Abstract
Atrazine (AZ), a widely used herbicide has drawn attentions for its potential impacts on amphibians. This study aims to investigate the toxicity of AZ in Bufo bufo gargarizans Cantor (B. bufo gargarizans), a species of toad commonly found in China and countries in East Asia. We treated tadpoles with 0.1, 1, 10 and 100 μg/L AZ for 85 days and examined related parameters. The results showed that the mortality of the toads in the treatment group increased dramatically in a U-shaped dose–response relationship. The hindlimb extension and metamorphosis rate of the toads were significantly inhibited by AZ at 10 and 100 μg/L. Under the same condition, there were significant progressive changes in the testicular structures. Moreover, we found that AZ has no significant effects on growth, sex ratios, gonadal morphology, forelimb emergence and histology in the ovaries. Our results support the idea that environmental contaminants including AZ may be relevant to global amphibian decline.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine, AZ) is one of the most widely used herbicides in China and other countries (Dong et al. 2006; Solomon et al. 1996). It is used to stop pre- and post-emergence of broadleaf and grassy weeds in major crops. Due to the widely application of AZ in agriculture, the environmental levels of AZ up to 108 μg/L have been reported in rivers of North America (US EPA 2002). In China, AZ concentrations in some areas exceed the standards for drinking water (2 μg/L) (GB 2006; Ren et al. 2002; Dong et al. 2006). AZ has been reported to cause endocrine disruption in fish, amphibians, reptiles, birds and mammals by affecting the normal reproductive functions and development in these organisms (Holloway et al. 2008; Gunderson et al. 2011; Kloas et al. 2009; de la Casa-Resino 2012). Due to their relatively ease of skin permeability and life cycles on land and in the water, amphibians are sensitive to environmental contaminants including AZ (Roy 2002). Recent studies showed that AZ has reproductive effects on amphibian species (Kloas et al. 2009; Langlois et al. 2010). Intriguingly, these studies have generated equivocal results. It has been reported that low concentration (0.1 μg/L) of AZ which is at environment levels (US EPA 2009), can affect metamorphosis in the tadpoles of two frog species (Langlois et al. 2010; Zaya et al. 2011). In contrast, other studies had shown that no effects were observed in tadpoles exposed to similar concentrations of AZ (Choung et al. 2011; Brodeur et al. 2013). These contradictories might be explained by differences in experimental design and/or their respective susceptibility of the testing species to AZ.
Bufo bufo gargarizans is a species of toads widely distributed in China, Russia and Korea. Unfortunately, the wide application of pesticides including AZ may cause contamination in water environment where larvae of B. bufo gargarizans live before completing their metamorphosis. It has been proposed that exposure to AZ may be one of the factors contributing to the declines of the population of amphibian through affecting their growth and metabolism, immune system regulation and blood cell processes (Langerveld et al. 2009). However, the effects of AZ on B. bufo gargarizans in China have not been studied. Therefore, our study aims to assess the effects of AZ on the growth, metamorphosis and gonadal condition in B. bufo gargarizans. To this end, we treated tadpoles with environmentally relevant concentrations of AZ and examined a series of parameters including the mortality, hindlimb extension and metamorphosis, forelimb emergence (FLE) and histologic changes in the reproductive organs. To our knowledge, this is the first study to investigate the effects of AZ in low and chronic exposure scenario on the indigenous species, B. bufo gargarizans.
Materials and Methods
AZ (98 % purity) and dimethyl sulfoxide (DMSO) were purchased from Sigma (Chemical Co. USA).
Three paired native sexually mature B. bufo gargarizans were collected (from Jinan Xiao Qing River, Shandong, China) into an aquarium on 27th February 2011 when it was in the midst of the breeding season. On the 29th February 2011, the spawns were collected and cultured in several tanks (dimensions: 25 × 20 × 20 cm). The offspring were kept in static dechlorinated tap water at pH 7.5 and 22 ± 2°C under natural light and fed on newly hatched brine shrimp spawns (Artemia nauplii) daily.
At stage 25–26 (Gosner 1960), tadpoles from the same pair of brood stock were treated with AZ at four concentrations (0.1, 1, 10 and 100 μg/L). Stock solutions of AZ were made with the reagent vehicle DMSO (0.05 %). Control groups were treated with the reagent vehicle DMSO (0.05 %) only. Each group had 200 tadpoles which were divided into eight replicate tanks (25 × 20 × 20 cm3) with 10 L of water each at 22 ± 2°C and pH 7.5. Animals were maintained on 12-h light/12-h dark cycles and treated for 85 days.
Test materials were applied in a static-renewal exposure regime. Test solutions were renewed by 50 % replacement every 48 h. At the first 9 days of exposure, water samples were taken immediately from each tank before and after exchange of the test solutions. Levels of AZ were measured using liquid chromatography–tandem mass spectrometry (LC–MS/MS) (Agilent 6410) as described by the method of Hua et al. (2006). The lower detection limit was 0.89 ng/L and the quantification limit was 2.67 ng/L (3 × DL). All concentrations are displayed as mean ± standard deviation (SD).
Larvae were observed daily for monitoring their morphological changes and health status. Dead or moribund animals were removed and recorded. Gosner stages of animals, the time and number of tadpoles reaching FLE and displaying hindlimb extension in each group were counted daily.
On day 23 (stage 31–33), tadpoles (n = 28) in each treatment group from eight replicate tanks were removed and processed for histopathological examination. For doing this, animals were euthanized by immersion in 3-aminobenzoic acid ethyl ester, rinsed in distilled water, and fixed in Bouin’s solution (71 % saturated picric acid, 24 % formaldehyde, 5 % glacial acetic acid) for routine paraffin embedding.
On day 49 (stages 39–42), tadpoles from each tank were rapidly transferred and weighed. The body and tail length were measured. Animals were returned to their home tank immediately after the measurements. Then, tadpoles (n = 28) from each group were dissected and the dorsal wall of the abdominal cavity with kidneys and gonads attached were collected and fixed in Bouin’s solution.
On day 85, all experimental animals were euthanized for body weight and length measurements, morphological assessment and tissue collection. The dorsal wall of the abdominal cavity with kidneys and gonads from males and females were collected and fixed in Bouin’s solution for histopathological examination.
Tissue samples fixed in Bouin’s solution were embedded in paraffin wax. The sections were cut in 5 μm and stained with hematoxylin and eosin (HE) for light-microscopic examination. Sex and gonadal morphology of B. bufo gargarizans from the treatments and control groups were determined by direct visual inspection on day 85. Sex ratios were expressed as percentage of male and female in each group. Intersex was referred to as gonadal abnormality.
Each tank was set as an experimental unit. Two-way ANOVA was used to analyze the mortality of tadpoles, one-way ANOVA was used to analyze the days when 70 % of the animals displaying hindlimb extension, the percentage of animals completing metamorphosis and the body sizes (body length, tail length) and the body weight on day 49 and 85. All data were expressed as mean ± SD. Sex ratios were analyzed using a 2-by-2 Contingency test. The percentage of B. bufo gargarizans reaching FLE at different time in each group was calculated. These data were analyzed for evaluating AZ-related effects using the repeated-measures ANOVA. All statistical analyses were performed on the STATA 10.0 (http://www.stata.com/) software package. Statistical probability of p < 0.05 was considered significant.
Results and Discussion
At the initiation of exposure (0 h), actual AZ concentrations in water samples treated with AZ (0.1, 1, 10, 100 μg/L) were 1.2 × 10−1 ± 0.4 × 10−1, 1.2 ± 0.2, 11.8 ± 1.6, and 104.6 ± 6.5 μg/L, respectively. At 48 h the AZ concentrations in the four treatment groups were 1.0 × 10−1 ± 0.3 × 10−1, 1.1 ± 0.3, 10.7 ± 0.9, and 99.5 ± 5.5 μg/L, respectively. Thus, the results showed that the measured concentrations varied generally less than 20 % from the nominal concentrations.
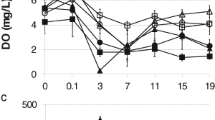
AZ has drawn more attentions for its potential environmental contamination and resultant risks to the ecological systems. As shown in Fig. 1, the mortality increased significantly in a U-shape dose–response manner when the tadpoles were exposed to AZ (p < 0.01). Especially, we found that AZ at concentration as low as 0.1 μg/L can induce a 20.1 % death of tadpoles with significant difference from that in 1 μg/L AZ treatment group (p < 0.01). However, study from Battaglin and Fairchild (2002) showed that AZ can cause mortality in chorus frogs (Pseudacris triseriata) at a higher dose (LC50 ≥ 410 μg/L). The various toxicities of AZ showed in different species of frogs suggest that a susceptibility to AZ may exist among species. Moreover, more studies are needed to elucidate the U-shaped dose–response relationship for AZ-induced the mortality in B. bufo gargarizans.
The effects of AZ on the body size and weight of tadpoles were studied. No significant difference in the size and weight of tadpoles was observed between the control and treatment groups on day 49 and day 85 respectively (p > 0.05). These results were consistent with another study of AZ at similar concentrations (Du Preez et al. 2008).
FLE and hindlimb development depend on thyroid-hormone (TH) in many anuran species (Marsh-Armstrong et al. 2004). Our results showed there was no significant effect of AZ on the percentage of B. bufo gargarizans reaching FLE on day 46, 48, 50 and 52 (p > 0.05). Recent studies showed that AZ has no effect on several frog species reaching FLE, which may suggest that AZ does not influence thyroid levels during development in anuran species (Hayes et al. 2002). In amphibians, before losing their tails at the peak of metamorphosis, tadpoles acquire a second spinal cord circuit to control leg flexion, extension and their mode of locomotion (Marsh-Armstrong et al. 2004). Therefore, we examined the development of hindlimb movement in B. bufo gargarizans and found that AZ at 10 and 100 μg/L showed to significantly delay the time (days) when 70 % of the animals displaying hindlimb extension (Fig. 2). At the same concentrations (10 and 100 μg/L), the percentage of animals completing metamorphosis was significantly reduced (p < 0.05) compared to that of the control group (Fig. 3). During the treatment period, when all the tadpoles in the control group just had completed metamorphosis, the gross abnormalities were still at stage 38–40 in the AZ treatments. The specific mechanism of how AZ affects the development and metamorphosis of B. bufo gargarizans remains to be explained by further investigations.
Histopathological changes were examined in the gonads of B. bufo gargarizans on day 23 when animals were in the early stage (stage 31–33). In the control animals, several primordial germ (PG) cells and a large amount of reproductive cells in the gonads were observed under the microscope, as shown in Fig. 4a1. Abnormal histological changes were not found in the gonads of animals exposed to low concentrations of AZ. Gonads from 10 to 100 μg/L AZ treatments showed much less PG and reproductive cells (Fig. 4a2). The histological development of gonads on day 49 (stage 39–42) was observed under light microscopy. Gonads from the control group showed normal development with a lot of reproductive cells as demonstrated in Fig. 4b1, b2. The gonad development was not affected by AZ at low concentrations (0.1 and 1 μg/L). However, we found only one gonad from several toads treated with 10 μg/L AZ (Fig. 4b3). AZ at 100 μg/L caused significant delay of the gonad development with reduced reproductive cells some of which showed necrosis characterized by dissolution of the nucleus and nuclear membrane (Fig. 4b4). Figure 4c1 showed a normal ovarian tissue from control in which a lot of vitellogenin oocytes (VO) and medullary cavity surrounded epithelial cells were observed. There was no significant abnormity in ovarian tissues of B. bufo gargarizans treated with AZ. The normal testis in control contained a few spermatocytes and many spermatoblasts at different stages as shown in Fig. 4c2. Obvious changes were found in testes from toads treated with 10 and 100 μg/L AZ including undeveloped testes without germ cells and disrupted histological architectures (Fig. 4c3), reduced testicular volume (Fig. 4c4), testicular oocytes (Fig. 4c5) and undeveloped lobules and rete testis (Fig. 4c6). Our results showed that AZ at 10 and 100 μg/L induced progressively structural changes in gonads of B. bufo gargarizans. Interestingly, we found the formation of testicular oocytes in the testes of toads on day 85 treated with 100 μg/L AZ. These findings suggested AZ can induce varying degrees of testicular degeneration and even feminization in B. bufo gargarizans. It has been reported that AZ at low concentration (0.1 μg/L) caused abnormalities in gonads of X. laevis during development (Hayes et al. 2003). However, we did not observe the same effects of AZ at such a low concentration. In addition, we did not find significant effect of AZ on ovary at all concentrations. This was in accordance with a previous study in which AZ was tested in American toads, gray treefrogs, southern leopard (Sara and Raymond 2008).
Microphotographs of gonad in B. bufo gargarizans from AZ-treated and control groups. AZ treated tadpoles were collected on day 23 (a1–2), 49 (b1–4), and 85 (c1–6). a1 Primordial germ (PG) cells (arrows) in the control group, HE ×400; a2 less PG cells (arrow) in the gonad from an AZ-treated toad (10 μg/L), HE ×400; b1 oocytes (arrow) in a normal developing ovary, HE ×400; b2 spermatocytes (arrow) in a normal developing testis, HE ×400; b3 only one gonad (arrow) from an AZ-treated toad (10 μg/L), HE ×50; b4 damaged germ cells (circle) in a gonad from an AZ-treated (100 μg/L) animal, HE ×400; c1 medullary cavity (arrows) in the normal ovarian tissues, HE ×50; c2 a normal testis, HE ×50; c3 undeveloped testis with empty spaces (arrows) from an AZ-treated (100 μg/L) male toad contained few germ cells, HE ×50; c4 delayed testis from an AZ-treated male toad (10 μg/L), HE ×50; c5 few spermatocytes and testicular oocytes (arrow) in a testis from an AZ-treated (100 μg/L) male toad, HE ×100; c6 undeveloped lobules and rete testis in a testis from an AZ-treated (100 μg/L) male toad, HE ×200
It has been suggested that AZ may act as an estrogen in vitro (Sanderson et al. 2000) through which AZ may exert its the potential impairment on the testicular development of B. bufo gargarizans as observed in this study. Hayes et al. (2010) found AZ can induce aromatase and increase circulating estrogens and cause the partially feminized amphibians. Therefore, the induction of aromatase and subsequent increases in estrogen synthesis represent another possible mechanism for the feminizing effect of AZ (Hayes et al. 2011). Anyway, our results implied that AZ may function as an estrogenic agent targeting the testis in B. bufo gargarizans.
In this study, we did not observe the effects of AZ on sex ratio of B. bufo gargarizans as shown in Fig. 5. Gonadal morphology abnormality was not found in any of the AZ-treated animals. This result was consistent with another study using X. laevis as a testing model (Kloas et al. 2009). We found that AZ induced histological abnormalities in the testis, but it did not cause complete feminization in male B. bufo gargarizans, which may be the reason that we did not find female-biased sex ratio and intersex gonads in B. bufo gargarizans treaded with AZ.
In conclusion, we found that AZ at environment-levels has significant effect on the mortality of B. bufo gargarizans in a U-shaped dose–response relationship. Moreover, AZ was found to induce a significant abnormality on metamorphosis and testicular histologic structure which may be a consequence of inhibition in the metamorphosis and testicular development of B. bufo gargarizans through targeting the central nervous system and pituitary hormone secretion. Its toxic mechanism remains to be further studied at the molecular level. The results of the present study, combined with data from frogs under field conditions, will provide an important basis for assessing the potential risk of AZ to B. bufo gargarizans and possibly other amphibians.
References
Battaglin W, Fairchild J (2002) Potential toxicity of pesticides measured in midwestern streams to aquatic organisms. Water Sci Technol 45:95–102
Brodeur JC, Sassone A, Hermida GN, Codugnello N (2013) Environmentally-relevant concentrations of atrazine induce non-monotonic acceleration of developmental rate and increased size at metamorphosis in Rhinella arenarum tadpoles. Ecotoxicol Environ Saf 92:10–17
Choung CB, Hyne RV, Mann RM, Stevens MM, Hose GC (2011) Developmental toxicity of two common corn pesticides to the endangered southern bell frog (Litoria raniformis). Environ Pollut 159:2648–2655
de la Casa-Resino I (2012) Endocrine disruption caused by oral administration of atrazine in European quail (Coturnix coturnix coturnix). Comp Biochem Physiol C Toxicol Pharmacol 156:159–165
Dong L, Chen L, Li Z, Gao H, Li J (2006) Quality assurance/quality control for monitoring and analysis of trace triazines in water. J Saf Environ 6:35–38 (in Chinese)
Du Preez LH, Kunene N, Everson GJ, Carr JA, Giesy JP, Gross TS, Hosmer AJ, Kendall RJ, Smith EE, Solomon KR, Van Der Kraak GJ (2008) Reproduction, larval growth, and reproductive development in African clawed frogs (Xenopus laevis) exposed to atrazine. Chemosphere 71:546–552
GB (2006) Standards of drinking water: GB 5749–2006. Beijing, China (in Chinese)
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Gunderson MP, Veldhoen N, Skirrow RC, Macnab MK, Ding W, van Aggelen G, Helbing CC (2011) Effect of low dose exposure to the herbicide atrazine and its metabolite on cytochrome P450 aromatase and steroidogenic factor-1 mRNA levels in the brain of premetamorphic bullfrog tadpoles (Rana catesbeiana). Aquat Toxicol 102:31–38
Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A (2002) Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci 99:5476–5480
Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A (2003) Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environ Health Perspect 111:568–575
Hayes TB, Khoury V, Narayan A, Nazir M, Park A, Brown T, Adame L, Chan E, Buchholz D, Stueve T, Gallipeau S (2010) Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc Natl Acad Sci 107:4612–4617
Hayes TB, Anderson LL, Beasley VR, de Solla SR, Iguchi T, Ingraham H, Kestemont P, Kniewald J, Kniewald Z, Langlois VS, Luque EH, McCoy KA, Muñoz-de-Toro M, Oka T, Oliveira CA, Orton F, Ruby S, Suzawa M, Tavera-Mendoza LE, Trudeau VL, Victor-Costa AB, Willingham E (2011) Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. Steroid Biochem Mol Biol 127:64–73
Holloway AC, Anger DA, Crankshaw DJ, Wu M, Foster G (2008) Atrazine induced changes in aromatase activity in estrogen sensitive target tissues. J Appl Toxicol 28:260–270
Hua W, Bennett ER, Metcalfe CD, Maio XS, Letcher RJ (2006) Seasonality effects on pharmaceuticals and s-triazine herbicides in wastewater effluent and surface water from the Canadian side of the upper Detroit River. Environ Toxicol Chem 25:2356–2365
Kloas W, Lutz I, Springer T, Krueger H, Wolf J, Holden L, Hosmer A (2009) Does atrazine influence larval development and sexual differentiation of Xenopus laevis? Toxicol Sci 107:376–384
Langerveld AJ, Celestine R, Zaya R, Mihalko D, Ide CF (2009) Chronic exposure to high levels of atrazine alters expression of genes that regulate immune and growth-related functions in developing Xenopus laevis tadpoles. Environ Res 109:379–389
Langlois VS, Carew AC, Pauli BD, Wade MG, Cooke GM, Trudeau VL (2010) Low levels of the herbicide atrazine alters sex ratios and reduces metamorphic success in Rana pipiens tadpoles raised in outdoor mesocosms. Environ Health Perspect 118:552–557
Marsh-Armstrong N, Cai L, Brown DD (2004) Thyroid hormone controls the development of connections between the spinal cord and limbs during Xenopus laevis metamorphosis. Proc Natl Acad Sci 101:165–170
Ren J, Jiang K, Zhou H (2002) The concentration and source of Atrazine residue in water of guanting reservoir. Environ Sci 23:126–128 (in Chinese)
Roy D (2002) Amphibians as environmental sentinels. J Biosci 27:187–188
Sanderson JT, Seinen W, Giesy JP, van den Berg M (2000) 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci 54:121–127
Sara IS, Raymond DS (2008) Variation in somatic and ovarian development: predicting susceptibility of amphibians to estrogenic contaminants. Gen Comp Endocrinol 156:524–530
Solomon KR, Baker DB, Richards P, Dixon KR, Klaine SJ, La Point TW, Kendall RJ, Giddings JM, Giesy JP, Hall JLW, Weisskopf CP, Williams W (1996) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15:31–76
US EPA (2009) EPA-HQ-OPP-2003-0367-0186
US EPA (2002) Reregistration eligibility science chapter for atrazine environmental fate and effects chapter. US EPA, Washington
Zaya RM, Amini Z, Whitaker AS, Kohler S, Ide CF (2011) Atrazine exposure affects growth, body condition and liver health in Xenopus laevis tadpoles. Aquat Toxicol 104:243–253
Acknowledgments
This work was supported by the National Science Foundation of China (30901214), Natural Science Foundation of Shandong (ZR2009CM114; 2010GSF10213), and Jinan Science and Technology bureau (201010005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sai, L., Wu, Q., Qu, B. et al. Assessing Atrazine-Induced Toxicities in Bufo bufo gargarizans Cantor. Bull Environ Contam Toxicol 94, 152–157 (2015). https://doi.org/10.1007/s00128-014-1441-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1441-0