Abstract
Heavy metal enrichment in the prey of red-crowned cranes in Zhalong Wetland, northeastern China was researched. Lead and Cd were the most abundant elements in the sediments; their concentrations ranged from 9.85 to 127 ppm and from 1.23 to 10.6 ppm, respectively. Six aquatic animal taxa contained detectable levels of heavy metals, in the decreasing order of Cyprinidae > Cobitidae > Dytiscidae > Odontobutidae > Viviparidae > Aeshnidae. Metal concentrations in these taxa followed the order: Zn > Cu > Cr > Pb > Hg > Cd. Metals in tissues of the red-crowned crane varied in the following order: Zn > Cr > Cu > Pb > Cd > Hg in feathers, and Zn > Cu > Hg > Cr > Pb > Cd in eggshells. Cadmium concentrations in the feathers of the red-crowned crane exceeded a level considered to be potentially toxic in birds (i.e., 0.22 ppm), ranging from 1.42 to 3.06 ppm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Special concerns have been raised on the excessive quantities of heavy metals regarding their health effects on waterfowl (Burger and Gochfeld 1993, 1997; Dauwea et al. 2003). Sediments are regarded as a basin for the accumulation of heavy metals that are discharged in aquatic environments according to Daskalakis and O’Connor (1995). According to previous studies, large quantities of the trace metals that enter aquatic environments are often attached to particulate matter (Daskalakis and O’Connor 1995), and are readily taken up by aquatic animals (Fisk et al. 2005). Therefore, it is important to monitor the concentrations of heavy metals in aquatic birds and their prey to determine if any adverse changes may be occurring in the ecosystems that they inhabit.
The red-crowned crane (Grus japonensis) has been listed in the International Union for Conservation of Nature and Natural Resources Red List as a threatened species since 2000 (BirdLife International 2012). Red-crowned cranes are omnivores and typically feed on aquatic plants (e.g., reed roots and stems) and water animals (e.g., fish, mollusks, and aquatic insects). Thus, their dietary intakes of toxic metals are significantly affected by their prey. These elements eventually accumulate in the bodies of the red-crowned cranes given that they roost and nest in stable sites for years. Thus, they may be chronically exposed to contaminated habitats and prey, as concluded by Koller (1980).
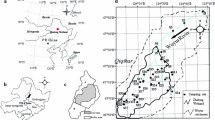
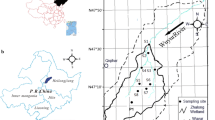
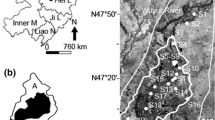
Wuyur River originates from the western foot of Xiaoxin’an Mountain, Northeastern China, wherein the watershed is an elongated area that extends through the main food production zone of Heilongjian Province in China (Fig. 1a). The lower reaches of the river are replaced by a large area of reed marsh after entering into the Zhalong National Nature Reserve (abbreviated as Zhalong Wetland). Zhalong Wetland covers an area of 2,100 km2 (123°51′–124°37′ E, 46°48′–47º32′N). The core area of approximately 700 km2 is the roosting and breeding site for endangered water birds, including the red-crowned cranes. A buffer zone of approximately 1,400 km2 surrounds the core area to serve as an area of protection for other common water birds (Fig. 1b). A Large area of pristine reed marsh in the wetland attracts approximately 500 migratory red-crowned cranes to inhabit and breed from late March to early November (approximately 8 months) every year. Tectonically, the wetland was formed by alluvial deposits with an average altitude of 140 m. Approximately 4,700 km2 of agricultural land surround the wetland (Fig. 1c). River feeding and precipitation are the major sources of water in this inland reed marsh. Various wastewaters from the surrounding residential area, agricultural land, and industrial workshops are discharged directly into the wetland without complete disposal treatment. These inputs contain several types of toxic contaminants, including heavy metals. The wastewater discharge volume increased from 0.17 × 108 m3 in 1993 to 0.45 × 108 m3 in 2010. Increased discharge volumes have resulted in elevated toxic element concentrations in sediment and aquatic biota (Luo et al. 2013). However, no research has been conducted on the dominant toxic elements and their enrichment in the habitat of migratory red-crowned cranes in the Zhalong Wetland.
Ecological health, safety and environmental quality are major concerns, and a better understanding of the degree of enrichment of heavy metals in the aquatic system is significant to conserve the endangered species. A complicating factor in determining this is the fact that all rare species in China, including the red-crowned crane, are protected in legislation, and any intentional killing of such species is prohibited. An alternative approach is to investigate the heavy metal concentrations in their habitat (i.e., sediment and prey) (Dauwea et al. 2003), and external tissues excreted by them (Burger and Gochfeld 1993, 1997; Fisk et al. 2005). The present research objectives were to monitor the concentrations of six heavy metals (Cu, Zn, Pb, Cd, Cr, and Hg) in the red-crowned crane and its prey to allow for an evaluation of possible adverse effects upon the crane.
Materials and Methods
Four areas including a total of 37 sampling sites were designed for sediment collection. The first set of three sample sites (i.e., S1–S3) were in the upper reaches of Wuyur River catchment; the second set of 16 sample sites (S4–S19) were in buffer zone A of the Zhalong Wetland; the third set of 10 sample sites (S20–S29) were in the core area of the wetland, and the remaining eight samples were in buffer zone B of the wetland (Fig. 1b, c). The surface sediment was collected by sediment grab sampler, and immediately packed in dark-colored polyethylene bags, refrigerated, and then transported back to the laboratory.
Six typical aquatic animal taxa, including three invertebrates [i.e., water beetle, Cybister japonicus Sharp (Dytiscidae), pond snail, Cipangopaludina chinensis (Viviparidae), and dragonfly, Aeshna mixta (Aeshnidae)] and three fish species with body size smaller than 10 cm, i.e., common carp, Cyprinus carpio Linnaeus (Cyprinidae), pond loach, Misgurnus mohoity Dybowski (Cobitidae), and Chinese sleeper, Perccottus glehnii Dybowski (Odontobutidae), that are typical prey of wild red-crowned crane in the wetland, were collected at four sampling sites (S10, S20, S22, and S26). All of the prey samples were rinsed thoroughly in the field with distilled water to remove pollutants attached to their bodies, then placed in a car refrigerator at −4°C and transported back to the laboratory.
All sediment samples were sieved through a 63 μm mesh after indoor air drying. They were then digested with acid, based on the method by Viklander (1998). The aquatic animals were first dried with filter paper, and then oven-dried to constant (48 h at 60°C). The dried samples were ground to homogenous powders in a quartz bowl for acid digestion. A total of 0.5 g of each sample type was acid digested in a microwave. Triplicate sub-samples of known dry weights were digested in acid mixture (3 mL HNO3 + 1 mL HCl), and evaporated slowly to near dryness. The residue was dissolved in 5 mL 1:1 diluted HCl, and then brought up to a volume of 25 mL for analysis after the solution cooled down to room temperature.
Four red-crowned crane carcasses were collected at four nesting sites: one adult crane was collected in early April of 2010 at site S10 [body weight (BW): 9.4 kg, male]; two sub-adult cranes were found in early November at site S20 (BW: 6.5 kg, female) and site S22 (BW: 6.8 kg, male), both of which had almost no food residues in their stomachs (only a few grass seeds, stems, fish bones and sediment grains were present). The fourth dead crane was an adult found in late October of 2012 at S26 (an obvious fracture injury was found in the right wing, BW: 8.3 kg, male). The direct death cause for these cranes was starvation due to a food shortage as a result of freezing conditions (Luo et al. 2014). Feathers were collected from the four red-crowned crane carcasses. After-hatch residual eggshells were collected from the field in late April and early May of 2012 at the four nesting sites. Similarly, the eggshells were washed with distilled water in the field and immediately transferred to the laboratory.
The determination of Cu, Zn, Cr, Cd, and Pb concentrations in the sediment, prey, external tissues (i.e., feathers and eggshells) was performed by ICP-MS (Agilent 7500ce, Agilent Technologies, Inc., Santa Clara, CA, USA). We determined total Hg concentration in sediments, prey tissues, and the external tissues of red-crowned cranes using a Mercury Analyzer (Tekran 2600 CVAFS, Corp., Knoxville, TN, USA) with a detection limit of 0.005 µg kg−1. We estimated the precision and accuracy of the analyses based on two certified reference materials (Beijing Shiji Ouke Bio-tech Co., Ltd): Pseudoscianea crocea (GBW08573) for Cu (1.36 ± 0.13 ppm), Zn (28.8 ± 1.4 ppm), Pb (8.8 ± 1.10 ppm), Cr (0.45 ± 0.04 ppm), and Cd (0.014 ± 0.001 ppm), and human hair (GBW–07601) for total Hg (0.36 ± 0.05 ppm). The results agreed with the certified values for all metals, with average recovery rates of 102 % for Cu, 94 % for Zn, 103 % for Pb, 95 % for Cr, 105 % for Cd and 92 % for Hg. All of the materials used for sampling and analysis were acid-washed. Moreover, all of the samples were analyzed in triplicate at a relative standard deviation lower than 1.5 %.
Pearson’s correlation coefficients were used to calculate correlations between the concentrations of six metals in the sediments and aquatic animal tissues. Analysis of variance (ANOVA) was employed to test whether the metal concentrations varied significantly between the sediments in the buffer zone and core area, and a post hoc comparison (Tukey method) was used as a follow-up test to ANOVA to show the statistical differences between sites. Possibilities <0.05 were considered statistically significant.
To describe the contamination of toxic substances in the sediment, two sets of sediment quality guidelines (SQGs) were applied as defined by McDonald et al. (2000):
-
(a)
The effect range low (ERL)/effect range median (ERM) values
-
(b)
The threshold effect level (TEL)/probable effect level (PEL) values.
To describe the ecotoxicology of combined metals, toxic units (TU) were established according to Pedersen et al. (1998), which are defined as the ratio of the determined concentration to the PEL value. If the sum of toxic units (ΣTUs) exceeds 6, the metal contamination would likely bring about high acute toxicity for the aquatic animal community, whereas a value below 4 for the ΣTUs would be indicative of no obvious toxicity for the community.
Results and Discussion
Concentrations of six heavy metals in the sediments of the study area are given in Table 1. Average concentrations of these metals were generally higher than natural background values, following the order: Zn > Pb > Cr > Cu > Cd > Hg. The maximum Pb, Cr, and Hg concentrations exceeded the tolerable level for agro-economic crops suggested by Kabata-Pendias (2001). Peak concentrations of Cr and Cd were detected at 182 ppm at S30 and 10.6 ppm at S37, respectively. The highest concentration of Hg was found at S8 at 0.41 ppm. Concentrations of Cd at the 37 sites exceeded the background value of 0.15 ppm in all cases. The Hg concentrations exceeded the background level in soil (0.06 ppm) in all cases, and the tolerable level in agro-economic products (0.15 ppm) in 36 of 37 sites. The concentrations of Cu, Pb, Cd and Hg in the buffer zone of the wetland were significantly higher than in the core area (F = 10.54, p = 0.003 for Cu, F = 5.84, p = 0.021 for Pb, F = 16.44, p < 0.001 Cd, and F = 5.21, p = 0.029 for Hg); however, the concentration differences for Zn and Cr between the buffer zone and core area were not significant (F = 1.63, p = 0.21 for Zn and F = 2.20, p = 0.15 for Cr).
Based on the SQGs, the incidence of toxicity by Cu and Zn are almost unexpected to occur in this area because the concentrations of these metals in the sediment at all of these sites were below the ERM and PEL values (Table 1). In contrast, adverse effects due to the sediment concentrations of Pb and Cd may frequently occur in the buffer zone of the marsh because most of the sites in these two sampling areas (12 for Pb and and 23 for Cd out of 27 sites in the buffer and upper reaches of Wuyur River catchment) were above the PELs or ERM. Almost no concentrations of the metals exceeded the risk level (ERM or PEL) in the core area, which indicated that the sediments in the core area of Zhalong Wetland are less toxic than those of the buffer zone or upper reaches of Wuyur River. In addition, the incidence of toxicity caused by Cr in the buffer zone (especially in buffer zone A) should give a significant concern because the concentrations at the five sample sites (four sites in zone A and one in zone B) exceeded the PEL. The average value of Hg concentrations in the upper reaches and buffer zone A were as high as 0.33 and 0.32 ppm respectively, which is almost twice the ERL or TEL, although not exceeding the PEL.
The TU and relative contributions of six heavy metals are presented in Fig. 2. TUs of the six elements are in the following order: Cd > Pb > Cr and Hg > Zn > Cu. The ∑TUs for Cd and Pb even exceeded the ∑TUs for the other four metals, indicating that Cd and Pb are heavy contributors to the incidence of metal toxicity to aquatic organisms in the study area. Heavy metal TU in the upper reaches and buffer zone were significantly elevated, i.e., ∑TUs at S8, S13, S30, S34, S36, and S37 exceeded 5, even reaching 5.7 at S3. The high summed values at these sites suggest that acute toxic effects upon the benthic fauna may be occurring. In comparison, ∑TUs were below 4 at all of the sites in the core areas, which suggest that almost no obvious toxicity is likely to occur in the core area.
Six aquatic animal taxa were detected to contain rather high concentrations of two essential elements (i.e., Cu and Zn), varying in decreasing concentrations in the order: Viviparidae > Dytiscidae > Aeshnidae > Odontobutidae > Cyprinidae > Cobitidae for Cu and Odontobutidae > Aeshnidae > Cyprinidae > Dytiscidae > Viviparidae > Cobitidae for Zn (Fig. 3a). Zinc and Cu were found to prevail in six aquatic animal taxa, ranging from 10.6 to 234 ppm and 0.53 to 4.66 ppm, respectively. Pretty high concentrations of Zn in aquatic animals were significantly correlated with Zn concentrations in the sediment (r 2 = 0.65, p = 0.001).
Lead was found in all of the aquatic animal groups, including Cyprinidae and Aeshnidae, which ranged from 8.37 to 170 ppb (Fig. 3b). This level was below the recommended limit (200 ppb) by the joint FAO/WHO food standards program (1990). Cadmium concentrations exceeded the recommended limit (i.e., 50 ppb) in the Viviparidae (61.6 ppb) and Aeshnidae (50.5 ppb) from site S10. The Hg concentrations in the Odontobutidae and Cyprinidae at site S10 approach the recommended limit of 200 ppb, suggesting the possible occurrence of toxic effects in fish.
Generally, the present research revealed that the organisms from the different habitats had varied concentrations of the nonessential elements (i.e., Pb, Cd and Hg) in their bodies. Concentrations of these elements were generally lower in prey from the core area (sites S20–S29) than the buffer zones (sites S4–S19 and S30–S37) or the upper reaches of the catchment (sites S1–S3)(Table 1). A similar trend was evident for the ΣTUs (Fig. 2). The Odontobutidae, Cobitadae and Viviparidae contained rather higher metal concentrations. The Cyprinidae contained relatively low toxic metal concentrations. The Aeshnidae had the lowest concentrations.
Based on SQGs, the probability for adverse effects upon sediment-inhabiting organisms would be highest in the buffer zones due to elevated concentrations of the nonessential elements Pb, Cd and Hg. The rather high toxic element contents in the sediment-inhabiting organisms may present a toxic risk to higher level predators, including red-crowned cranes, in this region because these sediment-inhabiting organisms are essential prey. These high concentrations may cause population reductions of these organisms, which in turn could negatively affect the cranes.
The concentrations of the six metals in the red-crowned cranes are presented in Table 2. In feathers, their concentrations followed the order: Zn > Cr > Cu > Pb > Cd > Hg; while in the eggshells, the order was: Zn > Cu > Hg > Cr > Pb > Cd. The concentration of Pb in crane feathers from site S10 exceeded the toxic level for birds (5 ppm), as reported by Burger and Gochfeld (1997). Cadmium concentrations in the feathers also exceeded the toxic level (0.22 ppm, Pain et al. 2005), ranging from 1.42 to 3.06 ppm. Total Hg contents in the feather and eggshell were below the toxic levels [5.0–11.0 ppm for feather (Burger and Gochfeld 1997), and 1.5–1.8 ppm for eggshell (Fisk et al. 2005)].
In the present research, the prey that is consumed by cranes is thought to have originated from areas near the nesting colony. Therefore, metal levels in the feathers and eggshell probably reflect contaminants acquired from the local environment. Given that any intentional killing of the wild cranes or collecting their eggs is prohibited by law, eggshells and feathers may be used as indicators for the toxic risk level posed on the rare birds (Burger and Gochfeld 1993, 1997; Leonzio et al. 2009). This study has shown that concentrations of certain heavy metals in the sediment, tissues of prey and external tissues of the red-crowned crane were sufficiently high to exceed thresholds of toxicity for the prey and cranes in this area. The inputs of heavy metals into the Wuyur River catchments should be reduced to ensure that this critical crane habitat is maintained in a healthy state for the long-term sustainability of the crane population.
References
BirdLife International (2012) Grus japonensis: IUCN 2012. IUCN red list of threatened species, version 2012. 4. http://www.birdlife.org/datazone/speciesfactsheet. Accessed 5 May 2013
Burger J, Gochfeld M (1993) Lead and cadmium accumulation in eggs and fledgling seabirds in the New York Bight. Environ Toxicol Chem 12:261–267
Burger J, Gochfeld M (1997) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75:160–172
Daskalakis KD, O’Connor TP (1995) Distribution of chemical concentrations in US coastal and estuarine sediment. Mar Environ Res 40:381–398
Dauwea T, Bervoets L, Pinxten R, Blust R, Eens M (2003) Variation of heavy metals within and among feathers of birds of prey: effects of molt and external contamination. Environ Pollut 124:429–436
Fisk A, deWit C, Wayland M et al (2005) Assessment of toxicological significance of anthropogenic contaminants in Canadian Arctic wildlife. Sci Total Environ 351–352:57–93
Joint FAO/WHO food standards programme (1990) Guideline levels for cadmium and lead in food. Codex committee of food additives and contamination, 22nd session, Hague, 19–24 March
Kabata-Pendias A (2001) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton
Koller LD (1980) Immunotoxicology of heavy metals. Int J Immunopharmacol 2:269–279
Leonzio C, Bianchi N, Gustin M, Sorace A, Ancora S (2009) Mercury, lead and copper in feathers and excreta of small passerine species in relation to foraging guilds and age of feathers. Bull Environ Contam Toxicol 83:693–697
Luo J, Yin X, Ye Y, Wang Y, Zang S, Zhou X (2013) Pb and Cd bioaccumulations in the habitat and preys of red-crowned cranes (Grus japonensis) in Zhalong Wetland, Northeastern China. Biol Trace Elem Res 156:134–143
Luo J, Ye Y, Yin X (2014) Bioaccumulation and dietary exposure of the red-crowned cranes (Grus japonensis) to arsenic in Zhalong Wetland, Northeastern China. Aquat Ecosyst Health Manag (in press)
McDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Pain DJ, Meharg AA, Ferrer M, Taggart M, Penteriani P (2005) Lead concentrations in bones and feathers of the globally threatened Spanish imperial eagle. Biol Conserv 121:603–610
Pedersen F, Sjφbrnestad E, Andersen HV, Kjφlholt J, Poll C (1998) Characterization of sediments from Copenhagen harbor by use of biotests. Water Sci Technol 37:233–240
Viklander M (1998) Particle size distribution and metal content in street sediments. J Environ Eng 124:761–766
Acknowledgments
This research was funded by the School of supporting youth’s academic backbone project in Heilongjiang province of China (Grant No. 1253G063).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, J., Ye, Y., Gao, Z. et al. Characterization of Heavy Metal Contamination in the Habitat of Red-Crowned Crane (Grus japonensis) in Zhalong Wetland, Northeastern China. Bull Environ Contam Toxicol 93, 327–333 (2014). https://doi.org/10.1007/s00128-014-1331-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1331-5