Abstract
Simazine was investigated for gene expression concurrent with simazine-induced phenotype changes during development of male Xenopus laevis. X. laevis tadpoles (Nieuwkoop-Faber stage 46) were exposed to 0.1, 1.2, 11.0 and 100.9 μg/L simazine for 100 days. The results showed that an increased mortality of X. laevis, decreased gonad weight and altered gonadosomatic index of males significantly (p < 0.05) when exposed to simazine at 11.0 and 100.9 µg/L. Significant degeneration in testicular tissues was observed when tadpoles were exposed to simazine at 100.9 µg/L. To investigate the molecular mechanisms behind the testicular degeneration by simazine, we evaluated gene expression in animals treated with 100.9 µg/L simazine and found that 1,315 genes were significantly altered (454 upregulated, 861 downregulated). Genes involved in the cell cycle control, and amino acid metabolism pathways were significantly downregulated. These results indicate that simazine affects the related gene expressions which may be helpful for the understanding of the reason for the reproductive toxicity of simazine on male X. laevis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Simazine is a widely used chlorotriazine herbicide in agriculture and in recreational park and garden areas when it has been commercially available since 1955. The breakdown of simazine can be through chemical, photochemical or biological processes. Degradation studies have shown DT50 values (Time required for concentration to decline by 50 %) of simazine in both soil and water, vary between a few days and 150 days. Temperature and humidity can affect the half-life of simazine. Low temperatures and drought may prolong the dissipation time by a factor of two or more (Strandberg and Scott-Fordsmand 2002). In 2009, simazine was tested in an endocrine disruptor screening program (EDSP) by the US EPA due to its multiple exposure pathways and high production volume (US EPA 2009). In China, simazine is one of the most commonly detected pesticides in underground and surface waters (Ma et al. 2006).
Simazine belongs to the group of environmental EDCs which are defined as “exogenous agents that interferes with the synthesis, secretion, transport, binding, action, or elimination of natural hormones that are responsible for the maintenance of homeostasis, reproduction, development, and behavior” (US EPA 2009; Brevini et al. 2005). To date, studies have shown that in vitro simazine has a stimulatory effect on the antiandrogen screen (Orton et al. 2009) and in vivo simazine produces adverse effects on the growth, development, immune and endocrine systems in a wide range of species (Kim et al. 2003; Ren et al. 2009; Turner 2003). Simazine was also found to affect the reproductive system in mammals and amphibians species (Zorrilla et al. 2010; Li et al. 2008).
Amphibians have a complex life cycle and permeable skin and can be exposed to environmental contaminants through multiple routes on land and in the water, which make them to be ideal model organisms for testing EDC exposure and resultant effects (Storrs and Kiesecker 2004). Recently, few studies employed amphibians for investigating the toxicity of simazine but none of them elucidated their effects at the molecular level which is important for understanding the underlying mechanisms of simazine-induced toxicity. Therefore, we treated Xenopus laevis at various concentrations for a range of biological responses measurements (such as body and organ weights) and studied the gene expression changes in developing male X. laevis chronically treated with 100.9 µg/L simazine for 100 days using the Affymetrix microarray and Q-RT-PCR.
Materials and Methods
Simazine (purity of 97 %) and dimethyl sulfoxide (DMSO) were obtained from Sigma (Chemical Co., USA). Trizol was supplied by Invitrogen (Carlsbad, USA). Agilent® Xenopus 4 × 44 K Gene Expression Microarrays and Agilent® p/n 5190-0442 One-Color Quick Amp Labeling Kit were obtained from Agilent (Technologies Inc., USA).
Three pairs of adult male and female X. laevis were purchased from the Chinese Academy of Sciences (Beijing, China). The offsprings were produced by the natural mating activities of the paired adult X. laevis. UV-treated and carbon-filtered laboratory freshwater was used for the acclimatization of frogs in the laboratory and for all subsequent exposures. The animals were kept at an average water temperature of 22 ± 2°C at pH 7.5, under 12 h light and 12 h dark cycle. Tadpoles were fed fairy shrimp (Artemia nauplii) eggs in a young age daily and pork liver (Qin et al. 2010) three times per week ad libitum when the tadpoles completed metamorphosis.
At Nieuwkoop-Faber (NF) stage 46, tadpoles (n = 600) from the same pair of brood stock were randomly divided into five groups (Nieuwkoop and Faber 1994). Each group (n = 120) was divided into eight replicate tanks (25 × 20 × 20 cm3), each containing 10 L water. The tadpoles were exposed to simazine dissolved in solvent vehicle DMSO (0.01 %) at designed dosages of 0.1, 1, 10 and 100 µg/L (measured as 0.1, 1.2, 11.0 and 100.9 µg/L respectively) for 100 days. The control tadpoles were treated with 0.01 % DMSO only.
Test materials were applied in a static-renewal exposure regime. Test solutions were renewed by 50 % replacement every 48 h. During the first 9 days of exposure, 20 mL water samples were taken immediately from each tank before and after exchange of the test solutions. Levels of simazine were measured using liquid chromatography-tandem mass spectrometry (LC–MS/MS) (Agilent 6410) as described by Hua et al. (2006). The detection limit was 1.07 ng/L and the quantification limit was 3.57 ng/L. All concentrations are displayed as mean ± standard deviation (SD).
Larvae were observed daily for monitoring their morphological changes and health status. Dead or moribund animals were removed and recorded.
After treatment, the frogs were euthanized for whole-body, liver and gonad weight, body length morphological assessment and tissue collection. The gonadosomatic index (GSI) (Brewer et al. 2008) and the hepatosomatic index (HSI) (Van der Oost et al. 2003) were calculated as GSI = gonad weight/body weight × 102 and HSI = liver weight/body weight × 102 respectively.
The testicular tissues of ten males from each group were isolated and fixed in Bouin’s solution (71 % saturated picric acid, 24 % formaldehyde, 5 % glacial acetic acid) and paraffin-embedded. The sections were cut at 5 µm serially and stained with hematoxylin and eosin (HE). The specimens were examined microscopically to assess the gonad development.
Tissues from the remaining males were flash-frozen in liquid nitrogen, and stored at −80°C for microarray expression analysis. On the basis of the results of the toxic endpoints six male control frogs, six male 100.9 µg/L simazine-treated frogs were selected for gene expression analysis. Total RNAs from individual testis was isolated using Trizol according to manufacturer’s instruction and the concentration and purity was measured using a NanoDrop® ND-100 spectrophotometer (NanoDrop Technologies Inc., USA). The integrity of RNA was determined by denaturing gel electrophoresis. RNA samples were further purified, converted to double-stranded cDNA and labeled using Agilent® p/n 5190-0442 One-Color Quick Amp Labeling Kit for microarray analysis which was conducted according to Agilent® Xenopus 4 × 44 K Gene Expression Microarrays protocols. After hybridization and washing, the chips were scanned with the Axon GenePix 4000B microarray Scanner (Molecular Devices, LLC, USA). Original fluorescent data were read by GenePix pro V6.0 software (Molecular Devices, LLC, USA) and standardized by the Agilent GeneSpring GX v11.5.1 Software (Agilent, USA) for further analysis. Differentially expressed genes were identified by the value of fold-change (FC) (≥2) and t test was used in comparison with those in the control group.
To determine whether specific biological pathways were differentially affected by simazine, we analyzed our microarray dataset using the Kyoto encyclopedia of genes and genomes (KEGG) pathway database (http://www.genome.jp/kegg/pathway.html).
We further validated the data from microarray analysis using Q-RT-PCR for which we selected six genes including arginine decarboxylase (adc), glutathione transferase zeta1 (gstz1), acid phosphatase 6 (acp6), glutamate-ammonia ligase (glul-b), argininosuccinate lyase (asl), WEE1 homolog 2 (wee2) with the primers listed in Table 1. The genes were chosen based on their function roles and respective fold change values. For Q-RT-PCR analysis, total RNA was converted into cDNA using the SuperScript® cDNA Synthesis kit (Invitrogen, USA). Reactions were performed according to the manufacturer’s instructions. Relative mRNA expression was calculated according to the 2−ΔΔ Ct method. The samples of control group were set as calibrator. The upregulated genes were identified at 2−ΔΔ Ct > 1, the downregulated genes were identified at 2−ΔΔ Ct < 1.
One-way ANOVA was used to analyze the mortality of tadpoles, the body, organ weights and the body length. All date are presented as mean ± SD. Sex ratios were analyzed using a 2-by-2 Contingency test. All statistical analyses were performed on the STATA 10.0 (http://www.stata.com/) software package. Group differences were evaluated by Fisher’s least significant difference (LSD) test. Statistical probability of p < 0.05 was considered significant.
Results and Discussion
The exposure was performed according to our designed concentrations which are 0.1, 1, 10, 100 μg/L. After treatment, we measured simazine concentrations at 0 and 48 h. The average measured concentrations were 0.1 ± 0.03, 1.2 ± 0.3, 11.0 ± 1.5, 100.9 ± 6.1 μg/L.
Simazine is the second most commonly detected pesticide in surface and ground waters in the US, Europe, and Australia, presumably due to relatively high persistence in soil and water. Concentrations in both surface and ground water are variable, since they are influenced by season and the extent of simazine-based herbicide use in the location investigated (Velisek et al. 2012). In US simazine levels can reach values up to 1,300 μg/L in surface water, and 800 μg/L in ground water (US EPA 1988). According to the water purity standards of the World Health Organization, tap water that is contaminated with 2 μg/L of simazine is considered harmful (Velisek et al. 2012). This study aims to address whether some environmentally-relevant concentrations of simazine might affect the physiological parameters of X. laevis and how simazine induce the toxicity effect. So we designed the concentrations of simazine as 0.1, 1, 10 and 100 μg/L which can include many kinds of environmentally-relevant concentrations of simazine and ensure that produce positive results for the further study on the mechanisms of simazine effects.
As shown in Table 2, the mortality increased significantly in the 11.0 and 100.9 μg/L simazine treatment groups (p < 0.05). The results indicated simazine at high concentrations may increase the pressure to the survival of X. laevis.
In this study, we did not observe the effects of simazine on sex ratio of X. laevis (52/54, 50/51, 51/52, 49/48, 47/50; p > 0.05). Gonadal morphology abnormality was not found in any of the simazine-treated animals. This result was consistent with another study using X. laevis as a testing model (Li et al. 2008). We propose that simazine is a weak EDC which may be the reason that we did not find female-biased sex ratio and intersex gonads in X. laevis treaded with simazine (Hecker et al. 2006).
Physiological parameters such as the changes in body and organ weights are useful and important indicators of toxicity in animal studies (Andersen et al. 1999; Bailey et al. 2004). In this study, we examined a total of 504 frogs which were treated with simazine at 0, 0.1, 1.2, 11.0 and 100.9 μg/L (n = 106, 101, 103, 97 and 97, respectively), but we did not observed significant changes in the body weight and length of frogs after exposure to simazine (p > 0.05). This indicated that simazine does not produce obviously phenotype effects on X. laevis.
HSI have been used as physiological biomarkers to reflect responses to toxicant exposure and provide information on energy reserves and the general health of the organism (Van der Oost et al. 2003). In the present study, liver weight and HSI did not show significant changes after treatments (p > 0.05), which suggested that no obvious dysfunctions in the liver of X. laevis occurred following exposure to simazine at a dosing concentration of up to 100.9 μg/L.
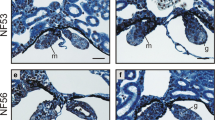
Interestingly, we found that the gonad weight and GSI were significantly reduced in male frogs exposed to simazine at 11.0 and 100.9 µg/L compared to the controls, but the gonad weight and GSI in females were not affected (Table 2). Meanwhile, histologic structure of ovaries was not changed, but the histologic structure of the testes was significantly changed in X. laevis treated with simazine over the entire dose range. Testes of frogs from the control group showed regular seminiferous lobules and spermatogenesis at all stages containing a few spermatogonias and spermatozoa as shown in Fig. 1a. However, irregular shape of seminiferous lobules, hypertrophic spermatogonias and large empty spaces (Fig. 1b, c) were observed in the frogs from the simazine treatment groups. Particularly, in testes of frogs from the 100.9 µg/L simazine treatment group, spermatogonias were hypertrophied and parts of the seminiferous lobules appeared pycnotic, a process involving necrosis in which the cell nuclei were characterized by condensation with hyperchromatic staining or pycnosis and sheet structure (Fig. 1d). This result is consistent with a previous study (Li et al. 2008). The results indicated that simazine has potential impairment on male reproduction of X. laevis since testis is the primary source of germ cells for the life of the organism (Saunders 1971).
Histological examinations in gonad of X. laevis from simazine-treated and control groups. a Regular seminiferous lobules and all stages of spermatogenesis are present in testis from a control frog, HE ×400; b The irregular shape of seminiferous lobules filled with hypertrophic spermatogonias (small arrow) and large empty spaces (arrow) are present in testis from a 0.1 μg/L simazine treated frog, HE ×200; c The irregular shape of seminiferous lobules filled with all stages of spermatogenesis and large empty spaces (arrow) are present in testis from a 1.2 μg/L simazine treated frog, HE ×100; d Parts of the seminiferous lobules involving necrosis (circle) are present in testis from a 100.9 μg/L simazine treated frog, HE ×100
We selected the 100.9 μg/L which was the typically effective concentration of male reproductive toxicity to the further study on the mechanisms of simazine effects. We have submitted the raw dataset of microarray into the NCBI Gene Expression Omnibus database (#17214940). We then analyzed gene expressions using microarray technology and found that the 1,315 genes were significantly altered (454 upregulated, 861 downregulated) in frogs exposed to 100.9 µg/L simazine. The results of genes expression were validated by Q-RT-PCR analysis which showed upregulation in gene expression for gstz1, glul-b, asl (2−ΔΔ Ct > 1) and downregulation in expression of adc, acp6, wee2 (2−ΔΔ Ct < 1). These results of Q-RT-PCR were consistent with the microarray data.
Many genes related to the reproductive system were found significantly downregulated at different levels which included acp2 (FC = −2.79), acp6 (FC = −13.45), mcm3 (FC = −8.14), wee1 (FC = −30.20), wee2 (FC = −45.22) and cdc20 (FC = −2.35). Studies have shown that acps are involved in immune, reproductive, digestive systems and apoptotic process (Bozzo et al. 2002; Yoneyama et al. 2004). Downregulated acps may play an important role in the simazine-induced adverse effects on the reproductive system observed in our study and other studies (Zorrilla et al. 2010) as well as other genes such as Rxfp1 (Park and Bae 2012). We also found genes involved in endocrine functions including ptgs2, stc1 and th were downregulated. PTGS2 is the key factor mediating the formation of prostaglandins which can also directly stimulate testicular Leydig cells to secrete testosterone (Thorn et al. 2011). Simazine (62.5 μM) was previously shown to elevate testosterone concentrations in vitro (Orton et al. 2009), however, we speculate that downregulation of ptgs2 may indirectly reduce the secretion of testosterone which has a negative impact on the reproductive system (Welsh et al. 2008). Stc1 is involved in steroidogenesis, and steroids have important physiological functions in the body (Luo et al. 2004). It may be speculated that simazine may disturb steroidogenesis by reducing the expression of related genes leading to further damage to a variety of body functions. Altered expression of these endocrine-related genes suggests simazine suppresses the endocrine system and related functions in endocrine. In a previous study (Rochira et al. 2001), estrogens have been found to inhibit the release of gonadotropin releasing hormone from hypothalamus resulting in reduced secretion of luteinizing hormone and follicle stimulating hormone from the pituitary in humans. However, we did not find significant changes of these genes (gnrh, lh, fsh) in X. laevis exposed to 100.9 µg/L simazine. It may be speculated that this mechanism works differently among species. Thus, this finding indicated that other potential mechanism(s) may exist in X. laevis to impair male reproductive system.
Based on KEGG analysis, we found five signaling pathways were obviously interrupted by simazine as shown in Table 3. These pathways include arginine/proline, alanine/aspartate/glutamate, riboflavin, tyrosine metabolism pathway and cell cycle pathway. The genes (oat, glul, adc) involved in arginine/proline metabolism were significantly downregulated by simazine. Mitochondrial enzyme ornithine aminotransferase participates in the synthesis of arginine (Kobayashi et al. 1995). Therefore, downregulation of oat may seriously interfere with the metabolism of arginine. Arginine decarboxylase can catalyze arginine into agmatine which is distributed in various organs and tissues and has important physiological functions including a role as a neurotransmitter, and as a regulator of cellular proliferation and inflammation (Zhu et al. 2004). Hence, downregulation of adc may block synthesis of agmatine leading to disrupted physiological functions. These results indicated that the inhibition of the arginine/proline pathway may interfere with the immune and endocrine function, etc. Genes (ccnb1, cdc20, mcm3, tfdp1-a, wee1-a, wee2, cdc6 and ywhaz-b) involving in cell cycle pathway was significantly affected by simazine. These genes are related with DNA replication and DNA damage control (Iizuka et al. 2006; Tominaga et al. 2006; Guida et al. 2005; Oehlmann et al. 2004). Therefore, downregulation of these cell cycle-related genes by simazine suggest it may affect cell replication and possible DNA damage repair which may contribute to the effects of simazine on the growth of X. laevis cells. In addition, alanine/aspartate/glutamate metabolism, riboflavin metabolism and tyrosine metabolism pathways were also found to be inhibited by simazine at 100.9 μg/L. We speculated that these pathways of metabolic regulation work together and plays an important role in the effect of simazine on male X. laevis.
In conclusion, our results demonstrated that simazine has potential impairment on male reproduction of X. laevis and caused irregular shape of seminiferous lobules, hypertrophic spermatogonias and large empty spaces in testes. By microarray analysis, expression of 1,315 genes (454 upregulated, 861 downregulated) was altered by at least twofold in the testes of X. laevis after simazine exposure. KEGG pathway analysis showed that these genes were enriched in the pathways of arginine/proline, alanine/aspartate/glutamate, riboflavin, tyrosine metabolism, cell cycle, etc. Among the altered genes, many related to the reproductive system and endocrine system, such as acp2, acp6, mcm3, wee2, ptgs2, stc1 and th were found significantly downregulated at different levels which may be helpful for the understanding of the reason for the reproductive toxicity of simazine on male X. laevis.
References
Andersen H, Larsen S, Spliid H, Christensen ND (1999) Multivariate statistical analysis of organ weights in toxicity studies. Toxicology 136:67–77
Bailey SA, Zidell RH, Perry RW (2004) Relationships between organ weight and body/brain weight in the rat: What is the best analytical endpoint? Toxicol Pathol 32:448–466
Bozzo GG, Raghothama KG, Plaxton WC (2002) Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate-starved tomato (Lycopersicon esculentum) cell cultures. Eur J Biochem 269:6278–6286
Brevini TA, Zanetto SB, Cillo F (2005) Effects of endocrine disruptors on developmental and reproductive functions. Curr Drug Targets Immune Endocr Metabol Disord 5:1–10
Brewer SK, Rabeni CF, Papoulias DM (2008) Comparing histology and gonadosomatic index for determining spawning condition of small-bodied riverine fishes. Ecol Fresh Fish 17:54–58
Guida T, Salvatore G, Faviana P, Giannini R, Garcia-Rostan G, Provitera L, Basolo F, Fusco A, Carlomagno F, Santoro M (2005) Mitogenic effects of the upregulation of minichromosome maintenance proteins in anaplastie thyroid carcinoma. J Clin Endoerinol Metab 90:4703–4709
Hecker M, Murphy MB, Coady KK, Villeneuve DL, Jones PD, Carr JA, Solomon KR, Smith EE, Van Der Kraak G, Gross T, Du Preez L, Kendall RJ, Giesy JP (2006) Terminology of gonadal anomalies in fish and amphibians resulting from chemical exposures. Rev Environ Contam Toxicol 187:103–131
Hua W, Bennett ER, Metcalfe CD, Maio XS, Letcher RJ (2006) Seasonality effects on pharmaceuticals and s-triazine herbicides in wastewater effluent and surface water from the Canadian side of the upper Detroit River. Environ Toxicol Chem 25:2356–2365
Iizuka M, Matsui T, Takisawa H, Smith MM (2006) Regulation of replication licensing by acetyltransferase Hbo1. Mol Cell Biol 26:1098–1108
Kim KR, Son EW, Hee-Um S, Kim BO, Rhee DK, Pyo S (2003) Immune alterations in mice exposed to the herbicide simazine. J Toxicol Environ Health A 66:1159–1173
Kobayashi T, Ogawa H, Kasahara M, Shiozaw Z, Matsuzawa T (1995) A single amino acid substitution within the mature sequence of ornithine aminotransferase obstructs mitochondrial entry of the precursor. Am J Hum Genet 57(2):284–291
Li H, Qin Z, Qin X, Xia X, Xu X, Ma B (2008) Effects of the herbicide simazine on the survival and gonadal development of African clawed frogs (Xenopus laevis). Asian J Ecotoxicol 3:280–285 (In Chinese)
Luo CW, Kawamura K, Klein C, Hsueh AJ (2004) Paracrine regulation of ovarian granulosa cell differentiation by stanniocalcin (STC) 1: mediation through specific STC1 receptors. Mol Endocrinol 18:2085–2096
Ma X, Gao N, Li Q, Xu B, Le L, Wu J (2006) Investigation of several endocrine disrupting chemicals in huangpu river and water treatment units of a waterworks. China Water Wastewater 19:1–4 (In Chinese)
Nieuwkoop PD, Faber J (1994) Normal table of Xenpous Laevis (Daudin). Garland Publishing Inc, New York
Oehlmann M, Score AJ, Blow JJ (2004) The role of Cdc6 in ensuring complete genome licensing and S phase checkpoint activation. Cell Biol 165:181–190
Orton F, Lutz I, Kloas W, Routledge EJ (2009) Endocrine disrupting effects of herbicides and pentachlorophenol: in vitro and in vivo evidence. Environ Sci Technol 43(6):2144–2150
Park HO, Bae J (2012) Disturbed relaxin signaling pathway and testicular dysfunction in mouse offspring upon maternal exposure to simazine. PLoS One 7(9):1–14
Qin X, Xia X, Yang Z, Yan S, Zhao Y, Wei R, Li Y, Tian M, Zhao X, Qin Z, Xu X (2010) Thyroid disruption by technical decabromodiphenyl ether (DE-83R) at low concentrations in Xenopus laevis. J Environ Sci 22(5):744–751
Ren R, Wang M, Zheng J, Zhang Y (2009) Effects of herbicide simazine on the immune system of rat. Chin J Ind Hyg Occup Dis 27:601–603 (In Chinese)
Rochira V, Balestrieri A, Madeo B, Baraldi E, Faustini-Fustini M, Granata AR, Carani C (2001) Congenital estrogen deficiency: in search of the estrogen role in human male reproduction. Mol Cell Endocrinol 178:107–115
Saunders JW Jr (1971) Patterns and principles of animal development. Collier-Macmillan, Toronto
Storrs S, Kiesecker J (2004) Survivorship patterns of larval amphibians exposed to low concentrations of atrazine. Environ Health Perspect 112:1054–1057
Strandberg MT, Scott-Fordsmand JJ (2002) Field effects of simazine at lower trophic levels-a review. Sci Total Environ 296:117–137
Thorn CF, Grosser T, Klein TE, Altman RB (2011) PharmGKB summary: very important pharmacogene information for PTGS2. Pharmacogenet Genom 21:607–613
Tominaga Y, Li C, Wang RH, Deng CX (2006) Murine wee1 plays a critical role in cell cycle regulation and preimplantation stages of embryonic development. Int Biol Sci 2:161–170
Turner L (2003) Simazine analysis of risks to endangered and threatened salmon and steelhead. Environmental Field Branch, Office of Pesticide Programs, p 31
US EPA (1988) Simazine: health advisory. Office of Drinking Water
US EPA (2009) EPA-HQ-OPP-2003-0367-0186
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Velisek J, Stara A, Machova J, Svobodova Z (2012) Effects of long-term exposure to simazine in real concentrations on common carp (Cyprinus carpio L.). Ecotoxicol Environ Saf 76(2):79–86
Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM (2008) Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118(4):1479–1490
Yoneyama T, Shiozawa M, Nakamura M, Suzuki T, Sagane Y, Katoh Y, Watanabe T, Ohyama T (2004) Characterization of a novel acid phosphatase from embryonic axes of kidney bean exhibiting vanadate-dependent chloroperoxidase activity. J Biol Chem 279:37477–37484
Zhu MY, Iyo A, Piletz JE, Regunathan S (2004) Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochim Biophys Acta 2:156–164
Zorrilla LM, Gibson EK, Stoker TE (2010) The effects of simazine, a chlorotriazine herbicide, on pubertal development in the female Wistar rat. Reprod Toxicol 29:393–400
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30901214), Natural Science Foundation of Shandong (2009ZRA01219; 2010GSF10213; 2013GNC11032), and Jinan Science and Technology Bureau (201010005). Also, we thank KangChen Bio-tech Inc. (Shanghai, China) for their excellent microarray services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sai, L., Liu, Y., Qu, B. et al. The Effects of Simazine, a Chlorotriazine Herbicide, on the Expression of Genes in Developing Male Xenopus laevis . Bull Environ Contam Toxicol 95, 157–163 (2015). https://doi.org/10.1007/s00128-015-1483-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1483-y