Abstract
Arsenic (As) is toxic to plants and animals. We tested the effects of arsenite and arsenate (0–16 mg/L) on seed germination, and on relative root and shoot length, α-amylase activity, reducing sugars and soluble total protein contents, and malondialdehyde content in barley seedlings. We also measured As accumulation in barley stems and roots. The α-amylase activity, relative root and shoot length, and seed germination decreased with increasing concentrations of arsenate and arsenite. The reducing sugars content in barley seedlings increased after 4 days of growth on media containing As. In general, the protein content in roots and seedlings decreased with increasing doses of As. Arsenic in the tissues was quantified by hydride generation–atomic absorption spectrophotometry. To confirm the accuracy of the method, we analyzed the certified reference material WEPAL-IPE-168. The limit of detection was 1.2 μg/L and the relative standard deviation was <2.0 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Arsenic (As) contamination of soil and groundwater is a worldwide problem. Common sources of As include semiconductor manufacturing, leaks, landfill, fertilizers, insecticides and herbicides, wood preservatives, fragrance-free paint production, mining, smelting, sewage, and coal combustion (Castillo et al. 2007; Li et al. 2007; Yadav 2010). The inorganic forms of As, arsenate [As(V)] and arsenite [As(III)], are usually dominant in As-contaminated soil, and in aerobic soils, the oxic conditions result in the prevalence of arsenate over arsenite. The arsenite in herbicides and insecticides is oxidized to arsenate (Smith et al. 2010). Both arsenite and arsenate have long been used in pesticides. Crop growth and development is inhibited by As (Duquesnoy et al. 2010). Crops grown in As-contaminated soil or irrigated with As-contaminated water accumulate As in the seeds or grain, and this is an increasingly important problem in many parts of the world. Because grains and seeds are used as foods and feed materials, As accumulated in plant roots and stems can reach humans via the food chain. In plants, As accumulates mainly in the root system, and to a lesser degree in the aboveground organs. It causes physiological changes in plants and reduces crop productivity (Stoeva et al. 2005). Studies have shown that As-sensitive plants show stress symptoms upon exposure to As, including reduced photosynthetic rates (Stoeva et al. 2003/2004), inhibited root growth, and death (Macnair and Cumbes 1987; Paliouris and Hutchinson 1991). Exposure to inorganic As results in the generation of reactive oxygen species (ROS; superoxide radicals, hydroxyl radicals, and hydrogen peroxide), which can directly damage proteins, amino acids, and nucleic acids, and can cause peroxidation of membrane lipids (Panda et al. 2010). Starch and sugar metabolism in plants are negatively affected under stress conditions.
The sensitivity of plants to various metals and the symptoms of metal toxicity depend on the types and concentrations of the toxicants and on the life-stage of the plant. Metals and other contaminants affect different biological processes (germination, seedling survival, vegetative growth) in different ways. For example, plants are more sensitive to metal pollution during the seed germination and early seedling growth stages, because some of the defense mechanisms have not yet developed fully. Therefore, the growth stage of the plant is an important consideration in toxicity assessment. Starch is the main form of stored polysaccharides in seeds (Liu et al. 2005); therefore, the effects of As on α-amylase, the key enzyme in starch degradation, should be considered. At present, little is known about the As-sensitivity of amylases.
In this study, we investigated the effects of various concentrations of arsenate and arsenite (0–16 mg/L) on some biochemical and physiological characteristics of barley during germination and the early stage of seedling growth. To evaluate the toxicity of these As compounds, we determined their effects on seed germination, seedling growth (relative root and shoot lengths), α-amylase activity, reducing sugar and soluble total protein contents in root and stem tissues, and malondialdehyde (MDA) contents in barley tissues. We also quantified the As that accumulated in barley root and shoot tissues.
Materials and Methods
Seeds of Hordeum vulgare (cv. Sladoran) were supplied by the Edirne Agricultural Research Institute. The As compounds used in these experiments were sodium arsenate (Na2HAsO47H2O, Merck) and sodium arsenite (NaAsO2, Merck).
To evaluate α-amylase activity, barley seeds were sterilized in 15 % sodium hypochlorite for 1 min, then washed several times with distilled water. The seeds were then incubated for 7 days at 20, 25, or 30°C in the dark. At various times during the germination period, enzyme extracts were prepared as described by Thevenot et al. (1991). The activity of α-amylase was determined as described by Ekinci and Aktaç (1996). Enzyme activity is expressed as units per milliliter (U/mL), as calculated from the rate of disappearance of the substrate (Ekinci and Aktaç 1996).
To test the effects of As on α-amylase activity in seeds, the barley seeds were soaked in freshly prepared solutions containing various concentrations of arsenate and arsenite (0–16 mg/L). The seeds were incubated in Petri dishes at 20°C in the dark. Seeds in the control were treated with distilled water. The α-amylase activity was measured on day 3 of the germination period (when the activity reached the highest level). The number of replicates in each test is eight.
To determine the effects of arsenate and arsenite on soluble protein contents in roots and stems, barley seeds were soaked in freshly prepared arsenate and arsenite solutions. Distilled water was used for the control group. Proteins were extracted from seeds on day 3 of the germination period using the method of Landry et al. (2001), with modifications. The radicals and coleoptiles were cut from the seeds and weighed, and then homogenized in 0.5 M NaCl (1 g tissue: 5 vol NaCl) at 4°C. The homogenate was incubated for 30 min and then centrifuged at 10,000g at 4°C for 10 min. The supernatant was removed (Supernatant 1) and the pellet homogenized in 0.5 M NaCl + 0.6 % (v/v) 2ME (mercaptoethanol) at 20°C. The mixture was incubated at room temperature for 30 min and then centrifuged at 10,000g at 4°C for 10 min. The supernatant was removed (Supernatant 2) and the pellet was homogenized in sodium acetate buffer, which was buffered in 0.5 M NaCl + 0.6 % (v/v) 2ME (pH 10) at room temperature. The mixture was incubated at room temperature for 30 min and then centrifuged at 10,000g at 4°C for 10 min. The supernatant was removed (Supernatant 3) and the pellet was homogenized in 55 % propanol + 0.6 % 2ME (v/v) at room temperature. The mixture was incubated at room temperature for 30 min and then centrifuged at 10,000g at 4°C for 10 min. The supernatant was removed (Supernatant 4). Protein amounts were spectrometrically measured according to Lowry method (Lowry et al. 1951).
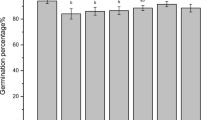
Seeds were germinated in Petri dishes on moist filter paper after surface sterilization and washing with distilled water as described above. Each Petri dish contained 100 seeds, which had been soaked in freshly prepared solutions containing various concentrations of arsenate or arsenite (0–16 mg/L) or distilled water (control). The seeds were incubated at 20°C in the dark. On day 3, the number of germinated seeds was counted (seeds with a radicle and plumula longer than 2 mm were considered to be germinated). Seed germination percentages of As-treated seeds were calculated relative to that of the control.
To examine the effects of As on plant growth, the root and stem lengths of arsenate-treated and arsenite-treated seedlings and controls were measured on day 3 of the germination period. The values are expressed as relative root and stem lengths compared with those of the control. The reducing sugars contents in roots and stems were measured on day 3 of the germination period using the 3–5 dinitrosalicylic acid method (Sanal et al. 2005).
The thiobarbituric acid (TBA) method was used to measure MDA content, which is indicative of lipid peroxidation, in root tissues (Sun et al. 2008). Root tissues were collected on day 4 of the germination period and immediately frozen at −80°C. Frozen root tissues (0.2 g) were homogenized in 10 % trichloroacetic acid containing 2.25 % TBA in a Teflon homogenizer. The mixture was incubated at 95°C for 30 min, cooled rapidly, and then centrifuged at 10,000g for 10 min. The absorbance of the supernatant was measured at 532 and 600 nm. The amount of MDA was calculated using an extinction coefficient of 1.55 mM/cm−1 and is expressed as mmol MDA per gram fresh weight.
To evaluate As accumulation in plant tissues, sterilized barley seeds were washed several times with distilled water, and then soaked in freshly prepared solutions of arsenate and arsenite in 2 mM MES-Tris buffer (pH adjusted to 5.8). In the control, seeds were soaked in the buffer without As. The seeds were incubated in Petri dishes at 20°C in the dark. On day 3 of the germination period, the germinated seeds were transferred into containers with Hoagland solution. The seeds were grown in these containers for 7 days at 20°C (mean light intensity, 50 lux). The test solution was changed every day to maintain the concentration of As compounds and the solution properties. The lengths of the longest roots and stems were measured on day 10. Data shown in figures and tables are the mean values of three replicates.
All samples were washed with cold distilled water to remove metal ions from the root surface. The root and stem tissues were dried at 80°C before As analyses. Arsenic accumulation in the tissues was measured by hydride generation–atomic absorption (HG-AAS) spectrophotometry after microwave-assisted acid digestion of samples. A CEM MARSXpress five closed-vessel microwave digestion system with pressure and temperature monitoring was used for microwave-assisted extraction of As. The HGAAS measurements were performed with a Perkin Elmer Analyst 800 atomic absorption spectrometer equipped with an MHS-15 (mercury hydride system) hydride generator. The HGAAS working program is shown in Table 1. All measurements were repeated three times. To evaluate the accuracy and the precision of the As analytical method, we analyzed a certified reference material, WEPAL-IPE-168.
Results and Discussion
Barley seeds were incubated at three different temperatures for 7 days to determine which temperature was optimal for these experiments. The activity of α-amylase was measured during the germination period to determine the period of peak activity. As shown in Table 2, in all of the temperature treatments, the highest α- amylase activities in barley seeds were observed on day 3 of the germination period. Among the three temperatures, 20°C was optimal, since the highest α-amylase activity was observed under these conditions (Table 2). Therefore, the temperature used in subsequent experiments was 20°C.
Next, we tested the effects of arsenate and arsenite on α-amylase activity in seeds on day 3 of the germination period. As shown in Table 3, arsenate and arsenite significantly inhibited α-amylase activity, compared with that in the control. The negative effect of arsenite on α-amylase activity was stronger than that of arsenate.
Arsenate and arsenite treatments significantly inhibited germination of barley seeds. Compared with that in the control (set to 100 %), the germination rate of seeds treated with 16 mg/L arsenate was inhibited by approximately 25 % and that of seeds treated with 16 mg/L arsenite was inhibited by approximately 40 % (Table 4).
The results shown in Tables 3 and 4 indicated that in terms of germination and α-amylase activity, higher concentrations of arsenate and arsenite generally resulted in greater inhibition, and arsenite had a stronger negative effect than arsenate. In another study, Liu et al. (2005) evaluated the effects of arsenate and arsenite toxicity on several wheat varieties, and found that high doses of arsenite and arsenate had similar effects on germination and total amylase activity (Liu et al. 2005). A similar result was reported in another study on wheat (Li et al. 2007).
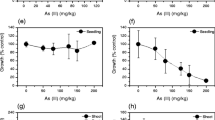
As shown in Table 5, arsenate and arsenite negatively affected the lengths of barley roots and stems. The 2 mg/L arsenate treatment showed the strongest negative effect, and higher concentrations of arsenate had a weaker negative effect. In the arsenite treatments, the root length was not affected by arsenite concentrations lower than 8 mg/L, but was negatively affected by concentrations higher than 8 mg/L. Similarly, the 2 mg/L arsenite concentration showed the strongest negative effect on stem length. Several previous studies showed that plants were inhibited by As in a dose-dependent manner (Shri et al. 2009; Singh et al. 2007; Stoeva et al. 2003/2004). Plants take up As in its arsenate and arsenite forms. These forms interact with phosphate in various metabolic pathways, and can replace or interact with sulfhydryl groups on proteins. Such processes can cause growth retardation in some plants (Shri et al. 2009). Several studies have reported that roots are more strongly affected than shoots by As. This is likely to be because they are the first point of contact with As compounds (Abedin and Meharg 2002). Environmental stresses negatively affect carbohydrate metabolism in plants. In the present study, we observed that arsenate and arsenite treatments increased the reducing sugars contents in the root and stem tissues compared with that in the control, and that higher doses of the As compounds generally resulted in greater increases (Figs. 1, 2, 3, 4). Jha and Dubey (2004) reported that As-treated rice seedlings showed decreased reducing sugars contents, increased activities of enzymes associated with sugar metabolism, and decreased activities of enzymes associated with sucrose synthesis.
The soluble protein concentration in the roots was significantly increased in response to 2 and 4 mg/L arsenate, compared with that in the roots of the control (Fig. 1). The soluble protein concentration in the roots of plants treated with 8 and 16 mg/L arsenate was similar to that in roots of the control. In stem tissues, the soluble protein concentration in plants treated with 0.5, 1, 2, 4, and 16 mg/L arsenate was lower than that in the control (Fig. 2). Similarly, the soluble protein concentration in roots decreased in response to 1 and 4 mg/L arsenite, but increased in response to 8 and 16 mg/L arsenite, compared with that in the control (Fig. 3). In the stem tissues, plants treated with 0.5, 1, 2, 4, and 16 mg/L arsenite showed lower soluble protein concentrations than that in the control (Fig. 4).
A combined supernatant was produced by mixing all of the supernatants obtained during the extraction procedure. The total protein content in this mixture was determined for all treatments and the control. The total protein content of the mixture obtained from plants treated with 0.5 mg/L arsenate was approximately three times that in the mixture obtained from the control (Table 6). All other concentrations of arsenate resulted in total protein contents less than that in the control. Among the plants treated with arsenite, those subjected to the 2 mg/L dose showed a total protein content approximately 2.2-times that in the control, while those subjected to other doses showed total protein contents similar to, or less than, that in the control (Table 6). When plants are exposed to As stress, they produce stress proteins to survive under the adverse environmental conditions (Seth et al. 2007). This may explain the increases in protein content in response to lower doses of arsenate (0.5 mg/L) and arsenite (2 mg/mL) (Table 6).
The protein content in roots increased in response to treatment with 4 mg/L arsenate, but decreased in response to higher doses. Similarly, Öztürk et al. (2010) reported that total protein content increased in response to low concentrations of arsenate (1, 3, 5, and 10 μM) but decreased significantly in response to a high concentration (50 μM).
Metal stress, including As stress, results in the formation of ROS, which trigger the formation of lipid peroxidation products such as MDA. Unsaturated fatty acids in the membrane induce peroxidation (Stoeva et al. 2003/2004, 2005; Singh et al. 2007; Shri et al. 2009; Khan et al. 2009). Measurement of MDA, the final product of lipid peroxidation, is commonly used to estimate the level of lipid peroxidation. We quantified MDA in roots, since they are the first point of contact with As. The MDA content in roots of plants treated with 0.5 and 1 mg/L arsenate was approximately 1.5–2 times that in roots of the control, but that in roots of plants treated with 2 mg/L arsenate was similar to that in roots of the control (Fig. 5). The MDA levels in roots of plants treated with 8 and 16 mg/L arsenate were higher than that in the control. Among the arsenite treatments, only the 4 and 16 mg/L concentrations resulted in significant increases in MDA levels in the roots, compared with that in the control (Fig. 5).
Khan et al. (2009) reported that the MDA content in mustard plants increased in response to 5 and 25 μM As, with a greater increase in response to the higher As dose. Li et al. (2007) treated wheat seedlings with increasing concentrations of As (0–20 mg/kg) and found that the increase in MDA content was dependent on the As dose; the increase was smaller in response to As concentrations lower than 1 mg/kg, but became more pronounced in response to higher As concentrations. Shri et al. (2009) observed that increasing concentrations of arsenate and arsenite increased the MDA content in rice tissues. This was reported to be related to an increase in the activity of the enzymes responsible for lipid peroxidation (Shri et al.2009). In root cells, arsenate is rapidly reduced to arsenite, which leaches into the outer environment, forms complexes with thiol peptides, or is transported to the stem. Arsenite has toxic effects on proteins as a result of binding to sulfhydryl groups and interacting with catalytic regions of enzymes (Zhao et al. 2009).
The data from this study and other studies suggest that plants are able to prevent lipid peroxidation by activating defense mechanisms in response to As at concentrations below a certain critical level. To further explore this topic, work is currently underway in our laboratory to analyze how oxidative stress enzymes in barley function under heavy metal stress, and to evaluate changes in the glutathione and phytochelatin levels under arsenite stress. It has been proposed that in plants subjected to high doses of As, the cell membrane composition changes, and the carriers in the membrane decrease transport of As to protect the cell against its toxic effects (Shri et al. 2009).
In this study, barley seeds were germinated and grown on Petri dishes with various concentrations of arsenate and arsenite (0–16 mg/L) and then further grown in Hoagland solution for 7 days. A dose-related accumulation of As was observed in the roots and stems of these plants. As shown in Table 7, among the plants treated with arsenate and arsenite, the roots accumulated more As than did shoots. The roots of arsenite-treated plants accumulated more As than did those of arsenate-treated plants. The highest concentration of As (3.35 mg/g FW) was detected in roots of plants treated with 16 mg/L arsenite.
To evaluate the accuracy and the precision of the As analytical method, we analyzed a certified reference material, WEPAL-IPE-168 (Wageningen Evaluation Programmes for Analytical Laboratories, Wageningen University, The Netherlands) by HGAAS under optimal conditions. There was good agreement between the detected value (69.1 ± 1.4 μg/kg) and the certified value (68.7 ± 2.2 μg/kg) (n = 5). The limit of detection was calculated as three times the standard deviation (3σ) of values obtained for blank samples (n = 10). The detection limit of As was 1.2 μg/L. The precision of the method was evaluated as the relative standard deviation (RSD), which was obtained by analyzing three replicates. The RSD values were lower than 2.0 %.
In their studies on mustard, Khan et al. (2009) showed that treatment with 5 and 25 μM As for 96 h resulted in increased As contents in the root and stem tissues. Shri et al. (2009) studied As accumulation in root and stem tissues of rice plants, and found that in plants treated with 100 μM arsenate, the As concentration in the stem was approximately twice that in the root. This suggested that the accumulation of As compounds varied among different plant tissues. Su et al. (2010) studied the effects of arsenite and arsenate on wheat, barley, and rice, and found that after a 5 μM arsenite or arsenate treatment, the arsenate contents in roots of rice and wheat roots were similar, and were 20 %–25 % higher than that in roots of barley. Among the rice, wheat, and barley plants treated with arsenite, the highest As concentration was in rice roots. Some studies have suggested that the phosphate transport pathway plays a significant role in transporting As, and that basal metabolism regulates arsenite efflux from the plant during As detoxification (Zhao et al. 2009; Logoteta et al. 2009), resulting in less arsenite being translocated to stem tissues (Su et al. 2010).
The effects of arsenate and arsenite can differ during the early development period of barley plants because they are metabolized differently. In conclusion, barley seedlings subjected to As treatments showed oxidative stress symptoms, such as an increase in lipid peroxidation. In addition, As treatments resulted in a decrease in α-amylase activity, and changes in the reducing sugars contents and total protein contents. These biochemical changes affected the plants and inhibited their growth, depending on the As dose. These data show that barley plants can use various defense mechanisms to protect themselves against As, up to a certain critical concentration.
References
Abedin MJ, Meharg AA (2002) Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil 243:57–66
Castillo-MH, Parsons JG, Peralta-Videa JR, Martinez- Martinez A, Dokken KM, Gardea-Torresdey JL (2007) Use of X-Ray absorbsiyon spectroscopy and biochemical techniques to characterize arsenic uptake and reduction in pea (Pisum sativum) plants. Plant Physiol Biochem 1–7
Duquesnoy I, Champeau GM, Evray G, Ledoigt G, Piquet-Pissaloux A (2010) Enzymatic adaptations to arsenic-induced oxidative stress in Zea mays and genotoxic effect of arsenic in root tips of Vicia faba and Zea mays. Biologies 333:814–824
Ekinci F, Aktaç T (1996) Buğday (Triticum aestivum L.) α-amilaz enziminin bazı biyokimyasal özelliklerinin belirlenmesi ve enzim aktivitesi üzerine endosülfanın etkileri. Turk J Biol 21:283–298
Jha AB, Dubey RS (2004) Carbohydrate metabolism in growing rice seedling under arsenic toxicity. J Plant Physiol 161:867–872
Khan I, Ahmad A, Iqbal M (2009) Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicol Environ Saf 72:626–634
Landry J, Delhaye S, Philippeau C, Michalet- Doreau B (2001) Isolation and quantitation of zein in waxy and amylase-extender and wild flint and dent maize endosperm using a new solvent sequence for protein extraction. J Agric Food Chem 49:164–169
Li C, Feng S, Shao Y, Jıang L, Lu X, Hou X (2007) Effects of arsenic on seed germination and physiological activities of wheat seedlings. J Environ Sci 19:725–732
Liu Xi, Zhang S, Shan X, Zhu YG (2005) Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere 61:293–301
Logoteta B, Xu X, Macnair MR, Mcgrath SP, Zhao FJ (2009) Arsenite efflux is not enhanced in the arsenate tolerant phenotype of Holcus lanatus. New Phytol 183:340–348
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Macnair MR, Cumbes Q (1987) Evidence that arsenic tolerance in Holcus lanatus L. is caused by an altered phosphate uptake system. New Phytol 107:387–394
Öztürk F, Duman F, Leblebici Z, Temizgül R (2010) Arsenic accumulation and biological responses of watercress (Nasturtium officinale R. Br.) exposed to arsenite. Environ Exp Bot 69:167–174
Paliouris G, Hutchinson TC (1991) Arsenic, cobalt and nickel tolerances in two populations of Silene vulgaris (Moench) Garcke from Ontario Canada. New Phytol 117:449–459
Panda SK, Uphadhyay RK, Nath S (2010) Arsenic stress in plants. J Agron Crop Sci 196:161–174
Sanal FE, Ertan F, Aktaç T (2005) Production of exo-inulinase from Alternaria alternate growth on Jerusalem artichoke and some biochemical properties. J Biol Sci 5:497–505
Seth CS, Chaturvedi PK, Misra V (2007) Toxic effect of arsenate and cadmium alone and in combination on giant duckweed (Spirodela polyrrhiza L.) in response to its accumulation. Environ Toxicol 22:539–549
Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110
Singh HP, Batish DR, Kohlo RK, Arora K (2007) Arsenic -induced root growth inhibition in mung bean (Phaseolus aureus) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53:65–73
Smith SE, Christophersen HM, Pope S, Smith FA (2010) Arsenic uptake and toxicity in plants: integrating mycorrhizal influences. Plant Soil 327:1–21
Stoeva N, Berova MA, Zlatev Z (2003/2004) Physiological response of maize to arsenic contamination. Biol Plant 47: 449–452
Stoeva N, Berova MA, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Su YH, McGrath SP, Zhao FJ (2010) Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 328:27–34
Sun Y, Li Z, Guo B, Chu G, Wei C, Liang Y (2008) Arsenic mitigates cadmium toxicity in rice seedlings. Environ Exp Bot 64:264–270
Thevenot C, Simond-Cote E, Daussant J (1991) Contribution of alueron layer and scutellum to α-amilase synthesis and secretion in wheat and rice grains. Physiol Plant 82:227–256
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Acknowledgments
This study was supported by Trakya University Research Fund. Project no. 2009-145.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanal, F., Şeren, G. & Güner, U. Effects of Arsenate and Arsenite on Germination and Some Physiological Attributes of Barley Hordeum vulgare L.. Bull Environ Contam Toxicol 92, 483–489 (2014). https://doi.org/10.1007/s00128-014-1214-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1214-9