Abstract
In this study, antioxidant responses including lipid peroxidation (LPO), superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST), were evaluated in the liver, gill and muscle tissues of pale chub (Zacco platypus) exposed to copper (Cu) and benzo[a]pyrene (BaP). Cu exposure induced significant antioxidant responses in Z. platypus, particularly in the liver, whereas BaP exposure had a negligible effect. Following Cu exposure, both SOD and CAT activity increased in a concentration-dependent manner, showing significant correlations with malondialdehyde (MDA) levels as a measure of LPO (r = 0.646 and 0.663, respectively). SOD, CAT and GST mRNA levels were also enhanced following Cu exposure, except at 20 μg L−1, although significant correlations with antioxidant enzyme activities were not found. The results of this study suggest that combined information on SOD and CAT activities together with LPO levels in the liver could be a useful indicator for assessing oxidative stress in freshwater fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Reactive oxygen species (ROS) occur naturally in organisms that use molecular oxygen for energy production (Li et al. 2008), and can induce toxic effects related to oxidative stress. The substrates that are most susceptible to autoxidation are polyunsaturated fatty acids in the cell membrane, which undergo rapid lipid peroxidation (LPO) (Zhang et al. 2004). This phenomenon may lead to muscle degradation, impairment of the nervous system, hemolysis, general deterioration of cellular metabolism and ultimately cell death (Frankel 1995). However, living organisms have evolved numerous defense mechanisms to neutralize the harmful effects of free radicals. Key components of this antioxidant defense system include enzymes such as catalase (CAT), superoxide dismutase (SOD) and glutathione S-transferase (GST) and other scavengers with low molecular weights (e.g., carotenoids, glutathione and vitamins E, K and C) (Martinez-Alvarez et al. 2005). These enzymes are found in virtually all tissues in fish, including the gills, liver and kidneys, but show different efficiencies in preventing oxidative stress (Eyckmans et al. 2011).
Antioxidant enzymes have been widely used to characterize defense mechanisms and to evaluate the toxicity induced by oxidative stressors (e.g., metals and other xenobiotics) (Regoli et al. 1998; Kim et al. 2009). This type of mechanistic knowledge has served as the basis for the development of bioanalytical tools, risk assessment procedures and methods for identifying causative toxic agents. However, implementing a combined transcriptional and enzymatic approach represents a key challenge in oxidative stress assessment (Eggen et al. 2004), although a limited number of studies have employed a multi-level biomarker approach, examining both gene- and protein-level responses to describe oxidative stress in freshwater fish. Thus, the present study investigates the antioxidant responses of various tissues, including the liver, muscles and gills, of pale chub (Zacco platypus), based on analyses of mRNA expression and the enzymatic activity of CAT, GST and SOD in addition to lipid peroxidation levels following exposure to copper (Cu) and benzo[a]pyrene (BaP).
Materials and Methods
Copper sulfate (CuSO4, 99 % pure) and benzo[a]pyrene (BaP, 99.9 % pure) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions of CuSO4 were prepared in dechlorinated tap water (pH, 6.9; alkalinity, 28.0 mg L−1 as CaCO3; total hardness, 47.8 mg L−1 as CaCO3) to produce nominal concentrations of 1.25, 5 and 20 μg Cu L−1), and dechlorinated tap water was used as a control. Stock solutions of BaP were prepared in N,N-dimethylformamide (10 mg L−1) and diluted with dechlorinated tap water to produce nominal concentrations of 0.5, 5 and 50 μg L−1, and N,N-dimethylformamide dissolved in dechlorinated tap water (10 mg L−1) was used as a control. N,N-dimethylformamide exhibits a low toxicity and bioaccumulation potential for aquatic organisms and is readily biodegradable (OECD SIDS 2001). Z. platypus (1-year old) with an average weight of 9 g and length of 20 cm were obtained from Damsunara company (Youngin, Korea) and were acclimated in culture tanks for 1 month prior to exposure experiments.
The experiments were conducted according to the guidelines of the Organization for Economic Co-operation and Development for the testing of chemicals, No. 203 (OECD 1992). A flow-through system receiving carbon-filtered and dechlorinated tap water was used in this study. Ten fish were held in each exposure tank under a 16:8 h (light:dark) photoperiod and were maintained at a water temperature of 22 ± 1°C, pH of 7.4 ± 0.3 and a dissolved oxygen level of 7.0 ± 0.4 mg L−1. The fish were not fed during the exposure experiments. At the end of the 4-day exposure, liver, muscle, and gill samples were collected from each fish, frozen in liquid nitrogen and stored at −80°C until analysis.
The concentrations of Cu and BaP in each test solution were determined at 0 and 4 days of exposure. BaP concentrations were measured via high-performance liquid chromatography (HPLC) according to Kim et al. (2008). The concentrations of the test substances were expressed in terms of the arithmetic means of the measured concentrations. The limit of detection was 0.1 μg L−1 for BaP. Cu concentrations were determined using a Perkin-Elmer inductively coupled plasma mass spectrometer (ICP-MS, ELAN DRC II, Perkin Elmer, Norwalk, CT, USA). The quantification of Cu was based on calibration curves of standard solutions of Cu (Sigma Aldrich, USA). The standard solutions were freshly prepared, and standard calibration curves with an r 2 > 0.995 were generated daily. The limit of detection was 1 μg L−1 for Cu.
Liver, muscle and gill tissues were homogenized in ice-cold phosphate buffer (100 mM, pH 7.8) containing 20 % glycerol and 0.2 mM phenylmethylsulfonyl fluoride (PMSF) as a serine protease inhibitor. The obtained homogenates were centrifuged at 15,000g at 4°C for 15 min, and the supernatants were used for biochemical assays. CAT activity was measured based on the decrease in absorbance at 240 nm due to H2O2 consumption (εmM = 0.0436) following Aebi (1974). SOD activity was determined using an indirect method involving the inhibition of cytochrome-c reduction (McCord and Fridovich 1969). GST activity toward 1-chloro-2,4-dinitrobenzene (CDNB) was measured as described by Habig et al. (1974). LPO was quantified via the TBARS assay, which measures the production of malondialdehyde (MDA) that reacts with thiobarbituric acid, using a commercial assay kit (OxiSelect™ TBARS Assay Kit, Cell Biolabs, Inc., San Diego, CA, USA).
Q-PCR was conducted to measure the relative mRNA expression of the CAT, SOD and GST antioxidant enzymes. Total mRNA was isolated from the liver tissues of Z. platypus (three individuals) following exposure to Cu and BaP using the Qiagen Total RNA Isolation kit (Hilden, Germany). Oligomeric primers were designed using Primer Express Software (Applied Biosystems, Forest City, CA, USA). Q-PCR was carried out using the Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems, CA, USA) and analyzed with the LightCycler 480 system (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer’s instructions. Expression levels were normalized to the mRNA levels of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and fold expression change in target genes (SOD, CAT, and GST) was calculated by comparing the expression levels between the control and treatment samples.
All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS), version 10.0 (SPSS, Inc., Chicago, IL, USA). Differences between the control and treatment samples were determined by one-way analysis of variance (ANOVA) followed by a Duncan’s test. p values < 0.05 were considered to be statistically significant. In addition, the Pearson test was applied to determine correlations among antioxidant responses.
Results and Discussion
Zacco platypus was exposed to environmentally relevant concentrations of Cu (1.25–20.0 μg L−1) and BaP (0.5–50.0 μg L−1). However, the measured concentrations of Cu and BaP (mean values of 1.0–15.0 and 0.4–29.5 μg L−1, respectively) were much lower than the nominal concentrations (Table 1). As explained in a previous report (Kim et al. 2013), this difference is most likely due to the sorption of the chemicals onto the circulation tubing or the walls of the tanks. The environmentally relevant concentrations of Cu and Bap were 0.5–1,000 μg L−1 (average concentration of 10 μg L−1) in lake water (Georgopoulos et al. 2001) and 0.001–6,000 (average concentration of 57 μg L−1) in wastewater (OEHHA 1997), respectively.
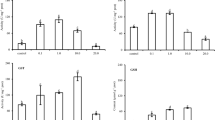
Following the exposure of Z. platypus to Cu, changes in antioxidant enzyme activities (SOD and CAT) and LPO were observed in liver tissues, whereas the muscle and gill tissues were less affected by oxidative stress (Fig. 1). Atli et al. (2006) reported that liver tissues tend to display a higher metabolic activity compared to other tissues, suggesting that liver tissues are more sensitive to toxic pollutants. In general, antioxidant defense systems preferentially develop in the liver, given this organ’s central role in detoxifying environmental pollutants and processing metabolic products for degradation (Li et al. 2010).
Antioxidant responses of a superoxide dismutase (SOD), b catalase (CAT), c glutathione S-transferase (GST) and d lipid peroxidation (MDA) in Zacco platypus following exposure to copper (Cu) over 4 days. Error bars represent standard deviations, and an asterisk indicates a significant difference (p < 0.05). The data for muscle and gill tissues came from a pooled sample (4 individuals) and the data for the most sensitive tissue (liver) came from three pooled samples (4, 3 and 3 individuals for each pool)
Unlike the results obtained following Cu exposure, BaP exposure had a negligible effect, regardless of the tissue type (Fig. 2). The antioxidant response in gill tissues may reflect adaptation, as the gills are in constant contact with ROS-inducible hazardous materials in aquatic ecosystems (Li et al. 2011). As indicated in Figs. 2 and 3, the intensity of the antioxidant response was minimal in muscle compared to liver and gill tissues, likely due to the low numbers of mitochondria (Li et al. 2011). These findings suggest that muscle and gill tissues may not represent ideal tissues for performing oxidative stress assessment but the liver is a good target organ for antioxidant response measurements.
Antioxidant responses of a superoxide dismutase (SOD), b catalase (CAT), c glutathione S-transferase (GST) and d lipid peroxidation (MDA) in Zacco platypus following exposure to benzo[a]pyrene (BaP) over 4 days. Error bars represent standard deviations. The data for muscle and gill tissues came from a pooled sample (4 individuals) and the data for the most sensitive tissue (liver) came from three pooled samples (4, 3 and 3 individuals for each pool)
Cu exposure induced concentration-dependent increases in SOD and CAT activities in the liver tissues of Z. platypus, whereas GST activity was not altered compared to the control (Fig. 1). SOD and CAT are both major antioxidant enzymes that are preferentially affected by Cu exposure (Atli et al. 2006). For instance, Craig et al. (2007) demonstrated that SOD activity increased significantly in the liver of zebrafish following Cu exposure (15 μg L−1). Furthermore, Sanchez et al. (2005) reported that CAT activity in three-spined stickleback increased following an increase in SOD activity after 4 days of exposure to Cu. SOD plays a critical role in the dismutation of superoxide anion radicals (O −·2 ) to hydrogen peroxide (H2O2), and CAT acts cooperatively as a scavenger of H2O2 (Martinez-Alvarez et al. 2005). Thus, the concomitant increase in SOD and CAT enzyme activities observed in this study reflects a cooperative antioxidant response against oxidative stress.
LPO also occurred at significant levels following Cu exposure (Fig. 1), suggesting that the increased SOD and CAT activities were not sufficient to effectively eliminate any oxidants present (O −·2 and H2O2). In particular, Cu induces redox cycles through the Fenton reaction, which can accelerate the formation of hydroxyl radicals and cause severe oxidative damage to cell membranes (Eyckmans et al. 2011). Interestingly, we found that MDA levels were positively correlated with both SOD and CAT activity (Table 2), suggesting that MDA and antioxidant enzyme levels may represent a sensitive diagnostic index of oxidative injury in Z. platypus following exposure to Cu.
During BaP exposure, no significant differences in the SOD, CAT, GST or LPO levels in liver tissues were observed among the treatment groups (Fig. 2). Fish can transform BaP to quinone metabolites such as benzo(a)pyrene-1,6-quinone, which may induce ROS formation through redox cycling (Di Giulio and Hinton 2008). However, the antioxidant response following BaP exposure was typically unclear (Pan et al. 2006).
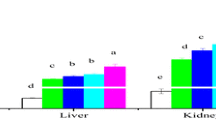
SOD, CAT and GST mRNA levels all appeared to increase following Cu exposure (Fig. 3). Several studies have demonstrated that antioxidant-related mRNA levels increase greatly following acute Cu exposure in bivalves (Bigot et al. 2011) and in HepG2 cells (Muller et al., 2007). In addition, Craig et al. (2007) reported that the mRNA expression of CAT in zebrafish showed a 2.5-fold increase after 2 days of exposure to Cu. However, the levels of all of the examined mRNAs decreased dramatically, to below control levels, following exposure to 15.0 μg Cu L−1 (Fig. 3). Bigot et al. (2011) showed that transient inhibition of SOD, CAT and GST mRNA expression occurs in bivalves following exposure to Cu at 50 μg L−1. Our findings suggest that Cu exposure at 15.0 μg L−1 may lead to sub-lethal effects in Z. platypus, potentially affecting the organism’s capacity to maintain the homeostasis of gene expression (Kim et al. 2010). This type of hormesis is commonly observed in toxicological studies (Calabrese and Blain 2005).
Significant correlations were not found between mRNA expression levels and the activities of the corresponding antioxidant enzymes (Table 2). Similar results have been reported elsewhere (Bagnall and Kotze 2004; Hansen et al. 2006). For example, Hansen et al. (2006) reported that the mRNA levels of SOD, CAT and glutathione peroxidase (GPx) were all increased in metal-exposed liver tissues of brown trout, whereas only the enzymatic activity of SOD was higher compared to controls. This result may be explained in part by the fact that enzyme activities are controlled not only by transcription but also by activation processes, specifically post-transcriptional modification (Hansen et al. 2006). Furthermore, a previous study showed that a time window most likely exists between transcription and translation when enzyme activities and gene expression are analyzed at the same point in time (Li et al. 2013).
In conclusion, Cu induced significant increases in the activities of antioxidant enzymes (SOD and CAT) and lipid peroxidation in Z. platypus, while BaP had no significant effects. In addition, liver tissues were more sensitive to Cu exposure compared to muscle and gill tissues. These findings suggest that the combined responses of SOD, CAT and LPO in liver tissues may represent a sensitive suite of indicators of oxidative stress in fish exposed to environmentally relevant concentrations of Cu (1.25–20.0 μg L−1).
References
Aebi H (1974) Catalase. Academic Press, London
Atli G, Alptekin Ö, Tükel S, Canli M (2006) Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp Biochem Physiol C 143:218–224
Bagnall NH, Kotze AC (2004) cDNA cloning and expression patterns of a peroxiredoxin, a catalase and a glutathione peroxidae from Haemonchus contortus. Parasitol Res 94:283–289
Bigot A, Minguez L, Giambérini L, Rodius F (2011) Early defense responses in the freshwater bivalve Corbicula fluminea exposed to copper and cadmium: transcriptional and histochemical studies. Environ Toxicol 26:623–632
Calabrese EJ, Blain R (2005) The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol 202:289–301
Craig PM, Wood CM, McClelland GB (2007) Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 293:R1882–R1892
Di Giulio RT, Hinton D (2008) The toxicology of fishes. CRC Press, New York
Eggen RI, Behra R, Burkhardt-Holm P, Escher BI, Schweigert N (2004) Challenges in ecotoxicology. Environ Sci Technol 38:58A–64A
Eyckmans M, Celis N, Horemans N, Blust R, De Boeck G (2011) Exposure to waterborne copper reveals differences in oxidative stress response in three freshwater fish species. Aquat Toxicol 103:112–120
Frankel EN (1995) Oxidation of polyunsaturated lipids and its nutritional consequences. In: Castenmiller WAM (ed) Proceedings of the 21st world congress of the international society of fat research. PJ Barnes and Associates, England, pp 265–269
Georgopoulos PG, Roy A, Lioy MJ, Opiekun RE, Lioy PJ (2001) Environmental copper: its dynamics and human exposure issues. J Toxicol Environ Health B 4:341–394
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. J Biol Chem 249:7130–7139
Hansen BH, Rømma S, Søfteland LIR, Olsvik PA, Andersen RA (2006) Induction and activity of oxidative stress-related proteins during waterborne Cu-exposure in brown trout (Salmo trutta). Chemosphere 65:1707–1714
Kim WK, Kim JH, Yeom DH, Lee SK (2008) Effect evaluation of benzo(a)pyrene on multiple biomarkers in common carp (Cyprinus carpio). J Environ Toxicol 23:171–178
Kim J, Park Y, Choi K (2009) Phototoxicity and oxidative stress responses in Daphnia magna under exposure to sulfathiazole and environmental level ultraviolet B irradiation. Aquat Toxicol 91:87–94
Kim J, Kim S, An KW, Choi CY, Lee S, Choi K (2010) Molecular cloning of Daphnia magna catalase and its biomarker potential against oxidative stresses. Comp Biochem Physiol C 152:263–269
Kim WK, Lee SK, Choi K, Jung J (2013) Integrative assessment of biomarker responses in pale chub (Zacco platypus) exposed to copper and benzo[a]pyrene. Ecotoxicol Environ Saf 92:71–78
Li C, Ni D, Song L, Zhao J, Zhang H, Li L (2008) Molecular cloning and characterization of a catalase gene from Zhikong scallop Chlamys farreri. Fish Shellfish Immunol 24:26–34
Li ZH, Zlabek V, Grabic R, Li P, Randak T (2010) Modulation of glutathione-related antioxidant defense system of fish chronically treated by the fungicide propiconazole. Comp Biochem Physiol C 152:392–398
Li ZH, Velisek J, Zlabek V, Grabic R, Machova J, Kolarova J, Li P, Randak T (2011) Chronic toxicity of verapamil on juvenile rainbow trout (Oncorhynchus mykiss): effects on morphological indices, hematological parameters and antioxidant responses. J Hazard Mater 185:870–880
Li M, Zheng Y, Liang H, Zou L, Sun J, Zhang Y, Qin F, Liu S, Wang Z (2013) Molecular cloning and characterization of cat, gpx1 and Cu/Zn-sod genes in pengze crucian carp (Carassius auratus var. Pengze) and antioxidant enzyme modulation induced by hexavalent chromium in juveniles. Comp Biochem Physiol C 157:310–321
Martinez-Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fisheries 15:75–88
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Muller P, van Bakel H, van de Sluis B, Holstege F, Wijmenga C, Klomp L (2007) Gene expression profiling of liver cells after copper overload in vivo and in vitro reveals new copper-regulated genes. J Biol Chem 12:495–507
OECD (1992) Guidelines for the testing of chemicals. Section 2: effects on biotic systems test no. 203: acute toxicity for fish. Organization for Economic Cooperation and Development, Paris
OECD SIDS (2001) Screening information data set (SIDS) of OECD. High production volume chemicals program: dimethylformaide. Organization for Economic Cooperation and Development, Paris
OEHHA (1997) Public health goal for benzo(a)pyrene in drinking water. Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, Sacramento, USA
Pan LQ, Ren J, Liu J (2006) Responses of antioxidant systems and LPO level to benzo(a)pyrene and benzo(k)fluoranthene in the haemolymph of the scallop Chlamys Ferrari. Environ Pollut 141:443–451
Regoli F, Nigro M, Orlando E (1998) Lysosomal and antioxidant responses to metals in the Antarctic scallop Adamussium colbecki. Aquat Toxicol 40:375–392
Sanchez W, Palluel O, Meunier L, Coquery M, Porcher JM, Aït-Aïssa S (2005) Copper-induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environ Toxicol Pharmacol 19:177–183
Zhang J, Shen H, Wang X, Wu J, Xue Y (2004) Effects of chronic exposure of 2,4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemophere 55:167–174
Acknowledgments
This study was supported by the Center for Aquatic Ecosystem Restoration (CAER) and the Eco-STAR project of the Ministry of the Environment, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, WK., Park, JW., Lim, ES. et al. Tissue-Specific Antioxidant Responses in Pale Chub (Zacco platypus) Exposed to Copper and Benzo[a]pyrene. Bull Environ Contam Toxicol 92, 540–545 (2014). https://doi.org/10.1007/s00128-014-1213-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1213-x