Abstract
Long-term, sublethal methylmercury exposure can cause reproductive depression, immune suppression, endocrine disruption and other problems in birds. We used two biomarkers to detect oxidative stress in livers of zebra finches (Taeniopygia guttata) developmentally exposed to sublethal levels of dietary methylmercury (0.0, 0.3, 0.6, 1.2, or 2.4 μg/g wet weight in diet). Our findings indicate that young adult finches exposed to environmentally relevant concentrations of mercury in ovo and through their diets, exhibited oxidative stress in their livers. We measured the ratio of the antioxidant glutathione in its reduced form (GSH) versus its oxidized form (GSSG) and the activity of the superoxide dismutase (SOD) enzyme suite. Blood total mercury served as a proxy for liver mercury concentration, and was on average 8.4 times the dietary dose (e.g., birds consuming 0.6 μg/g had blood mercury levels of ~5 μg/g on a wet weight basis). Consistent with what is known from large, aquatic bird species, there was a significant, negative relationship between GSH/GSSG ratios and tissue mercury concentrations, which is indicative of oxidative stress. This relationship was driven by a significant increase in the oxidized glutathione in the livers of birds with higher blood mercury levels. SOD activity was also found to have a significant, negative relationship with blood mercury.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lethality of mercury (hereafter Hg) in high doses to wildlife has been well established (Eisler 2006). Due to its persistence and potential for biomagnification, sites contaminated with Hg can remain a threat to humans and wildlife for centuries (Henny et al. 2002). Contamination may spread beyond initially impacted rivers or lakes into broad floodplains and throughout terrestrial food webs (Cristol et al. 2008; Singer et al. 2013). Atmospheric Hg can also bioaccumulate in wildlife of upland regions (Townsend et al. 2013; Keller et al. 2014). Thus, it is important to know the full extent of the impact of Hg and to develop effective bioassays to detect its physiological effects on aquatic and terrestrial wildlife.
Birds are often exposed to Hg according to their trophic level and metabolism, so they have frequently been used as bioindicators (Frederick et al. 2002). Much of the research on Hg and birds has been conducted on fish-eating birds due to historical contamination of aquatic sites with Hg. Recent work has shown that terrestrial songbirds accumulated harmful doses of Hg from invertebrate food webs in a riparian habitat adjacent to a polluted river (Cristol et al. 2008). There have been negative effects reported in songbird reproduction, immune and endocrine systems, and behavior (Hallinger et al. 2010; Hawley et al. 2009; Wada et al. 2009). Similar effects were also reported in Hg-dosed songbirds (Lewis et al. 2013; Moore et al. 2014; Varian-Ramos et al. 2014). In an effort to find the physiological basis for the various effects of Hg in songbirds, we examined two different oxidative stress biomarkers in zebra finches (Taeniopygia guttata) exposed to a range of dietary Hg concentrations: the hepatic glutathione antioxidant system, and the activity of the superoxide dismutase (SOD) enzyme suite.
The liver and its ability to handle oxidative stress were the focus of this study. Oxidative stress refers here to the inability of the liver to process and dispose of free radicals or reactive oxygen species (ROS) in tissues. ROS can be beneficial to cells in small concentrations, but when they exist in excess they can damage cell contents such as lipids and DNA (Valko et al. 2007). ROS are the byproducts of normal cellular activities, and antioxidant systems exist in the body to dispose of them and prevent them from harming tissues. When a redox-inactive metal such as mercury or lead enters cells, it can disrupt the body’s normal antioxidants, leading to the accumulation of excess ROS that could then overwhelm the remaining antioxidant enzymes (Ercal et al. 2001).
The liver is particularly important to the study of antioxidant systems because it processes toxins for excretion as they are carried from the gastrointestinal tract to the liver via the hepatic portal system. Hg and other heavy metals such as lead and cadmium accumulate in high concentrations in the livers of exposed organisms. Hg may contribute to oxidative stress by binding directly to the antioxidant enzyme itself and rendering it dysfunctional, or by stimulating the overproduction of reactive byproducts from cellular reactions (Ercal et al. 2001).
Glutathione is a commonly used biomarker for oxidative stress because it is a thiol compound that facilitates the scavenging of ROS and the reduction of peroxides into water, and it is the most abundant non-protein antioxidant in animal tissues (Townsend et al. 2003). One can measure either total glutathione itself, or the activity of enzymes that act on it such as glutathione reductase (GR), the enzyme responsible for converting the oxidized form of glutathione (GSSG) back to the reduced form (GSH) following its reduction of free radicals. Typically, the levels of GSH are much higher in the body of a healthy individual as opposed to levels of GSSG, because GR constantly converts GSSG back to GSH (Townsend et al. 2003). We measured the ratio of GSH/GSSG in the livers of songbirds exposed to Hg, thereby directly measuring oxidized and reduced glutathione and indirectly measuring the enzyme activity. If oxidative stress is occurring, GSSG concentrations will increase in cells as more glutathione is utilized and GR reaches maximum capacity to convert GSSG to GSH (Townsend et al. 2003). Additionally, GR may be dysfunctional due to direct binding with Hg, allowing relative GSSG concentrations to rise (Ercal et al. 2001). We hypothesized that Hg causes oxidative stress in the liver and predicted that GSSG levels would increase with increasing blood Hg and GSH levels would decrease. Therefore, a decreased GSH/GSSG ratio overall would indicate increased oxidative stress (Townsend et al. 2003).
SODs are a group of antioxidant metalloenzymes that transform the superoxide radical into hydrogen peroxide and oxygen, which the body can then dispose of (McCord and Fridovich 1969). This prevents the free radicals or ROS from causing tissue damage over extended periods of time. Because the superoxide radical is a byproduct of normal cellular activities, and the SODs are present throughout the body in order to dispose of it, they are good indicators of overall oxidative stress (Gutteridge 1995). Changes to the enzyme activity of SOD indicate a departure from healthy antioxidant defenses of the body (McCord et al. 1971). We hypothesized that SOD activity would be reduced by excess substrate in Hg dosed birds, or rendered dysfunctional due to Hg interacting with its metal cofactors or some other feature of its molecular structural activity, and thus predicted that SOD activity would be negatively related to tissue mercury concentration.
Materials and methods
Animals and tissue sample collection
We collected livers from young, captive-bred zebra finches that had recently become independent of their parents (45–60 days after hatching; 47 females for glutathione analysis, 61 males and 61 females for SOD analysis). These finches were the offspring of parents dosed with Hg throughout their adult lives as part of another study on the reproductive effects of dietary Hg (see husbandry and dosing methods in Varian-Ramos et al. 2014). Thus, subjects had been exposed in ovo and throughout the nestling and fledgling periods to one of the following dietary concentrations of methylmercury cysteine: 0.0, 0.3, 0.6, 1.2, or 2.4 μg/g wet weight food (hereafter “ppm”). Parents and offspring within each treatment group were given the same dietary dose. In ovo exposure was estimated based on the mean and standard deviation of dry weight total mercury concentration of the first egg (freeze-dried minus shell) in the previous clutch by this set of parents (8.38 ± 1.87, 15.50 ± 3.49, 29.89 ± 7.08, 55.86 ± 16.47 ppm, respectively; unpublished data). These concentrations span and exceed the range of concentrations reported to affect hatching success in dosed mallards, which are a species relatively insensitive to dietary or embryonic exposure to mercury (Heinz et al. 2010). Finches were euthanized by rapid decapitation. A small sample of whole blood (50 μL) was collected in a heparinized capillary tube at the time of sacrifice to determine blood total Hg concentration at time of death. The liver was dissected from the carcass within 3 min of death, divided in half, and placed in two 1.8 mL centrifuge tubes to be immediately snap frozen in liquid nitrogen. After collection, liver tissues were stored at −80 °C until use.
Tissue sample preparation
Glutathione
Liver tissues were removed from −80 °C and a portion of liver weighing approximately 100 mg (103 ± 10 mg, range 80–139 mg) was rapidly weighed at room temperature. All differences in liver mass were accounted for in final data calculations. The weighed sample was placed into a 1.8 mL homogenizing centrifuge tube containing 300 mL of ice cold 5 % metaphosphoric acid and homogenized on ice using a pestle within the tube until no solid tissue particles were visible.
We used the Oxford Biomedical Microplate Assay for GSH/GSSG and followed the manufacturer’s protocol with minor adjustments as noted below. The homogenized mixture was split into two aliquots: 100 μL of the homogenized mixture was placed in a clean microcentrifuge tube to become the GSH sample, and 200 μL was placed in a clean tube to become the GSSG sample. 60 μL of kit scavenger solution was added to the GSSG sample. Both the GSH and GSSG samples were then mixed for approximately 20 s at room temperature, and then placed on ice. After all samples had been homogenized, divided into aliquots, and mixed, they were centrifuged at 4,000 rpm (1310×g) at 4 °C for 10 min.
After centrifuging, 50 µL of each GSSG sample supernatant was collected and placed in clean centrifuge tubes containing 700 μL of kit assay buffer. 12 μL of each GSH sample was collected and placed in new tubes containing 1.5 mL of assay buffer. These samples were placed on ice until the microplate was prepared.
SOD assay
A solution of 20 mM HEPES buffer was prepared using 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose. The final pH of the solution was approximately 7.2. The same solution was used to prepare all samples. Liver tissues were removed from −80 °C and a sample weighing approximately 50 mg (52 ± 5 mg, range 43–63 mg) was removed and washed with cold, 1× PBS buffer. The sample was then homogenized in 250 µL of cold 20 mM HEPES buffer and placed on ice. When all samples were properly homogenized, they were centrifuged at 3,000×g for 5 min at 4 °C. 25 μL of the supernatant were then added to 100 μL of the HEPES buffer. The dilute supernatant was then separated into two aliquots in clean tubes, each containing approximately 63 μL of liquid, and placed on ice until the microplate was prepared.
Microplate assay preparation and protocol
Glutathione assay
The microplate assay was performed as described in the assay procedure for the Oxford Biomedical Microplate Assay (Ann Arbor, MI) for GSH/GSSG with the following additional specifications. Samples were run in duplicate and assayed in groups of 15–20 individuals at a time. A standard curve was created for each assay using the 10 μM GSSG standard working solution in the specified concentrations. After loading, the plate was inserted into the Multiskan FC microplate reader (Thermo Fisher Scientific Inc.) for a 5 min incubation using the shake function set at 30 s intervals. Following the addition of the final reagent, the plate was immediately inserted into the plate reader set to the kinetic function to record the absorption at 450 nm in each well at 1-min intervals for 10 min. The t = 0 min recording was taken immediately after placing the plate into the reader.
SOD
The microplate assay was performed as specified by the SOD Assay Kit from Cayman Chemical Company (Ann Arbor, MI), with the following modifications. The samples were run in duplicate and assayed in groups of 20–40 individuals per microplate. A standard curve was created for each microplate used in the assay according to the specifications of the kit. The plate was allowed to incubate at room temperature for 20 min, before being immediately inserted into the Multiskan FC microplate reader and read one time at 450 nm.
Estimation of concentrations
Glutathione
The microplate reader kinetic calculation function was used to calculate the average rate for each sample as follows: average rate = (slope) (GSH concentration) + intercept.
To obtain the GSH and GSSH concentration, the average rate for each duplicate sample was multiplied by the sample dilution factor (GSH: 488, GSSG: 30). The ratio of GSH/GSSG was calculated as: GSH/GSSG ratio = (GSHt − 2GSSG)/GSSG where GSHt equals total glutathione, and GSSG equals GSSG concentration for that sample. The calculated values of GSH and GSSG were normalized to represent the concentration in μM enzyme per 100 mg of tissue.
SOD assay
The average absorption value of the method blank wells was subtracted from all standard and unknown values. The absorption values for each duplicate sample and each duplicate standard were averaged and used in subsequent calculations. To find the level of SOD activity, the linearized rate (LR) was calculated by dividing each absorption value for each sample and standard into the value of Kit Standard A (equal to zero units/mL). After each LR was determined, the values for each of the standards were then graphed against the seven known SOD activity levels (units of activity/mL) for the kit standards: 0, 0.025, 0.05, 0.1, 0.15, 0.2, and 0.25 U/mL. The slope and Y-intercept of this standard curve was used to calculate the activity of the SOD in each sample as follows:
SOD units/mL = [((sample LR − yintercept)/slope)) × 0.23 mL/0.01 mL] × dilution.
All reported sample values were normalized to Units per 50 mg tissue.
Assay quality control
To assess the variability in the glutathione assay, we ran duplicates for all samples and found relative percent difference for GSSG of 20.2 ± 25.2 % (n = 47 pairs of duplicates), and for GSH of 18.4 ± 17.9 % (n = 47 pairs of duplicates). To evaluate consistency across the three runs of the glutathione assay, we selected two intermediate concentrations of the standard stock solution that were used across all three runs and calculated the relative percent difference across the runs (GSSG: 8.0 ± 4.7 %, n = 6 sets of standards; GSH: 3.9 ± 3.7 %, n = 6 sets of standards).
To assess the variability in the SOD assay, we ran duplicates for all samples and found relative percent difference of 2.5 ± 2.3 % (n = 112 pairs of duplicates). To evaluate consistency across the three runs of the SOD assay, we selected two intermediate concentrations of the standard stock solution that were used across all three runs and calculated the relative percent difference (21.1 ± 14.6 %, n = 6 sets of standards).
Hg analysis
Hg concentrations in artificial diet, blood and liver were analyzed using a direct mercury analyzer (Milestone DMA 80, Shelton, CT), which measures total mercury content. Both dosed food and bird tissues were assayed on a wet weight basis, livers were freeze dried and reported on a dry weight basis. All samples were analyzed using the quality control procedures standardized in our lab (Varian-Ramos et al. 2011). Briefly, standard reference samples (DORM-3, DORM-4, DOLT-3, DOLT-4, National Research Institute, Canada) and machine and sample blanks were run every 20 samples. The mercury analyzer was recalibrated every 2 months or as necessary. The calculated minimum detection limit (based on seven spiked chicken egg samples near estimated limit) was at or below 0.009 ppm during the assay period. Standard recovery samples and spiked samples were run throughout the study to verify repeatability (DOLT-3: n = 8, 98.4 ± 0.03 %; DOLT-4: n = 38, 97.3 ± 1.8; DORM-3: n = 24, 92.2 ± 4.8; DORM-4: n = 24, 95.6 ± 3.0 %; spiked blood: n = 14, 97 ± 2.5 %; spiked liver: n = 4, 102 ± 2.2 %). The mean relative percent difference between pairs of tissue samples taken from the same bird and run as duplicates was 5.5 ± 6.8 % (n = 9 liver and 6 blood duplicates).
Statistical analyses
Because insufficient liver tissue was available to determine Hg levels for most birds, we used blood Hg level as a proxy for liver Hg in testing for relationships between glutathione/SOD and Hg level. To test whether blood and liver Hg within the same individuals were correlated, we used a subset of birds for which we had both liver Hg and blood Hg values, and tested the relationship for statistical significance using ANOVA and linear regression.
We used a linear mixed model with a scaled identity matrix and assay run as a random effect to test for a relationship between blood Hg concentration and GSH concentration, GSSG concentration, GSH/GSSG ratio and SOD concentration. Birds were on one of five doses of dietary Hg, and individual Hg concentrations of different treatment groups barely overlapped, but there was great individual variation within treatment groups. Therefore, individual blood Hg concentration was used as a fixed effect in all analyses as opposed to analyzing by dietary doses.
Results
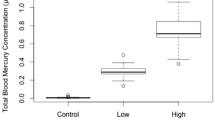
The concentration of Hg in liver and blood of the same individuals were significantly, positively related (P < 0.001; r2 = 0.92; y = 2.978x + 3.185; Fig. 1) indicating that blood total Hg was a valid proxy for relative liver total Hg. The ratio of the two forms (GSH/GSSG) had a significantly negative relationship with blood Hg (t42.2 = −2.107, P = 0.041; Fig. 2a), indicating that birds with more blood Hg had more of their glutathione in the form of GSSG than birds with less exposure. There was no relationship evident between blood Hg and the liver concentration of the reduced form of glutathione (GSH: t44.0 = −0.325, P = 0.747; Fig. 2b). Birds with higher blood Hg concentrations had higher concentrations of oxidized glutathione (GSSG) in their livers than birds with lower blood Hg (t42.0 = 3.39, P = 0.002; Fig. 2c). The concentration of SOD was also significantly negatively related to the concentration of blood Hg (t73.0 = −2.214, P = 0.030; Fig. 3). Unlike glutathione, for SOD we used male and female subjects, but there was no sex difference (means and standard deviations equal to two decimal places, t110 = 0.28, P = 0.39).
a The ratio of liver GSH/GSSG decreased significantly with increasing blood total Hg in dosed individuals, consistent with oxidative stress in the liver. b Liver GSH concentration (μM/100 mg) was not related to blood total Hg concentration. c Liver GSSG concentration (μM/100 mg) significantly increased with blood total Hg concentration in dosed individuals, suggesting that GSSG was not being properly converted back to GSH
In terms of dietary dose, rather than individual mercury level, blood mercury increased in step with diet, approximately doubling with each doubling of dose (Table 1). The effects of dietary dose on GSH/GSSG ratio and SOD, however, were apparent only for the highest doses (1.2 and 2.4 μg/g; Table 1).
Discussion
We measured the ratio of the oxidized form of glutathione (GSSG) to the reduced form (GSH) in the livers of Hg-dosed zebra finches and found that the GSH/GSSG ratio had a negative relationship with blood Hg. Decreases in this ratio are indicative of oxidative stress (Townsend et al. 2003). The data therefore are consistent with the hypothesis that Hg exposure during development and young adult life induced oxidative stress in the livers of zebra finches. Changes in the GSH/GSSG ratio were essentially due to the increased concentration of GSSG, which likely accumulated as GR was unable to convert it back into GSH at a normal rate. This made the ratio of GSH/GSSG in dosed finches smaller than would be expected in healthy individuals (Townsend et al. 2003).
While the accumulation of GSSG suggests that the livers of Hg-dosed zebra finches are under oxidative stress, this is not a direct measure of oxidative stress. To increase our confidence that Hg causes oxidative stress, we assayed the liver enzyme superoxide dismutase (SOD) as a second, independent biomarker for oxidative stress. We also found a significant, negative relationship between blood Hg levels and SOD activity. Individuals with higher Hg had lower levels of SOD activity. This relationship suggests that experimental, environmentally relevant doses of Hg contributed to lowered antioxidant defense capabilities (McCord et al. 1971).
Whereas the data were analyzed in terms of the individual blood mercury levels, when examined on the basis of dietary dose, the effects on oxidative repair systems appears to have been limited to the two highest doses (1.2 and 2.4 μg/g; Table 1). Because individual blood mercury concentrations varied within dose treatment groups, the appropriate statistical analysis is by individual level, but it is clear from the analysis by dose that the most profound effects occurred only at the higher doses, which were designed to mimic cases of extreme point source contamination.
While it is known that binding of cell materials with heavy metals may damage cellular membranes and disrupt cellular functions, the exact mechanism of this oxidative stress remains to be discovered. Without this information it cannot be stated whether the data shown here represent effects from a structurally dysfunctional GR enzyme, or simply an excess of GSSG substrate that exceeds the enzyme’s functional capacity. Likewise in the case of SOD, it is unknown whether or not the SOD enzyme suite is being structurally altered or overwhelmed by anion substrate.
While antioxidant systems, particularly the GSH and GSSG system, have been well-studied as indicators of oxidative stress due to heavy metal toxicity in the past, until the last decade they were mainly studied in mammals. In studies of large aquatic birds collected from areas with high levels of Hg in the food chain, mercury has been consistently associated with changes in various biomarkers for oxidative damage of the liver in adult and developing birds, including diminished GSH concentration (ducks: Hoffman and Heinz 1998; herons: Custer et al. 1997; terns: Hoffman et al. 2011) and GSH/GSSG ratio (ducks: Hoffman et al. 1998; herons: Henny et al. 2002; terns: Hoffman et al. 2011). When wild great egret nestlings were dosed with mercury in the field, Hg exposure altered glutathione-related activities and thiol concentrations in the liver (Hoffman et al. 2005). In a study similar to the present study, captive mallards dosed with 4× the maximum dose used here exhibited reduced GSH/GSSG ratios. Another study on dosed, captive-reared Common Loons (Gavia immer), employing doses similar to those reported here (0.4 and 1.2 ppm), revealed oxidative damage of the liver via GSH and GSSG biomarkers (Kenow et al. 2008). Thus, our results are consistent with a sizeable body of recent literature on waterbirds, in both field-exposure and dosing studies. We extend the finding that lifelong sublethal mercury exposure causes hepatic oxidative damage to young adult songbirds, in a situation where additional pollutants and the age and existing health of the individuals were well controlled.
The data presented support the conclusion that Hg affects the organ systems and cellular functions of avian species exposed to sublethal, environmentally relevant doses. In terms of wildlife management and conservation, it is important to consider that toxins may produce subtle, yet persistent effects in individuals at concentrations that are not lethal and do not produce overt symptoms. The doses used in this study correspond with those found at various contaminated sites worldwide (Varian-Ramos et al. 2014). Songbirds are declining worldwide, and many are exposed to Hg as well as other contaminants. While Hg has been implicated in only a few cases of widespread avian population decline, it is possible that subtle reductions in fitness due to oxidative stress and other effects of Hg are occurring on a much larger scale than realized.
References
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL et al (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335. doi:10.1126/science.1154082
Custer TW, Hines RK, Melancon MJ, Hoffman DJ, Wickliffe JK et al (1997) Contaminant concentrations and biomarker response in great blue heron eggs from 10 colonies on the upper Mississippi River, USA. Environ Toxicol Chem 16:260–271. doi:10.1002/etc.5620160223
Eisler R (2006) Mercury: hazards to living organisms. CRC: Taylor and Francis, Boca Raton
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539. doi:10.2174/1568026013394831
Frederick PC, Spalding MG, Dusek R (2002) Wading birds as bioindicators of mercury contamination in Florida, USA: annual and geographic variation. Environ Toxicol Chem 21:163–167. doi:10.1002/etc.5620210123
Gutteridge JM (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 41:1819–1828
Hallinger KK, Zabransky DJ, Kazmer KA, Cristol DA (2010) Song differs between birds on mercury-polluted and reference sites. Auk 127:156–161. doi:10.1525/auk.2009.09058
Hawley DM, Hallinger KK, Cristol DA (2009) Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology 18:499–503. doi:10.1007/s10646-009-0307-4
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR (2010) Reproduction in mallards exposed to dietary concentrations of methylmercury. Ecotoxicology 19:977–982. doi:10.1007/s10646-010-0479-y
Henny CJ, Hill EF, Hoffman DJ, Spalding MG, Grove RA (2002) Nineteenth century mercury: hazard to wading birds and cormorants of the Carson River, Nevada. Ecotoxicology 11:213–231. doi:10.1023/A:1016327602656
Hoffman DJ, Heinz GH (1998) Effects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks. Environ Toxicol Chem 17:161–166. doi:10.1002/etc.5620170204
Hoffman DJ, Ohlendorf HM, Marn CM, Pendleton GW (1998) Association of mercury and selenium with altered glutathione metabolism and oxidative stress in diving ducks from the San Francisco bay region, USA. Environ Toxicol Chem 17:167–172. doi:10.1002/etc.5620170205
Hoffman DJ, Spalding MG, Frederick PC (2005) Subchronic effects of methylmercury on plasma and organ biochemistries in great egret nestlings. Environ Toxicol Chem 24:3078–3084. doi:10.1897/04-570.1
Hoffman DJ, Eagles-Smith CA, Ackerman JT, Adelsbach TL, Preston KR (2011) Oxidative stress response of Forster's terns (Sterna forsteri) and caspian terns (Hydroprogne caspia) to mercury and selenium bioaccumulation in liver, kidney, and brain. Environ Toxicol Chem 30:920–929. doi:10.1002/etc.459
Keller RH, Xie L, Buchwalter DB, Franzreb KE, Simons TR (2014) Mercury bioaccumulation in Southern Appalachian birds, assessed through feather concentrations. Ecotoxicology 23:304–316. doi:10.1007/s10646-013-1174-6
Kenow KP, Hoffman DJ, Hines RK, Meyer MW, Bickham JW et al (2008) Effects of methylmercury exposure on glutathione metabolism, oxidative stress, and chromosomal damage in captive-reared common loon (Gavia immer) chicks. Environ Pollut 156:732–738. doi:10.1016/j.envpol.2008.06.009
Lewis CA, Cristol DA, Swaddle JP, Varian-Ramos CW, Zwollo P (2013) Decreased immune response in zebra finches exposed to sublethal doses of mercury. Arch Environ Contam Toxicol 64:327–336. doi:10.1007/500244-012-9830-2
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
McCord JM, Keele BB, Fridovich I (1971) An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci USA 68:1024–1027
Moore CS, Cristol DA, Maddux SL, Varian-Ramos CW, Bradley EL (2014) Lifelong exposure to methylmercury disrupts stress-induced corticosterone response in zebra finches (Taeniopygia guttata). Environ Toxicol Chem 33:1072–1076. doi:10.1002/etc.2521
Singer MB, Aalto R, James LA, Kilham NE, Higson JL, Ghoshal S (2013) Enduring legacy of a toxic fan via episodic redistribution of California gold mining debris. Proc Natl Acad Sci USA 110:18436–18441. doi:10.1073/pnas.1302295110
Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57:145–155. doi:10.1016/s0753-3322(03)00043-x
Townsend JM, Rimmer CC, Driscoll CT, McFarland KP, Iñigo-Elias E (2013) Mercury concentrations in tropical resident and migrant songbirds in Hispaniola. Ecotoxicology 22:86–93. doi:10.1007/s10646-012-1005-1
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84. doi:10.1016/j.biocel.2006.07.001
Varian-Ramos CW, Condon AM, Hallinger KK, Carlson-Drexler KA, Cristol DA (2011) Stability of mercury concentrations in frozen avian blood samples. Bull Environ Contam Toxicol 86:159–162. doi:10.1007/s00128-010-0164-0
Varian-Ramos CW, Swaddle JP, Cristol DA (2014) Mercury reduces avian reproductive success and imposes selection: an experimental study with adult- or lifetime-exposure in zebra finch. PLoS ONE 9:e95674. doi:10.1371/journal.pone.0095674
Wada H, Cristol DA, McNabb FMA, Hopkins WA (2009) Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol 43:6031–6038. doi:10.1021/es803707f
Acknowledgments
This study was funded by E. I. DuPont de Nemours and Company and completed with oversight from the South River Science Team, which is a collaboration of state and federal agencies, academic institutions and environmental interests. Additional funding was provided by the Howard Hughes Medical Institute Undergraduate Science Education Program grant and a Llanslo-Sherman award from the Department of Biology at the College of William and Mary to KAH.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The experiments conducted in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the College of William and Mary.
Rights and permissions
About this article
Cite this article
Henry, K.A., Cristol, D.A., Varian-Ramos, C.W. et al. Oxidative stress in songbirds exposed to dietary methylmercury. Ecotoxicology 24, 520–526 (2015). https://doi.org/10.1007/s10646-014-1400-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1400-x