xia X, Xu X, Ma B (2008) Effects of the herbicide simazine on the survival and gonadal development of African clawed frogs (Xenopus laevis). Asian J Ecotoxicol 3:280–285 (in chinese)
Ma X, Gao N, Li Q, Xu B, Le L, Wu J (2006) Investigation of several endocrine disrupting chemicals in Huangpu River and water treatment units of a waterworks. China Water Wastewater 19:1–4 (in chinese)
Ren R, Wang M, Zheng J, Zhang Y (2009) Effects of herbicide simazine on the immune system of rat. Chin J Ind Hyg Occup Dis 27:601–603 (in chinese)
Sai L, Liu Y, Qu B, Yu G, Guo Q, Bo C, Xie L, Jia Q, Li Y, Li X, Ng JC, Peng C (2015a) The effects of simazine, a chlorotriazine herbicide, on the expression of genes in developing male Xenopus laevis. Bull Environ Contam Toxicol 95:157–163
Sai L, Wu Q, Qu B, Bo C, Yu G, Jia Q, Xie L, Li Y, Guo Q, Ng CJ, Peng C (2015b) Assessing atrazine-induced toxicities in Bufo bufo gargarizans Cantor. Bull Environ Contam Toxicol 94:152–157
Shi YB (2000) Amphibian metamorphosis: from morphology to molecular biology. Wiley-Liss, New York
Stara A, Machova J, Velisek J (2012) Effect of chronic exposure to simazine on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.). Environ Toxicol Pharmacol 33:334–343
Storrs S, Kiesecker J (2004) Survivorship patterns of larval amphibians exposed to low concentrations of atrazine. Environ Health Perspect 112:1054–1057
Turner L (2003) Simazine analysis of risks to endangered and threatened salmon and steelhead. Environmental Field Branch, Office of Pesticide Programs, p 31
US EPA (2009) EPA-HQ-OPP-2003-0367-0186
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Velisek J, Stara A, Machova J, Svobodova Z (2012) Effects of long-term exposure to simazine in real concentrations on common carp (Cyprinus carpio L.). Ecotoxicol Environ Saf 76:79–86
Yang L, Zha J, Zhang X, Li W, Li Z, Wang Z (2010) Alterations in mRNA expression of steroid receptors and heat shock proteins in the liver of rare minnow (Grobiocypris rarus) exposed to atrazine and p, p'-DDE. Aquat Toxicol 98:381–387
Zorrilla LM, Gibson EK, Stoker TE (2010) The effects of simazine, a chlorotriazine herbicide, on pubertal development in the female Wistar rat. Reprod Toxicol 29:393–400
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30901214; 81470145; 81573198), Natural Science Foundation of Shandong (2009ZRA01219; 2010GSF10213; ZR2015YL045), and Jinan Science and Technology Bureau (201010005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sai, L., Qu, B., Li, Y. et al. Continued Studies on the Effects of Simazine on the Liver Histological Structure and Metamorphosis in the Developing Xenopus laevis . Bull Environ Contam Toxicol 97, 517–520 (2016). https://doi.org/10.1007/s00128-016-1897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1897-1