Abstract
Chromium-resistant bacteria isolated from industrial wastes can be used to detoxify toxic chromium from contaminated sources. From effluent of Shafiq Tannery, Kasur, Pakistan, bacterial strain STCr-1 that could endure 40 mg mL−1 of potassium chromate in nutrient agar medium was isolated. STCr-1, identified as Ochrobactrum anthropi by 16S rRNA gene sequence homology, demonstrated substantial Cr(VI) reduction at pH 7 and temperature 37°C. It completely reduced 250 μg mL−1 of Cr(VI) and showed 71.2 % Cr(VI) reduction at Cr(VI) concentrations of 550 μg mL−1. Rate of Cr(VI) reduction increased with increase in cell and Cr(VI) concentration. The presence of Cu2+, Co2+ and Mn2+ significantly stimulated Cr(VI) reduction. Assay with cell free extracts clearly indicated that Cr(VI) reduction was solely associated with the soluble fraction of the cell.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The extensive industrial usage of chromium compounds and subsequent release of effluents in the environment contaminated the ecosystem. The chromium discharge from industries like metal finishing industry, petroleum refining, leather tanning, iron and steel industries, textile manufacturing and paper industry elevated its concentration in aquifers and ground water, which poses a serious threat to human health. The discharged effluents from these industries have been found to contain high concentrations of this metal (Mir and Hai 1999). In spite of its crucial role in biological life, above crucial level chromium is known to have toxic, mutagenic, carcinogenic and genotoxic effects (Chen et al. 2001). Toxic effects of chromium are valence dependent. Hexavelant chromium is highly soluble, mutagenic and carcinogenic whereas the trivalent form is less soluble, thus less mobile and less toxic (Ackerley et al. 2006; Xu et al. 2009).
It is not only exigent to extract the toxic chromium from effluents before discharging in the environment but also to detoxify the contaminated lands and aquifers. The routine methods for treatment of chromium pollution generally involve the chemical reduction of Cr6+ to Cr3+ and subsequent precipitation of less soluble Cr3+ at or near neutral pH. These require high inputs of energy or expensive chemicals (Xu et al. 2009). Hence more practical and economical methods are being explored (Bailey et al. 1999). Bacterial potential for enzymatic reduction of Cr6+ to Cr3+ (Michel et al. 2001; Cheung and Gu 2003; Pal and Paul 2004; Camargo et al. 2005; Sultan and Hasnain 2007; Desai et al. 2008; He et al. 2009; Xu et al. 2009; Zhang and Li 2011) offer an ecofriendly alternate for treatment of contaminated sources. The isolation of Cr-resistant bacteria and assessment of their Cr-detoxification capabilities are primary steps in developing processes for bioremediation. But the availability of efficient bacterial strains is the main bottleneck in developing a bioremedial process. To this end we are isolating chromium-resistant bacteria from effluents of industrial wastes for their possible use in the bioremediation of Cr containing wastewater. Presently Cr(VI) resistance and reduction by whole cells and cell free extract of Cr-resistant Ochrobactrum anthropi strain STCr-1 from tannery effluent is being described.
Materials and Methods
Chromium-resistant bacterial strain STCr-1 was isolated from an effluent of tannery (Sultan and Hasnain 2005) and was resistant to 40 mg mL−1 potassium chromate in nutrient agar. The strain was routinely cultured in nutrient agar (pH 7) at 37°C. Effect of chromium on the growth of bacterial strain was determined in nutrient broth [0–40 mg mL−1 Cr(VI)] and M9 minimal medium [0–6 mg mL−1 Cr(VI)] (contained g/L: Na2HPO4, 6; KH2PO4, 3; NH4Cl, 1; NaCl, 0.5; MgSO4·7H2O, 0.246; CaCl2, 0.01). Culture flasks containing 50 mL medium and Cr(VI) salt were inoculated with 100 μL from overnight bacterial culture and incubated at 37°C with shaking at 150 rpm. Growth was measured at definite time intervals in terms of optical density at 600 nm (Megharaj et al. 2003) in a spectrophotometer (S-300D, R&M Marketing, UK).
Cr(VI) reduction ability of the chromate resistant bacterial strain was estimated in the medium used by DeLeo and Ehrlich (1994) (contained g/L: tryptone, 10.0; yeast extract, 5.0; NaCl, 5.0; citric acid, 1.0; Na2HPO4, 6.9). The effect of temperature (28, 37, 42°C), pH (6, 7, 8), initial cell concentration (8 × 105–1 × 108 cells mL−1) and Cr(VI) concentration (100–1,000 μg mL−1) on Cr(VI) reduction was investigated as described earlier (Sultan and Hasnain 2006). Cr(VI) reduction was studied in aerobic batch cultures in 250 mL conical flasks containing 50 mL medium. The autoclaved medium in flasks was amended with appropriate amount of Cr(VI) and was inoculated with overnight bacterial culture (containing desirable number of cells mL−1) and incubated at desirable temperature with shaking (150 rpm). Samples were aseptically removed at definite time intervals, centrifuged (6000 rpm for 10 min) and Cr(VI) reduction was monitored over time by measuring the disappearance of Cr(VI) in the supernatant fluid by using diphenylcarbazide method (APHA 1995). For each Cr(VI) reduction experiment cell free controls were also employed to monitor any abiotic Cr(VI) reduction. The effect of other metals (Co2+, Cd2+, Cu2+, Mn2+, Ni2+, Pb2+, Zn2+) on Cr(VI) reduction was also checked as described above.
Cell free extracts were prepared following Megharaj et al. (2003). Bacterial cells were grown in L-broth, harvested at mid exponential phase by centrifugation at 6,000×g for 10 min at 4°C. Cells were washed two times with 10 mM Tris–HCl buffer (pH 7.2) and resuspended in the same in 5 % of original culture. The cells were disrupted in an ice bath with a sonifier (MSE Soniprep 150, UK) (6 1-min pulses). The resultant homogenate was centrifuged at 12,000×g for 15 min at 4°C to pellet unbroken cells and obtain supernatant (S12). The S12 supernatant was then centrifuged at 30,000×g for 40 min at 4°C to prepare supernatant (S30) and pellet (P30). The pellet (P30) was suspended in Tris–HCl buffer at 5 % of original culture. Cr(VI) reduction assays were conducted with soluble fractions (S12, S30) and pellet suspension (P30) at 37°C with shaking. Autoclaved fractions (S12, S30, P30) served as control.
Genomic DNA was extracted from overnight inoculated bacterial cultures at 37°C in L-broth. DNA extraction was carried out by using genomic DNA extraction kit (BIORAD). PCR amplification of 16S rDNA was performed following the method described by Hasnain and Thomas (1996). A ~1.7-kb DNA fragment containing 16S rRNA gene was amplified with universal primers 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1522r (5′-AAGGAGGTGATCCA(AG)CCGCA-3′) (Johnson 1994). To 0.1 μg of chromosomal template DNA, 0.25 μM of each primer, 200 μM deoxynucleoside triphosphate and 1 unit of Taq polymerase (Mullis et al. 1986) were added. Initial denaturation was carried at 94°C for 5 min. Then 30 cycles were carried out for denaturation at 94°C (20 s), primer annealing at 50°C for 20 s and primer extension at 72°C for 2 min. The product was purified using Aqua Pure Gel Extraction Kit (Fermantas) and sequenced using 27f primer on automatic sequencer, ABI PRISM-3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
All experiments were performed in triplicate. Results were statistically analyzed for standard error of the means and Duncan’s New Multiple Range Test following Steel et al. (1996).
Results and Discussion
Chromium-resistant bacterial strain STCr-1 exhibiting a very high level of resistance to chromate i.e., 40 mg mL−1 of potassium chromate in nutrient agar was previously isolated from the effluent of Shafiq Tannery, Kasur, Pakistan (Sultan and Hasnain 2005). The isolate was gram-negative, motile and aerobic rod. When the bacterial strain was grown at varying concentrations of potassium chromate (zero to 40 mg mL−1) in nutrient broth, the growth of the strain decreased with increasing concentration of chromate (Fig. 1a). The OD of STCr-1 was drastically reduced after 20 mg mL−1 chromate. In M9 minimal medium the strain was able to tolerate lower concentrations of chromate (4 mg mL−1) (Fig. 1b). The strain could grow well up to 2.5–3 mg mL−1 of potassium chromate in M9 minimal medium with maximum population density between 0.5 and 1 mg mL−1. The relatively low tolerance level in minimal medium is comprehensible. In nutrient rich medium complexing of the Cr-salt might be lowering the level of available/free chromium, hence leading to apparent increased resistance of this strain in rich medium. In general bacterial population density decreased with increase in Cr(VI) concentration. The decreased population density at higher concentrations of Cr(VI) might be attributed to increase in generation time, decrease in cell division/cellular multiplication (Al-Aoukaty and Appana 1990) and alteration of genetic material (Losi et al. 1994). Cr(VI) resistance level of this strain was quite high as compared to strains reported by other workers (Megharaj et al. 2003; Elangovan et al. 2006; Desai et al. 2008; He et al. 2009; Xu et al. 2009; Zhang and Li 2011).
Cr(VI) reduction potential of this strain was assessed in medium used by DeLeo and Ehrlich (1994) by using different Cr(VI) salts i.e., K2CrO4, K2Cr2O7 and Na2Cr2O7 at 100 and 200 μg mL−1 concentrations. The strain STCr-1 manifested very good reduction potential with all Cr(VI) salts used (Fig. 2). At 100 μg mL−1 STCr-1 reduced 84 %, 74 % and 79 % of K2CrO4, K2Cr2O7 and Na2Cr2O7, respectively, within 24 h incubation and completely reduced 100 μg mL−1 of each Cr(VI) salt within 48 h incubation. At 200 μg mL−1 concentration this strain reduced 52 %, 43 % and 51 % within 24 h, and 86 %, 77 % and 83 % within 48 h of K2CrO4, K2Cr2O7 and Na2Cr2O7, respectively. Cr(VI) occurs in the aquatic environment either as CrO4 2− or Cr2O7 2− (McLean and Beveridge 2001; Thacker and Madamwar 2005) and this bacterial strain was able to reduce Cr(VI) in any form (CrO4 2− or Cr2O7 2−).
Cr(VI) reduction capability of strain STCr-1 was characterized by studying effect of temperature, pH, cell density and initial Cr(VI) concentration. Cr(VI) reduction by STCr-1 occurred reasonably well from 28 to 42°C (35–91 %) and from pH 6–8 (72–91 %) with optimum at 37°C and pH 7 (results not shown). Similar temperature and pH optima for Cr(VI) reduction were also shown by Brucella sp. (Thacker et al. 2007) and Serratia sp. (Zhang and Li 2011). Cell density has profound influence on Cr(VI) reduction. Effect of initial cell density ranging from 8 × 105 to 1 × 108 cells mL−1 was investigated. Cr(VI) reduction by STCr-1 was proportional to the initial cell density (Fig. 3). There was a noteworthy difference in chromate reduction occurring after 24 h at initial cell densities from 8 × 105 to 1 × 108 cells mL−1. About 100 μg mL−1 of Cr(VI) was completely reduced at initial cell density of 1 × 108 and 2 × 107 cells mL−1 within only 32 h of incubation. A high initial cell density has been recommended for significant Cr(VI) reduction to occur (Wang and Xiao 1995; Pattanapipitpaisal et al. 2001).
The rate of Cr(VI) reduction is greatly influenced by the initial Cr(VI) concentration but complete reduction of Cr(VI) is of rare occurrence even at the lowest concentration (Pattanapipitpaisal et al. 2001). The effect of Cr(VI) concentration was assessed over a range of 111.5–1,075 μg mL−1 of Cr(VI). STCr-1 completely reduced 111.5 μg mL−1 of Cr(VI) within 32 h while reduction of 250 μg Cr(VI) mL−1 was achieved in 72 h of incubation (Fig. 4). Arthrobacter sp. reduced nearly 30 μg mL−1 of chromate during 46 h and Bacillus sp. could reduce only up to 10 μg chromate mL−1 (Megharaj et al. 2003). Bacillus sphaericus failed to cause complete reduction even at initial concentration of 10 μg mL−1 (Pal and Paul 2004). Providencia sp., however, reduced 200 μg mL−1 Cr(VI) in 96 h (Thacker et al. 2006). Substantial Cr(VI) reduction was also observed at higher initial Cr(VI) concentrations. There was 71.2 % and 42.8 % reduction at higher initial Cr(VI) concentration of 550 and 1,075 μg mL−1, respectively with 96 hours of incubation (Fig. 4). Initial Cr(VI) concentrations used in this study are very high as compared to other workers (Pattanapipitpaisal et al. 2001; Megharaj et al. 2003; Pal and Paul 2004; Thacker et al. 2006).
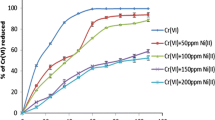
Effect of additional heavy metals on Cr(VI) reduction by STCr-1 revealed that the presence of Cu2+, Co2+, and Mn2+ significantly promoted Cr(VI) reduction. Presence of Cd2+ and Zn2+, however, inhibited Cr(VI) reduction while it was not affected by the presence of Ni2+ and Pb2+ (Table 1). Cr(VI) reduction by B. sphaericus was inhibited by the presence of additional metals such as Ni2+, Co2+, Cd2+ and Pb2+ and Ni2+ at the lowest concentration of 20 μg mL−1 was the most toxic (Pal and Paul 2004). Cr(VI) reduction by E. coli, however, was not affected by Cd2+ and Pb2+ at 1–20 μg mL−1 (Shen and Wang 1994). In Pseudomonas aeruginosa, Zn2+ had inhibitory while Cu2+ had stimulatory effect on Cr(VI) reduction (Xu et al. 2009). The mechanism of stimulatory effect of Cu2+ and other metals on Cr(VI) reduction activity is not clear. But Cu2+ is a prosthetic group for many reductase enzymes. The main role of Cu2+ has been described to be related to electron transport protection or acting as electron redox center and, in some circumstances, as a shuttle for electrons between protein subunits (Abe et al. 2001).
Cr(VI) reduction was also demonstrated with cell free extracts of STCr-1 under aerobic conditions. The soluble fractions (S12 and S30) proved to be very effective in reducing Cr(VI) and S30 fraction was relatively more efficient than the S12 fraction in Cr(VI) reduction (Fig. 5). The insoluble fraction (P30) as well as autoclaved soluble fractions (S12 and S30) did not show any Cr(VI) reduction. These findings substantiate the previous studies with E. coli (Shen and Wang 1994) and Bacillus (Campos et al. 1995; Pal and Paul 2004). Membrane associated chromate reduction activity was detected in anaerobic E. cloacae (Wang et al. 1990). Lack of Cr(VI) reduction activity by autoclaved soluble fractions of STCr-1 clearly indicate the enzymatic nature of Cr(VI) to Cr(III) reduction by this strain. Enzymatic Cr(VI) reduction associated with soluble fractions has also been shown in other bacterial strains (Michel et al. 2001; Thacker et al. 2007; Desai et al. 2008).
On the basis of high Cr(VI) reduction potential, strain STCr-1 was selected for ribotyping to ascertain its taxonomic identity. Based on the 16S rRNA gene sequence homology, the strain STCr-1 showed 99.7 % similarity with O. anthropi strains CCUG 44770 (AM114410). Hence this strain was designated as O. anthropi STCr-1. The partial 16S rRNA gene sequence of STCr-1 was submitted and the GenBank accession number of this strain was DQ989207. Cr(VI) resistant Ochrobactrum strains have been reported previously but they are resistant to quite lower concentration of Cr(VI) (Francisco et al. 2002; Branco et al. 2004; He et al. 2009). All these features make this strain a model contestant to be used in developing a bioremidial process for Cr(VI)-contaminated environments under a broad range of environmental conditions. Efficiency of its Cr(VI) reductase could further be enhanced by genetic/biochemical engineering.
References
Abe F, Miura T, Nagahama T, Inoue A, Usami R, Horikoshi K (2001) Isolation of a highly copper-tolerant yeast, Cryptococcus sp., from the Japan trench and the induction of superoxide dismutase activity by Cu2+. Biotechnol Lett 23:2027–2034
Ackerley DF, Barak Y, Lynch SV, Curtin J, Matin A (2006) Effect of chromate stress on Escherichia coli K-12. J Bacteriol 188:3371–3381
Al-Aoukaty A, Appana VD (1990) Sensitivity of Pseudomonas syringae to various metals complexed to citrate. Microbios Lett 45:105–111
APHA (1995) Standard methods for the examination of water and waste water, 19th edn. American Public Health Association, Washington
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low cost sorbents for heavy metals. Wat Res 33:2469–2479
Branco R, Alpoim MC, Morais PV (2004) Ochrobactrum tritici strain 5bvl1-characterization of a Cr(VI)-resistant and Cr(VI)-reducing strain. Can J Microbiol 50:697–703
Camargo FAO, Okeke BC, Bento FM, Frankenberger WT (2005) Diversity of chromium-resistant bacteria isolated from soils contaminated with dichromate. Appl Soil Ecol 29:193–202
Campos J, Martinez-Pacheco M, Cervantes C (1995) Hexavalent-chromium reduction by a chromate resistant Bacillus sp. strain. Anton Leeuwenhoek 68:203–208
Chen F, Vallyathan V, Castranova V, Shi X (2001) Cell apoptosis induced by carcinogenic metals. Mol Cell Biochem 222:183–188
Cheung KH, Gu JD (2003) Reduction of chromate by an enrichment consortium and an isolate of marine sulfate-reducing bacteria. Chemosphere 52:1523–1529
DeLeo PC, Ehrlich HL (1994) Reduction of hexavelant chromium by Pseudomonas fluorescens LB300 in batch and continuous cultures. Appl Microbiol Biotechnol 40:756–759
Desai C, Jain K, Madamwar D (2008) Evaluation of In vitro Cr(VI) reduction potential in cytosolic extracts of three indigenous Bacillus sp. isolated from Cr(VI) polluted industrial landfill. Bioresour Technol 99:6059–6069
Elangovan R, Abhipsa S, Rohit B, Ligy P, Chandraraj K (2006) Reduction of Cr(VI) by a Bacillus sp. Biotechnol Lett 28:247–252
Francisco R, Alpoim MC, Morais PV (2002) Diversity of chromium resistant and reducing bacteria in a chromium-contaminated activated sludge. J Appl Microbiol 92:837–843
Hasnain S, Thomas CM (1996) Two related rolling circle replication plasmids from salt-tolerant bacteria. Plasmid 36:191–199
He Z, Gao F, Sha T, Hu Y, He C (2009) Isolation and characterization of a Cr(VI)-reduction Ochrobactrum sp. strain CSCr-3 from chromium landfill. J Hazard Mat 163:869–873
Johnson JL (1994) Similarity analysis of rRNAs. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. American Society for Microbiology, Washington, pp 683–700
Losi ME, Amrhein C, Frankenberger WT (1994) Environmental biochemistry of chromium. Rev Environ Contam Toxicol 36:91–121
McLean J, Beveridge TJ (2001) Chromate reduction by a pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol 67:1076–1084
Megharaj M, Avudainayagam S, Naidu R (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47:51–54
Michel C, Brugna M, Aubert C, Bernadac A, Bruschi M (2001) Enzymatic reduction of chromate: comparative studies using sulfate-reducing bacteria. Key role of polyheme cytochrome c and hydrogenases. Appl Microbiol Biotechnol 55:95–100
Mir S, Hai SMA (1999) Pollution due to hazardous waste-water discharge by the local industry and its control. Sci Vision 4:1–7
Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol 51:263–273
Pal A, Paul AK (2004) Aerobic chromate reduction by chromium-resistant bacteria isolated from serpentine soil. Microbiol Res 159:347–354
Pattanapipitpaisal P, Brown NL, Macaskie LE (2001) Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI)-contaminated site. Appl Microbiol Biotechnol 57:257–261
Shen H, Wang YT (1994) Biological reduction of chromium by E. coli. J Environ Eng 120:560–570
Steel RGD, Torrie JH, Dickey DA (1996) Principles and procedures of statistics, a biometrical approach, 3rd edn. McGraw Hill International Book Company, New York
Sultan S, Hasnain S (2005) Plasmid mediated chromate resistance in bacteria isolated from industrial waste. Pak J Biologic Sci 8:1771–1777
Sultan S, Hasnain S (2006) Characterization of an Ochrobactrum intermedium strain STCr-5 manifesting high level Cr(VI) resistance and reduction potential. Enzyme Microb Technol 39:883–888
Sultan S, Hasnain S (2007) Reduction of toxic hexavalent chromium by Ochrobactrum intermedium strain SDCr-5 stimulated by heavy metals. Bioresour Technol 98:340–344
Thacker U, Madamwar D (2005) Reduction of toxic chromium and partial localization of chromium reductase activity in bacterial isolate DM1. World J Microbiol Biotechnol 21:891–899
Thacker U, Parikh R, Shouche Y, Datta M (2006) Hexavalent chromium reduction by Providencia sp. Process Biochem 41:1332–1337
Thacker U, Parikh R, Shouche Y, Datta M (2007) Reduction of chromate by cell free extract of Brucella sp. isolated from Cr(VI) contaminated sites. Bioresour Technol 98:1541–1547
Wang YT, Xiao C (1995) Factors affecting hexavalent chromium reduction in pure cultures of bacteria. Water Res 29:2467–2474
Wang P-C, Mori T, Toda K, Ohtake H (1990) Membrane-associated chromate reductase activity from Enterobacter cloacae. J Bacteriol 172:1670–1672
Xu WH, Liu UG, Zeng GM, Li X, Song HX, Peng QQ (2009) Characterization of Cr(VI) resistance and reduction by Pseudomonas aeruginosa. Trans Nonferrous Met Soc China 19:1336–1341
Zhang K, Li F (2011) Isolation and characterization of a chromium-resistant bacterium Serratia sp. Cr-10 from a chromate-contaminated site. Appl Microbiol Biotechnol 90:1163–1169
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sultan, S., Hasnain, S. Chromium (VI) Reduction by Cell Free Extract of Ochrobactrum anthropi Isolated from Tannery Effluent. Bull Environ Contam Toxicol 89, 152–157 (2012). https://doi.org/10.1007/s00128-012-0648-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0648-1