Abstract
This study examined the spatial distribution of total mercury (THg) and total arsenic (TAs) in water, soil and cassava (Manihot esculenta) (leaves and roots) samples taken from areas in Rwamagasa village in northwestern Tanzania where daily living activities occur in close proximity to extensive artisanal and small scale gold mining. Results indicated that 33.3 % of the water sources had THg levels above the WHO guideline of 1.0 µg/L for safe drinking water, and 12.5 % had TAs levels above 10 µg/L. Cassava leaves were found to have higher THg (ranging from 8.3 to 167 µg/kg) and TAs (ranging from 60 to 1,120 µg/kg) levels than cassava roots, which ranged between 1.2–8.3 µg/kg for THg and 25–310 µg/kg for TAs. Concentrations of THg and TAs in soil samples ranged between 5.8–1,759 and 183–20,298 µg/kg, respectively. Both THg and TAs were found to be distributed throughout Rwamagasa village.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Artisanal mining is increasing in many parts of the world with more than 30 million active artisanal miners in more than 55 countries (GEUS 2000; ILO 1999). An estimated 0.5–1.5 million people in Tanzania rely on informal mining activities for their livelihoods (Jønsson and Bryceson 2009). Artisanal and small scale gold mining (ASGM) activities are rising in many areas of Tanzania, including the Lake Victoria Goldfields. Most artisanal mining is conducted in a haphazard manner with minimal waste management and little regard for environmental, occupational or human exposure. Thus, these activities result in widespread release of mercury (Hg) and arsenic (As), raising significant human health concerns for local communities (Jønsson and Bryceson 2009; Smedley et al. 1996).
Gravimetric material flow analyses have shown that for every kilogram (kg) of gold produced, 1.2–1.5 g of Hg is introduced into the environment (Van Straaten 2000a). Based on this estimate, approximately 3–4 tonnes of Hg are released into the air in the Lake Victoria Goldfields of Tanzania each year (Van Straaten 2000a). Arsenopyrite (FeAsS), orpiment (As2S3) and realgar (As4S4) often has gold locked in its mineral matrix (Keshavarzi et al. 2012). The liberation of gold through mechanical and chemical means results in the release of As into the environment.
Previous studies in the Lake Victoria Goldfields have indicated that dispersion of Hg from tailings is relatively restricted because of iron-rich laterites and seasonal swamps, which act as natural barriers or sinks (facilitating accumulation of Hg), attenuating the widespread dispersion of Hg in soils (Campbell et al. 2003; Taylor et al. 2005). However, when amalgamation ponds overflow and tailings are left unrestricted (which is common in this area), the possibility of contaminating other areas is higher, hence increasing the potential for Hg and As to be transferred to other trophic levels. Contamination of seasonal swamps is of particular concern as they are the primary water sources for the local shallow water wells that provide drinking water for humans and livestock. They are also used for growing food crops because of the relatively fertile soil that surrounds them and the fact that they retain moisture. Seasonal swamps are also one of the sources of water used in artisanal mining activity; it is easier to transport a bag of ore on a bike or donkey than buckets of water. Miners take gold ore to seasonal swamps or water sources for processing.
Elevated levels of Hg and As have been found in air, surface water, plants and soils in gold mining watersheds (Adjorlolo-Gasokpoh et al. 2012; Campbell et al. 2003; Ikingura et al. 2006; Taylor et al. 2005), threatening human and environmental health. To better understand the potential risk for Hg and As exposure to people in ASGM in Tanzania, the present study examined the spatial distribution of Hg and As in water, soil and cassava (leaves and roots) samples taken from areas in Rwamagasa village in northwestern Tanzania, an area that has a long history of ASGM. The samples were obtained from areas where daily living activities occur in close proximity to extensive ASGM mining.
Materials and Methods
Water, soil and cassava plant samples were collected at Rwamagasa village, which is located in Geita District, northwestern, Tanzania, where ASGM has been actively taking place since 1972. The total population of Rwamagasa village is 7,768 (3,764 males and 4,004 females), according to village records. The majority of people in the village use water from unprotected sources (such as rivers, natural hand dug wells, and constructed wells). Only 12 % of the population has access to a protected public well in Rwamagasa area (Wagner 2003). Water from unprotected sources is also used for livestock consumption and mining activities. The investigators obtained the support of the miners and the community for the study by visiting, introducing, and explaining the purpose and relevance of the study to the Rwamagasa community. The miners were also provided with personnel protective gear, including dust masks and latex gloves.
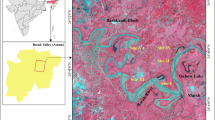
The environmental risk assessment involved a one-time sampling of water, soil, and cassava plants throughout the community near the mining sites (water supplies for drinking, fields used to cultivate food, food sources) in areas with a high degree of human use. Sampling was undertaken towards the end of the rainy season in June–July, 2011. The samples collected included: (1) 92 soils samples collected using a staggered grid sampling method after the top layer (litter) of the soil being removed and at a depth of 10–30 cm, which was in the plough zone (the depth of the soil that can grow plants) (Fig. 1); (2) 24 water samples collected from community water sources, and (3) 28 samples from cassava plants (Manihot esculenta) (14 from leaves and 14 from the roots of the same plants) from 14 different farms; the cassava plants sampled were identified using a grid sampling technique and consisted of the uprooted leaves and roots of one plant, which were analyzed separately. Cassava plants were chosen because they are a staple food and because their growing period is more than a year, and includes both a rainy season and a dry season; hence there is a longer time for the absorption of Hg and As. Guidelines used for sample collection were adopted from the British Columbia Guidance for assessing contaminated sites (2010), the American Public Health Association (Eaton et al. 2005), and the United States Environmental Protection Agency (1994) guidelines.
To ensure quality compliance, the following quality controls (QC) were adhered to: (1) all field data were documented on field data sheets/checklist and sample bags were labeled on spot using a permanent marker; (2) the principal investigator (ECN) supervised all the associated activities; (3) water samples were preserved by 1 %v/v HNO3 and 20 %w/v K2Cr2O7 for Hg samples, 1 % HCl for As, and stored below 4°C (Eaton et al. 2005); (4) plant samples were air/sun dried, homogenized and vacuum packed to prevent rotting during transit; (5) soil samples were air/sun dried, homogenized, pulverized, and sieved to <2 mm prior to acid digestion; (6) the soil sampling auger was washed with metal free water (18.2 MΩ cm) after each sampling site, (7) the cassava roots from one farm were treated as one sample and hence washed with metal free water, peeled separately then pounded and homogenized followed by air drying, and (8) laboratory known QC, duplicated samples, blanks (18.2 MΩ cm de-ionized water) and Certified Reference material were read along with the environmental samples.
Soils and cassava plant samples, and water samples were analyzed in an ISO/IEC 17025:2005 accredited laboratories (OMAC laboratories in Ireland and African Assay Environmental laboratories in Tanzania respectively). Total Hg (THg) in water was determined by Cold vapor atomic absorption spectrophotometry (CVAAS) using Tin(II) chloride (25 % SnCl2) as reductant (Eaton et al. (2005). Total As (TAs) in water was determined by hydride generation atomic absorption spectrophotometry (HGAAS) using sodium-borohydride (0.30 % NaBH4) and sodium hydroxide (0.25 % NaOH) as reductant (Eaton et al. (2005). Total Hg and TAs in soils and plants were determined by inductively coupled plasma mass spectrometry (ICP-MS).
Environmental samples were taken and their respective coordinates were recorded using a global positioning system (GPS). Maps were produced using ArcGIS 9.2 software (ESRI 2010). Base maps were obtained from the Tanzanian Land Survey Department (1994). The contoured soil maps were created by kriging using Surfer 9 software (Golden Software Inc. 2010).
Ethical approval was obtained from Catholic University of Health and Allied Sciences and Bugando Medical Centre joint Research Ethical Committee. Permission to conduct research in Geita District was obtained from the respective authorities at the regional, district and village levels.
Results and Discussion
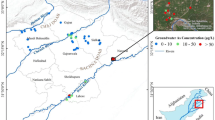
The results from unprotected water sources give cause for concern (Table 1). Laboratory results revealed that some of the sampled water sources had THg and TAs above the background levels established by Taylor et al. (2005) (0.04–0.05 and 0.10–0.50 µg/L, respectively). Eight (33.3 %) water sources were above the WHO guidelines (2004) (1.0 µg/L) for safe drinking water with THg ranging from (2–920) µg/L. Three water sources had TAs above the WHO (2004) guideline (10 µg/L) for safe drinking water and ranged from (43–110) µg/L. Two water sources were above the recommended Tanzanian guideline (TBS 2003) of 50 µg/L for As in drinking water.
The presence of THg and TAs in water sources used for human consumption could be due to dispersion of these toxicants from unprotected tailings and amalgamation ponds mostly located in the south west and few in south east of Rwamagasa close to Insingile River, a water source. The amalgamation ponds do overflow especially during rainfall. The findings of this study are in consistence with those of Taylor et al. (2005). Heavy rainfall, which could result in dilution of these toxicants in water sources, cannot be relied on as a solution to Hg and As contamination. Rather, it appears to result in translocating the contaminants from one area to another. This movement of contaminants could magnify the level of contamination in areas that are distant from mining activity.
For the 14 surveyed cassava farms (Table 2), THg values were higher in the leaves ranging from 8.3 to 167.1 µg/kg compared to the roots, which ranged between 1.2 and 8 µg/kg. TAs was found in all of the leaf (ranging from 60.0 to 1,120 µg/kg) and root samples (ranging from 25.0 to 310 µg/kg) with concentrations in the leaf samples being relatively higher. Similar results were observed for THg at a Bogoso mining site in Ghana (Adjorlolo-Gasokpoh et al. 2012). Studies have also indicated the elevated levels of the metal such as Hg in leaves around mining areas could be due to the plant uptake of the metal from the atmosphere through leaf surfaces (Alloway and Ayres 1994; Essumang et al. 2007).
The iron-rich laterites and the seasonal swamps in which farming takes place in Rwamagasa act as natural barriers that attenuate dispersion of Hg and As in soils (Ikingura et al. 2006; Taylor et al. 2005). Mercury and As analytes concentrate in these areas and cultivated plants such as cassava may absorb these contaminants from the soils as they grow (Leonard et al. 1998). The findings in this study indicate that cassava, a major food source in Rwamagasa, is a significant route for exposure to these toxicants. Our findings suggest that consumption of cassava leaves puts individuals at a higher health risk than consumption of cassava roots; however, both are consumed as staple foods. Leaves are used to make stews and roots are either cooked fresh or pounded to make flour used for porridge. We do not know, however, if food preparation methods impact THg and TAs concentrations in this staple food. Therefore, future research is needed that examines whether food preparation methods alter the concentrations of THg and TAs in cassava leaves and roots.
Laboratory assay results revealed 94.6 % (n = 87) of the soil samples had THg ranging between 5.8–1,759 µg/kg and 97.8 % (n = 90) had TAs concentrations ranging between 183 and 20,298 µg/kg (Table 3). Soil samples, which were above the method detection limit <0.005 and <0.10 mg/kg were also above the Rwamagasa background levels of up to 0.06 and 0.30 mg/kg for Hg and As respectively (Taylor et al. 2005). Typically, the abundance of THg in soils is very low (20.0–60.0) µg/kg (Environmental Agency 2009a; Taylor et al. 2005). This study revealed elevated concentrations of THg and TAs in Rwamagasa and that these elevated concentrations are distributed throughout the Rwamagasa area (Table 3; Fig. 2a, b).
In northwestern Rwamagasa, there was a generalized trend of decreasing THg concentration levels from specified hot spots (high concentration zone); very low localized concentrations of Hg were found in the eastern part or the study area where mining activities are minimal. Similar trends of decreasing Hg with distance from the mine site was found for the Bogoso mining site in Ghana and the Muteh-Isfahan mining site in Iran (Adjorlolo-Gasokpoh et al. 2012; Keshavarzi et al. 2012). Arsenic was widely distributed around hot spots, which were mainly in the south western and some eastern parts of the study area. The intermittent distribution of TAs in the study area could be attributed to human activities associated with gold mining (such as pulverization and amalgamation), as in most areas where little or no mining activity took place the concentrations were relatively low. The results of our study suggest that ASGM is associated with increased environmental contamination of soils in areas in close proximity to mining sites.
The findings of this study indicate that individuals living in communities near ASGM sites in Rwamagasa are at risk for exposure to THg and TAs from various sources including water, soil and plants consumed for food. Unprotected water sources were a primary source of exposure to THg. The concentration of THg and TAs in soil samples varied greatly depending on the location the sample was obtained from. Cassava leaves consistently had higher levels of both THg and TAs. The level of risk for miners and community residents is of concern. Long-term contamination of water, soil and food by these toxicants could result in an increase in the environmental burden of disease to the local population around the mining area. Taken together, our findings suggest a continued need for the monitoring of water sources, along with increasing the number of protected water sources. Further, water sources for human consumption (such as wells), should be built upstream from water sources or watershed drainage areas affected by ASGM activities. Future studies should investigate other food sources (both seasonal and perennial crops) and food preparation approaches to establish levels of contamination.
References
Adjorlolo-Gasokpoh A, Golow AA, Kambo-Dorsa J (2012) Mercury in the surface soil and cassava, Manihot esculenta (flesh, leaves and peel) near goldmines at Bogoso and Prestea, Ghana. Bull Environ Contam Toxicol 89:1106–1110
Alloway BJ, Ayres DC (1994) Chemical principles of environmental pollution. London: Blackie Academic and Professional
British Columbia (2010) Recommended guidance and checklist for ecological risk assessment of contaminated sites in British Columbia—Chapter 6.Urban Park. http://www.env.gov.bc.ca/epd/remediation/policy_procedure_protocol/protocols/tier1/chapter6.htm. Accessed 20 June 2013
Campbell LM, Hecky RE, Muggide R, Dixon DG, Ramlal PS (2003) Variation and distribution of total mercury in water, sediment and soil from northern Lake Victoria, East Africa. Biogeochemistry 65:195–211
Eaton AD, Clesceri LS, Rice EW, Greenberg AE (Eds) (2005) Standard methods for the examination of water and wastewater. 21st edition. Washington, D.C.: American Public Health Association: Method 3111B, D, 3112B & 3114B.3:19–34. American Public Health Association (APHA), American Water Works Association (AWWA), Water Environmental Federation (WEF)
Environmental Agency (2009a) Contaminants in soil: updated collation of toxicological data and intake values for humans. Mercury. Science Report SC 050021/SR TOX 7. Environmental Agency, Bristol
Environmental Systems Research Institute (ESRI) (2010) ArcGIS 9.2. ESRI, Redlands
Essumang DK, Dodoo DK, Obiri S, Yaney JY (2007) Arsenic, cadmium and mercury in cocoyam (Xanthosoma sagittifolium) and wateryam (Colocasia esculenta) in Tarkwa a mining community. Bull Environ Contam Toxicol 79(4):377–397
Geological Survey of Denmark and Greenland—GEUS (2000) Small scale mining. http://www.geus.dk/program-areas/common/int_tz01-uk.html. Accessed 20 June 2012
Golden Software Inc (2010) Surfer 9. Golden Software Inc, Golden
Ikingura JR, Akagi H, Mujumba J, Messo C (2006) Environmental assessment of mercury dispersion, transformation and bioavailability in the Lake Victoria Goldfields, Tanzania. J Environ Manage 81:167–173
ILO (1999) Social and labour issues in small-scale mines. Report for discussion at the tripartite meeting on social and labour issues in small-scale mining (TMSSM). International Labour Office, Geneva
Jønsson JB, Bryceson DF (2009) Rushing for gold: mobility and small scale mining in East Africa. Dev Change 40(2):249–279
Keshavarzi B, Moore F, Rastmanesh F, Kermani M (2012) Arsenic in the Muteh gold mining district, Isfahan, Iran. Environ Earth Sci 67:959–970
Leonard TL, Taylor GE, Gustin MS, Fernandez GCJ (1998) Mercury and plants in contaminated soils: uptake, partitioning, and emission to the atmosphere. Environ Toxicol Chem 17(10):2063–2071
Smedley PL, Edmunds WM, Pelig-Ba KB (1996) Mobility of arsenic in groundwater in the Obuasi gold-mining area Ghana: some implications for human health. In: Appleton JD, Fuge R, McCall GJH (eds) Environmental geochemistry and health, geological society special publication, vol 113. Chapman & Hall, London, pp 163–181
Tanzania Bureau of Standards (TBS) (2003) National Environmental Standards: TZS789:2003—Drinking (Portable) water—specification. Tanzania Dar es Salaam: Bureau of Standards
Tanzanian Land and Survey Dept (1994) Quarter Degree Sheet (QDS) 46/1 1:50, 0000. Government of Japan (JICA), For the Government of the United Republic of Tanzania. Dar es Salaam
Taylor H, Appleton JD, Lister R, Smith B, Chitamweba D, Mkumbo O (2005) Environmental assessment of mercury contamination from the Rwamagasa artisanal gold mining centre, Geita District Tanzania. Sci Total Environ 343(1–3):111–133
U.S. Environmental Protection Agency (EPA) (1994) Standard operating procedure (SOP) for sampling in hazardous waste sites. Terrestrial plant sampling guidance. SOP#: 2037 Rev. 0.0. 19/10/1994. http://clu-in.org/download/ert/2037-R00.pdf. Accessed 15 May 2012
Van Straaten P (2000) Human exposure to mercury due to small scale gold mining in northern Tanzania. Sci Total Environ 259(1–3):45–53
Wagner S (2003) Socio-economic survey of Rwamagasa mining site in Geita district. Removal of Barriers to Introduction of Cleaner Artisanal Gold Mining and Extraction Technologies UNIDO—Socio-Economic Survey of Rwamagasa Mining Site in Geita District, Tanzania. Vienna, Austria: Global Mercury Project. GEF/UNDP/UNIDO, pp 3–5. http://www.unites.uqam.ca/gmf/intranet/gmp/countries/tanzania/sociological_report/Tanzania_Sociological_Report.pdf. Accessed 15 Mar 2012
WHO (2004) Guideline for drinking water quality, vol 1. World Health Organization, Geneva. http://www.who.int/water_sanitation_health/dwq/fulltext.pdf. Accessed 12 Dec 2011
Acknowledgments
The authors acknowledge the Catholic University of Health and Allied Sciences-Bugando, the University of Calgary and the University of Colorado, Denver. A special thank you goes to African Eagle Resources who contributed funding to support this project. The authors would also like to thank all the participants in this study. The authors also declare that they have no competing interests. It should be noted that Twigg Gold is not engaged or associated with artisanal mining in the Rwamagasa belt or anywhere else.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nyanza, E.C., Dewey, D., Thomas, D.S.K. et al. Spatial Distribution of Mercury and Arsenic Levels in Water, Soil and Cassava Plants in a Community with Long History of Gold Mining in Tanzania. Bull Environ Contam Toxicol 93, 716–721 (2014). https://doi.org/10.1007/s00128-014-1315-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1315-5