Abstract
A rapid and robust LC–MS/MS method for the analysis of cyazofamid and its metabolite, 4-chloro-5-p-tolylimidazole-2-carbonitrile (CCIM), in environmental samples (soil and water) and a variety of crops (apple, mandarin, Kimchi cabbage, green pepper, potato and soybean) was established in this study. Those compounds were analyzed by selected reaction monitoring with electrospray ionization (positive mode) on LC–MS/MS. Method limit of quantitations were 2 ng g−1 (cyazofamid) and 5 ng g−1 (CCIM) for soil/crop samples, while 0.02 ng mL−1 (cyazofamid) and 0.05 ng mL−1 (CCIM) were achieved for water samples. Matrix effect (%) was different depending on sample matrices. For recovery tests, soil/crop samples were treated with QuEChERS method and water samples were extracted with dichloromethane. The recoveries of target analytes in the environmental and crop samples were 80.2 %–105.1 % for cyazofamid and 75.1 %–99.1 % for CCIM (coefficients of variation; ≤16.4 %).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cyazofamid (4-chloro-2-cyano-N,N-dimethyl-5-p-tolylimidazole-1-sulfonamide, Fig. 1a) is a sulfonamide fungicide (Tomlin and British Crop Protection Council 2009) which has been used for protection of several vegetables and fruits from various diseases (Pesticide Handbook 2011).
It has very low mammalian toxicity (LD50 for rats: >5,000 mg kg−1) and ecological effect [LC50 (96 h) for carps: >0.14 mg L−1] (Tomlin and British Crop Protection Council 2009). In aerobic soil, cyazofamid degraded rapidly (DT50 in soil: 3–5 days) into the major degradates such as CCIM (Fig. 1b), 4-chloro-5-p-tolylimidazole-2-carboxamide (CCIM–AM), and 4-chloro-5-p-tolylimidazole-2-carboxylic acid (CTCA) which were covalently bound to organic matter (Evaluation Report Cyazofamid 2004). When cyazofamid was treated on field for crop residue trial, CCIM was found as a major metabolite (Evaluation Report Cyazofamid 2004).
Only few reports were available for the analysis of cyazofamid residues in the limited environmental or crop/food samples including grapes, ginseng, soil and water (Choi et al. 2007; González-Álvarez et al. 2012; González-Rodríguez et al. 2009, 2011; Lee et al. 2012; Tandon and Singh 2012). Such methods utilized HPLC–UVD (Choi et al. 2007; Lee et al. 2012; Tandon and Singh 2012) or GC–ITMS (González-Álvarez et al. 2012; González-Rodríguez et al. 2009, 2011) after conventional sample treatment, and only cyazofamid was analyzed without metabolites.
The objective of this study is to establish a rapid and effective analytical method of cyazofamid and its metabolite, CCIM, using LC–MS/MS after QuEChERS sample treatment. Upland soil and deionized water were used for environmental samples, while apple, mandarin, Kimchi cabbage, green pepper, potato, and soybean were selected for the representative crops.
Materials and Methods
Cyazofamid (98.4 %) was purchased from Fluka™ (Buchs, Switzerland) and its metabolite, CCIM was kindly donated from the manufacturer. All of solvents (HPLC grade) were obtained from Burdick and Jackson® (Korea) and other reagents were purchased from Sigma Aldrich (USA). Water was distilled and purified with a LaboStar™ TWF UV7 Ultra-pure water system (Siemens Water Technologies LLC, USA). Upland soil sample was collected from a local province. Apple, mandarin, Kimchi cabbage, green pepper, potato, and soybean of ‘Residue free grade’ were purchased from a local market. The QuEChERS materials were obtained from commercial suppliers. For extraction of crops, ‘Ultra QuECh extract kit’ (Ultra Scientific, USA), which is containing 4 g of MgSO4, 1 g of NaCl, 1 g of Na3Citrate·2H2O and 0.5 g of Na2HCitrate·1.5H2O was used. For the dispersive SPE (d-SPE) cleanup of crop extracts from apple, mandarin Kimchi cabbage, green pepper, and potato samples, ‘Ultra QuECh dSPE-General’ (2 mL centrifuge tubes containing 150 mg of MgSO4 and 25 mg of PSA, Ultra Scientific, USA) was used. For soybean samples, ‘Agilent Dispersive SPE 2 mL Fatty Samples’ (2 mL centrifuge tubes containing 150 mg of MgSO4, 25 mg of PSA and 25 mg of C18, Agilent Technologies, USA) was chosen.
Standard stock solutions of cyazofamid and CCIM were prepared at the concentrations of 1,000 and 100 mg L−1 with acetonitrile, respectively. Those two stock solutions were combined to make standard mixture solution, and then it was diluted with acetonitrile to prepare the standard working solutions of various concentrations. The calibration curves were constructed in solvent (acetonitrile), or in matrix extract for matrix matched calibration at 5, 10, 20, 50, 100, and 200 ng mL−1 levels. Matrix effect (ME, %) was calculated by the equation of ME (%) = [(Sm/Ss)−1] × 100 (Lozano et al. 2012). Ss is the slope of solvent calibration curve, while Sm is the slope of matrix matched calibration curve.
A 10 g of homogenized crop sample was placed in a 50 mL conical tube. And the sample was fortified with the standard mixture solution at 10 and 100 µg kg−1 levels before the crop was extracted with acetonitrile (10 mL) by shaking with the reciprocal shaker (SA-2 s, Taitec Corporation, Japan) at 250 rpm for 10 min. Then, ‘Ultra QuECh extract kits’ were added in the conical tubes, shaken for 2 min, and centrifuged for 5 min at 3,500 rpm (Combi 408, Hanil Science Industrial Co., Ltd., Korea). In the case of soybean sample, it was soaked in water (10 mL) for 20 min before extraction. An aliquot (1 mL) of extract was transferred in 2 mL d-SPE tube, before centrifuged for 2 min at 15,000 rpm (Micro 17TR, Hanil Science Industrial Co., Ltd., Korea). The aliquots (400 µL) of crop extracts from d-SPE cleanup were also mixed with 50 µL acetonitrile with 1 % formic acid and 50 µL of acetonitrile for matrix matching before analyzed with LC–MS/MS. For crop matrix matched calibration, an aliquot (400 µL) of control crop extract was mixed with 50 µL acetonitrile which contains 1 % formic acid and 50 µL of standard working solution. For the true sample applications, grapes were purchased from local market and 10 g of homogenized grape sample were processed and analyzed according to the crop method.
A dried and sieved (2 mm) soil (10 g, sandy loam, pH 6.5) was placed into a 50 mL conical tube, and was fortified with the standard mixture solution at 10 and 100 µg kg−1 levels. And then it was extracted with 10 mL acetonitrile containing 1 % acetic acid by shaking at 250 rpm for 5 min with the reciprocal shaker. Four grams of MgSO4 and 1 g of NaCl were added into extraction mixture, and the mixture was shaken with the reciprocal shaker for 5 min, before centrifuged at 3,500 rpm for 5 min. The aliquots (800 µL) were mixed with 200 µL of acetonitrile, and analyzed with LC–MS/MS. For soil matrix matched calibration, an aliquot (800 µL) of control soil extract was mixed with 200 µL of standard working solution. For the field sample applications, orchard soil was collected and the dried and sieved (2 mm) soil (10 g, sandy loam, pH 6.5) was processed and analyzed according to the soil method.
The water sample (500 mL) was placed into a 1,000 mL separatory funnel and fortified with the standard mixture solution at 0.1 and 1 µg L−1 levels. Then the sample was added with 20 g of NaCl before extracting with 100 and 50 mL of dichloromethane successively, by hand shaking. Two dichloromethane extracts were combined, dried over anhydrous Na2SO4, and concentrated with rotary evaporator (Rotavapor R-114, Büchi, Switzerland) under reduced pressure. The residue was dissolved with 5 mL of acetonitrile and an aliquat (400 µL) was mixed with 50 µL of acetonitrile which contains 1 % formic acid and 50 µL of acetonitrile before analyzed with LC–MS/MS. For water matrix matched calibration, an aliquot (400 µL) of control water sample extract was by mixed with 50 µL of acetonitrile which contains 1 % formic acid and 50 µL of standard working solution.
LC–MS/MS analysis was performed on LCMS-8030 (Shimadzu, Japan) coupled to Nexera UHPLC (Shimadzu, Japan) with electrospray (ESI, positive mode). The analytical column was a Kinetex C18 (100 × 2.1 mm i.d., 2.6 µm, Phenomenex, USA) and the column oven temperature was 40°C. The injection volume was 4 µL and the mobile phases were eluted at a 0.2 mL min−1. Mobile phases were 0.1 % formic acid in water (A) and 0.1 % formic acid in acetonitrile (B). For gradient elution, the initial combination was 40:60 (A:B, v/v) and the B solution was increased to 100 % in duration of 2 min, holding for 1 min. To establish the selected reaction monitoring (SRM) condition on LCMS-8030, precursor ions, product ions, Q1 and Q3 pre bias voltage, and collision voltage were optimized through the flow injection of cyazofamid and CCIM standard solutions (1 µg mL−1).
Results and Discussion
On LC–MS/MS, the protonated molecular ion [M + H]+ at m/z = 325.0 for cyazofamid, and m/z = 218.0 for CCIM were obtained in full scan spectra at 4,500 v of needle voltage at the positive ESI mode. And product ions of cyazofamid and CCIM were selected in product scan during the SRM optimization of Q1 pre bias, Q3 pre bias and collision voltages (Table 1). The other conditions such as DL temperature, nebulizing gas flow, heat block temperature were set at the recommended values of the instrument. Good and clear separation was observed on SRM for cyazofamid and CCIM in a variety of samples, showing better sensitivity for cyazofamid than CCIM (Fig. 2).
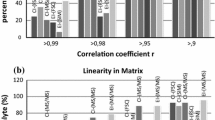
Method limit of quantitations (MLOQs) were 2 ng g−1 (cyazofamid) and 5 ng g−1 (CCIM) for soil/crop samples, while 0.02 ng mL−1 (cyazofamid) and 0.05 ng mL−1 (CCIM) for water samples. Linearity of matrix matched standard solutions (5–200 ng mL−1) of cyazofamid and CCIM was good, showing higher than 0.999 of coefficients of linear determination (Table 2). In water samples ME (%) was generally enhanced, while those of upland soil, mandarin, green pepper, and soybean samples were suppressed for the both of cyazofamid and CCIM. On the other hand in apple, Kimchi cabbage, and potato samples, enhancement for cyazofamid, and suppression for CCIM were observed.
The recoveries of crop samples were from 80.2 % to 105.1 % for cyazofamid (C.V; 0.5 %–16.4 %) and from 75.1 % to 99.1 % for CCIM (C.V; 0.8 %–15.1 %). For soil recovery tests, good recoveries from 91.1 % to 96.5 % (C.V; 1.0 %–9.5 %) were obtained, while the recoveries were from 91.3 % to 99.0 % (C.V; 1.7 %–4.7 %) for water samples (Table 3; Fig. 2).
Most of the previous analytical studies dealt with only cyazofamid (Choi et al. 2007; González-Álvarez et al. 2012; González-Rodríguez et al. 2009, 2011; Lee et al. 2012; Tandon and Singh 2012) however, in this study, metabolite CCIM was also analyzed together with cyazofamid because it has been known as the major and common metabolite in soil and crop (Pesticide Fact Sheet 2004; Evaluation Report Cyazofamid 2004) and in the processed food (e.g. wine) (European Food Safety Authority 2013).
Generally, LC–MS/MS offers very sensitive, selective and rapid analysis compared to the conventional HPLC and GC. When SRM mode was used in this study few ppb levels for soil and crop samples, and sub-ppb levels for water samples were observed as MLOQs.
For the rapid and efficient sample treatment, QuEChERS method was used because the conventional extraction, liquid–liquid partitioning, column chromatography purification and concentration procedures are tedious and time/labor consuming. The popular QuEChERS method was originally introduced in 2003 by Dr. Steven Lehotay’s team of USDA ARS (Anastassiades et al. 2003) for the multiresidue analysis of pesticides in food and cops, and was modified into the acetate-buffering version (Lehotay et al. 2005) and the citrate-buffering versions (Anastassiades et al. 2007). This method applied successfully in not only crops but also environmental samples (Kvíčalová et al. 2012; Liu et al. 2014) for pesticide residue analysis.
In this study for crop samples, citrate-buffering QuEChERS version was used successfully, giving a reasonable recoveries (75.1 %–105.1 %) and precisions for two target analytes. The other studies (Lee et al. 2012; Choi et al. 2007) with the conventional sample treatment and HPLC analysis of crops reported that the recoveries were 75.3 %–98.5 % for several crops and 80.2 %–80.6 % for ginseng. As expected, the analytical time, cost, reagents and labor were saved greatly compared to conventional procedures, still keeping reasonable recovery rates. When grape sample from market was analyzed, crop method was applied successfully and no cyazofamid and CCIM was observed.
In soil recovery tests, the modified QuEChERS method (Caldas et al. 2011) which utilizes MgSO4 and NaCl in extraction process was used without d-SPE procedure since the extract was less complicated than crop extracts. By omitting d-SPE step, the analytical time and cost were reduced again, maintaining of good recoveries (91.1 %–96.5 %). In case of field soil analysis, the established soil method was applied nicely and no cyazofamid and CCIM was observed. Tandon and Singh (2012) also obtained the reasonable recoveries (86.4 %) of cyazofamid from soil samples after the conventional extraction and SPE cartridge purification.
However for water samples, the traditional liquid–liquid partitioning of large volumes of sample (500 mL) and concentration step were carried out in order to reach the sub-ppb level of MLOQ because the analytes concentration in field water samples could be significantly low by dilution. Recoveries from 91.3 % to 99.0 % were obtained with MLOQs of 0.02–0.05 ng mL−1 in this study, while 95.3 % of recovery of cyazofamid was reported by Tandon and Singh (2012), which used the partitioning with chloroform of water samples. However, MLOQ of this study was significantly lower than that of their work (Tandon and Singh 2012).
As a conclusion, a rapid and effective LC–MS/MS method for the analysis of cyazofamid and its metabolite CCIM in environmental samples (soil and water) and crops (apple, mandarin, Kimchi cabbage, green pepper, potato and soybean) was established in this study. By employing QuEChERS process for the crop and soil sample treatment, and SRM on positive mode ESI for MS/MS analysis, the method was very sensitive, quick and selective. Matrix matched calibration was used for the proper quantitation and the recoveries of analytes from matrix samples satisfied the criteria of CODEX guideline (2010). Applicability and robustness of established method was proved through true samples (grapes and soil) analysis.
References
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Anastassiades M, Tasdelen B, Scherbaum E, Stajnbaher D (2007) Recent developments in QuEChERS methodology for pesticide multiresidue analysis. Pesticide chemistry: crop protection, public health, environmental safety Wiley-VCH, Weinheim
Caldas SS, Bolzan CM, Cerqueira MB, Tomasini D, Furlong EB, Fagundes C, Primel EG (2011) Evaluation of a modified QuEChERS extraction of multiple classes of pesticides from a rice paddy soil by LC–APCI–MS/MS. J Agric Food Chem 59(22):11918–11926. doi:10.1021/Jf202878s
Choi J-H, El-Aty AMA, Park Y-S, Cho S-K, Shim J-H (2007) The assessment of carbendazim, cyazofamid, diethofencarb and pyrimethanil residue levels in P. ginseng (C. A. Meyer) by HPLC. Bull Korean Chem Soc 28(3):369–372
European Food Safety Authority (2013) Reasoned opinion on the modification of the existing MRLs for cyazofamid in grapes. EFSA Journal, vol 11
Evaluation Report Cyazofamid (2004) Pesticides experts committee. Food Safety Commission, Tokyo, Japan
González-Álvarez M, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2012) Impact of phytosanitary treatments with fungicides (cyazofamid, famoxadone, mandipropamid and valifenalate) on aroma compounds of godello white wines. Food Chem 131(3):826–836. doi:10.1016/j.foodchem.2011.09.053
González-Rodríguez RM, Cancho-Grande B, Simal-Gándara J (2009) Multiresidue determination of 11 new fungicides in grapes and wines by liquid–liquid extraction/clean-up and programmable temperature vaporization injection with analyte protectants/gas chromatography/ion trap mass spectrometry. J Chromatogr A 1216(32):6033–6042. doi:10.1016/j.chroma.2009.06.046
González-Rodríguez RM, Cancho-Grande B, Simal-Gándara J (2011) Decay of fungicide residues during vinification of white grapes harvested after the application of some new active substances against downy mildew. Food Chem 125(2):549–560. doi:10.1016/j.foodchem.2010.09.047
Guidelines on good laboratory practice in pesticide residue analysis (2010) vol CAC/GL 40-1993. Codex Committee on Pesticide Residues (CCPR)
Kvíčalová M, Doubravová P, Jobánek R, Jokešová M, Očenášková V, Süssenbeková H, Svobodová A (2012) Application of different extraction methods for the determination of selected pesticide residues in sediments. Bull Environ Contam Toxicol 89(1):21–26. doi:10.1007/s00128-012-0622-y
Lee H, Kim E, Moon J-K, Zhu Y-Z, Do J-A, Oh J-H, Kwon K, Lee Y, Kim J-H (2012) Establishment of analytical method for cyazofamid residue in apple, mandarin, korean cabbage, green pepper, potato and soybean. J Korean Soc Appl Biol Chem 55(2):241–247. doi:10.1007/s13765-012-1042-8
Lehotay SJ, Mastovska K, Lightfield AR (2005) Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J AOAC Int 88(2):615–629
Liu H, Guo B, Wang H, Li J, Zheng L (2014) Determination of bromothalonil residues and degradation in apple and soil by QuEChERS and GC–MS/MS. Bull Environ Contam Toxicol 92(4):451–454. doi:10.1007/s00128-014-1224-7
Lozano A, Rajski L, Belmonte-Valles N, Ucles A, Ucles S, Mezcua M, Fernandez-Alba AR (2012) Pesticide analysis in teas and chamomile by liquid chromatography and gas chromatography tandem mass spectrometry using a modified QuEChERS method: validation and pilot survey in real samples. J Chromatogr A 1268:109–122. doi:10.1016/j.chroma.2012.10.013
Pesticide Fact Sheet (2004) U.S. Environmental Protection Agency, Washington
Pesticide Handbook (2011) Korean crop protection association, Seoul, Korea
Tandon S, Singh N (2012) Method development for determination of cyazofamid in soil and water by HPLC. J Liq Chromatogr Relat Technol 35(7):924–936. doi:10.1080/10826076.2011.613145
Tomlin C, British Crop Protection Council (2009) The pesticide manual: a world compendium, 15th edn. British Crop Protection Council, Alton, Hampshire
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H., Kim, E., Lee, JH. et al. Analysis of Cyazofamid and its Metabolite in the Environmental and Crop Samples Using LC–MS/MS. Bull Environ Contam Toxicol 93, 586–590 (2014). https://doi.org/10.1007/s00128-014-1369-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1369-4