Abstract
The effects of selected polycyclic aromatic hydrocarbons on the marine calanoid copepod Acartia tonsa were tested in laboratory short-term toxicity tests in order to facilitate risk assessment of those compounds to the marine pelagic environment. Photo-induced toxicity of pyrene was also investigated under naturally relevant UV light regimes. Lethal and sublethal effects on egg production rate, hatching and potential recruitment rate were evaluated after 48 h exposure to fluoranthene, phenanthrene and pyrene. The 48 h-median lethal concentrations (LC50) reducing survival by 50% were 594, 2,366 and >640 nM for fluoranthene, phenanthrene and pyrene, respectively, whilst lower concentrations induced different sublethal effects. Median effective concentrations (EC50) affecting the egg production rate and the recruitment rate were 433 and 385 (fluoranthene), 1,245 and 1,012 (phenanthrene) and 306 and 295 nM (pyrene), respectively. An increase in toxicity of pyrene was detected after incubation under UV light, resulting in LC50 values of 201 nM (24 h) and 138 nM (48 h) and EC50 values of 79 nM (egg production rate) and 41 nM (recruitment rate). Finally, a comparison between effective concentrations and worst-case environmental concentrations reported in literature indicated that pyrene may pose a threat to A. tonsa from exposure in the field, and that the risk of adverse effects is high for fluoranthene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are persistent planar molecules consisting of two or more six-membered (benzene) rings directly linked together, common in aquatic ecosystems (Kennish 1992; Walker et al. 2001). Major sources of PAHs are anthropogenic like industrial effluents, petroleum spillage or the refining and combustion of fossil fuels (Albers 1995; Walker et al. 2001), and global PAHs discharges into the aquatic environment from all sources, both natural and anthropogenic, have been estimated at 80,000–230,000 tonnes per year (Kennish 1992; Wright and Welbourn 2002).
Polycyclic aromatic hydrocarbons constitute the most toxic components of oil for the marine biota with genotoxic, carcinogenic, or reproductive effects, and may be bioaccumulated in marine organisms (Corner et al. 1976; Kennish 1992; Albers 1995; Manahan 2001; Pane et al. 2005). While lowest molecular weight PAHs are the most toxic, they are generally unimportant because of their volatility and accordingly short half-life in water (Walker et al. 2001). Intermediate molecular weight aromatics (two to four ring PAHs) are more persistent and their solubility in water is high enough to cause toxicity to pelagic organisms. On the other hand, higher molecular weight PAHs are well known carcinogenics, but they do not pose a short-term risk to pelagic organisms because of their extremely low solubility in water (Albers 1995).
The present study focused on the toxicity of intermediate molecular weight PAHs to marine mesozooplankton. Although the toxicity of intermediate molecular weight PAHs has been reported for a variety of freshwater organisms, few studies have addressed their effects on marine zooplankton organisms. For example, phenanthrene was reported to cause toxic effects in the range 1–5,000 μg l−1 (6–28,000 nM) (Steevens et al. 1999), fluoranthene was found toxic to marine invertebrates between 64 and 3,300 μg l−1 (320–16,000 nM) (Pelletier et al. 1997; Spehar et al. 1999), and pyrene has been reported to exert toxicity in the range 25–12,000 μg l−1 (120–59,000 nM) (Pelletier et al. 1997; Lyons et al. 2002).
Polycyclic aromatic hydrocarbons water concentrations in offshore sites are usually below those values reported in literature to be acutely toxic. However, higher concentrations have been found in polluted coastal and estuarine areas. For instance, Law et al. (1997) reported 1–2 μg l−1 for fluorene, phenanthrene, fluoranthene or pyrene, and 7 μg l−1 for naphthalene in polluted coastal areas around England and Wales. Maskaoui et al. (2002) and Zhou and Maskaoui (2003) registered 1–2 μg l−1 for naphthalene and phenanthrene, 2–3 μg l−1 for fluoranthene and pyrene and 6.6 μg l−1 for fluorene in polluted estuaries from the east coast of China. Furthermore, growing evidence suggests that ultraviolet (UV) light may enhance the toxicity of certain PAHs (Arfsten et al. 1996; Peachey 2005). Thus, anthracene, fluoranthene and pyrene are two to three orders of magnitude more toxic to marine invertebrates in the presence of UV light than under fluorescent light (Pelletier et al. 1997; Spehar et al. 1999; Lyons et al. 2002).
The choice of a biological response to be measured in toxicity tests depends on a compromise between sensitivity and feasibility (His et al. 1999). Lethal responses may be simple from a methodological point of view, but their sensitivity is lower than that of sublethal endpoints. Thus, to evaluate the risk of chemical pollutants on marine ecosystems it is necessary to consider both lethal and sublethal effects of those compounds on sensitive organisms. Ecologically relevant sublethal effects of toxicants such as petroleum hydrocarbons (Berdugo et al. 1977; Cowles and Remillard 1983), metals (Hutchinson et al. 1994), sediment-associated PAHs (Lotufo 1997), and surfactants (Christoffersen et al. 2003) on the reproduction of marine copepods, have been reported at lower levels than those affecting survival, and the use of reproductive endpoints in toxicity testing has been encouraged.
Copepods constitute roughly 80% of the total marine zooplankton biomass worldwide and Acartia tonsa is one of the most common neritic copepods with a wide distribution within coastal plankton communities. A. tonsa feeds omnivorously on phytoplankton and pelagic protozoans, serves as food for fish larvae and represent an ecologically important species playing a significant role in the pelagic food web of the world ocean (Reeve and Walter 1977). This species has been the focus of intense studies of zooplankton energetics and much is known about egg production, egg hatching and larval development of A. tonsa and how these variables are influenced by environmental factors such as temperature, salinity, food concentration and food quality (Kiørboe et al. 1985; Berggreen et al. 1988; Durbin et al. 1990; Kleppel and Burkart 1995; Besiktepe and Dam 2002; Jones et al. 2002; Hazzard and Kleppel 2003; Calliari et al. 2006). A. tonsa is a free spawner and releases eggs directly into the water so egg production rates and success of egg hatching (which usually occurs within 24 h after spawning), are easily measurable parameters. Moreover, A. tonsa is relatively easy to culture in laboratory and has been proved to be a sensitive organism to a wide range of toxic compounds (e.g. Kusk and Petersen 1997; Andersen et al. 1999; Medina et al. 2002; Christoffersen et al. 2003; Medina and Barata 2004). This copepod is therefore a suitable test organism for hazard identification of chemicals in the marine environment and is included in standardised toxicity test protocols (ISO 1999; OECD 2004; Medina and Barata 2004).
In the present study, we carried out a laboratory study on the effects of three individual PAHs, phenanthrene (Phe), fluoranthene (Fluo) and pyrene (Py), on mortality rate and sublethal responses such as egg production rate, egg hatching success and potential recruitment rate of A. tonsa, in order to contribute to the evaluation of the risk to the marine environment of those compounds.
Materials and methods
Experimental solutions
Stock solutions were made by dissolving analytical grade fluoranthene (Fluo), phenanthrene (Phe) and pyrene (Py) (Aldrich Steinheim) in acetone. The experimental concentrations were obtained by diluting the stock solutions in 0.3 μm filtered natural seawater (FSW). During this dilution, equal amounts of acetone (less than 200 μl l−1), found not to be toxic to A. tonsa in preliminary tests, were added to each experimental beaker with PAHs solutions. All glassware was acid-washed (HNO3 10% vol.) and rinsed with acetone and distilled water before the experiments.
Experimental concentrations were chosen on the basis of preliminary trials and on data from the literature. Two separate experiments were conducted for Fluo and Phe (a range-finding trial and a definitive test) and one experiment for Py. The dosing concentrations in the range-finding trials were 10, 50, 250 and 1,250 nM for Fluo and 2.4, 24, 240 and 2,400 nM for Phe, whereas tested concentrations in definitive trials were 200, 400 and 800 nM for Fluo and 200, 600 and 1,800 nM for Phe. Py tested concentrations were 40, 80, 160, 320 and 640. In addition an experiment was carried out with Py (2.5, 25, 250 nM) under UV light. UV light was provided by a QPanel 313 (QPanel Co.) and UV light regimes, measured with a spectroradiometer (OL-754, Optronic Laboratories, Inc.), were 0.08 W m−2 (UVA) and 0.119 W m−2 (UVB). Tested concentrations for each compound were below their water-saturation levels (Nagpal 1993).
Biological material
Acartia tonsa obtained from a culture at the Danish Institute for Fisheries Research were maintained in laboratory at the Kristineberg Marine Research Station (Sweden) under indirect natural light conditions in FSW at 18°C and 30 psu salinity. Copepods were fed the cryptophyte Rhodomonas baltica ad libitum. R. baltica were cultured continuously in FSW on f/2 medium (Guillard and Ryther 1962) at a 12 h:12 h light:dark cycle, 18°C and 30 psu salinity.
Experimental procedure
Prior to all experiments, female adult copepods were acclimatised for 48 h to the experimental food concentration of 2.7 × 104 cells ml−1 R. baltica corresponding to 1,000 μg C l−1 (Mullin et al. 1966). To ensure fertilisation of the female copepods, males were added during the acclimatisation. For the experiments, groups of five adult fertilised females were collected under a dissecting microscope and transferred to 320 ml Pyrex® glass bottles containing 2.7 × 104 cells ml−1 R. baltica and the PAH solutions. Experimental treatments consisted of four controls (FSW, no PAH) and four replicates of each PAH concentration. The bottles were sealed with airtight Teflon-lined screw caps and were incubated in darkness for 48 h on a rotating plankton wheel (0.5 rpm). After 24 h, the experimental solutions were renewed and the eggs produced by the copepods were removed by sieving copepods onto a 200 μm nylon mesh. The number of dead females was recorded. At the end of the 48 h incubation period the copepods and eggs produced were collected on a 60 μm mesh. The number of eggs produced in each bottle was counted under a dissecting microscope and the number of dead females recorded. Subsequently, all eggs were collected and kept in Petri dishes containing 15 ml of experimental solutions for 24 h. Unhatched eggs and nauplii were then counted to calculate egg hatching success. The potential recruitment rate was estimated as egg production rate × egg hatching success.
Statistical analyses
Statistical analyses were conducted using the SPSS® version 12.0 statistical software. Differences in egg production rates, hatching success, recruitment rate and survival among treatments were tested for significance by means of one-factor analysis of variance (ANOVA). When differences among groups were significant the Dunnett’s test was employed to compare the control group and each of the experimental groups for calculation of the Lowest Observed Effect Concentrations (LOEC). The EC10 and EC50 and their 95% confidence intervals (95CI) were calculated according to the Probit method after normalising data to the control response mean percentage using Abbot’s formula (Emmens 1948). Data of the two experiments for Fluo and Phe were pooled together after control normalisation for EC10 and EC50 calculations. For analysis, hatching success and survival data were first arcsine-transformed to achieve normality (Hayes Jr 1991). Statistical tests were performed according to Sokal and Rohlf (1995) and Newman (1995).
Results
Effects on the egg production rate, hatching success and recruitment rate
Acartia tonsa females exposed to PAHs showed significantly lower egg production rates than controls after 48 h exposure (Table 1, Fig. 1). Fluo caused a reduction in egg production rates from 25 to 3 eggs female−1 day−1 at 800 nM, whilst the EC10 and EC50 were 227 and 433 nM, respectively (Table 2). Phe was less toxic with EC10 and EC50 values of 613 and 1,245 nM. Here, the reduction in egg production rate from 25 to 5 eggs female−1 day−1 was induced at concentrations above 1,800 nM. Py showed the highest toxicity, with an EC10 of 107 nM, and reduced the egg production rate from 50 to 20 eggs female−1 day−1 at 320 nM. The calculated EC50 was 306 nM (Table 2). The toxicity of Py on the egg production rate was thrice elevated under UV light, with EC10 and EC50 values of 33 and 79 nM, respectively. No significant effects of UV light exposure on the egg production rate were detected.
Significant effects were also observed on egg hatching success after 24 h exposure to PAHs (except for Py + UV), although, in general, a small decrease of this biological response was observed (Table 1, Fig. 2). Fluo reduced egg hatching by 60% at 1,250 nM, and yielded EC10 and EC50 values of 202 and 793 nM (Table 2). Significant effects of Phe on egg hatching were detected only at 2,400 nM whilst the EC10 was 926 nM. On the other hand Py caused a 20% reduction in hatching success at 320 nM and the EC10 was 221 nM. No effects of Py on hatching success were registered when incubations were conducted under UV light at the experimental concentrations. However, statistical differences were observed when comparing controls with UV light exposed controls (p < 0.001, F = 35.1).
Recruitment rate was in general the most sensitive response variable. Fluo showed toxicity on the recruitment rate at concentrations above 400 nM (Table 1) and EC10 and EC50 values were 222 and 385 nM (Table 2). Phe was less toxic, reducing the recruitment rate from 19 to 3 ind day−1 at 1,800 (Fig. 3), and showing EC10 and EC50 values of 385 and 1,012 nM. Py reduced recruitment rate significantly by 33 ind day−1 at 320 nM, with EC10 and EC50 values of 134 and 295 nM (Tables 1 and 2, Fig. 3). Under UV light, the EC10 and EC50 values decreased to 8.2 and 41 nM, respectively. Also, significant differences were detected between controls and UV light exposed controls (p < 0.05, F = 14.0).
Effects on survival
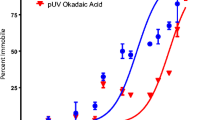
Polycyclic aromatic hydrocarbons exposure also significantly decreased survival (Table 1) but survival constituted, in general, a much less sensitive response parameter. Fluo caused 47% mortality at 800 nM after 24 h exposure, and the EC10 and EC50 values were 463 and 824 nM (24 h), and 426 and 594 nM (48 h), respectively. Phe caused a 47 and 52% increase in mortality after 24 and 48 h exposure to 2,400 nM (Fig. 4). The EC10 and EC50 values were 1,800 and 2,436 nM (24 h) and 1,772 and 2,366 nM (48 h) (Table 2). Py caused a decrease of 26% in survival at 640 nM after 48 h exposure (Fig. 4), whilst calculated EC10 was 209 nM (Table 2). No significant differences in survival were observed at 24 h exposure. Effects on survival were detected after 24 and 48 h exposure to Py under UV light at 250 nM (Table 1), with EC10 and EC50 values of 98 and 201 nM (24 h), and 77 and 138 nM (48 h), respectively (Table 2). No significant effects of UV light exposure on survival were observed.
Survival of A. tonsa females exposed to Fluo (A), Phe (B), Py (C) and Py + UV (D) for 24 h (circles) and 48 h (triangles). Error bars represent the standard deviation for each treatment (n = 4). *Significant differences at p < 0.05; **significant differences at p < 0.01; ***significant differences at p < 0.001
Discussion
Little information is available in literature about the toxicity of PAHs to marine pelagic invertebrates in general, and to copepods in particular. Ott et al. (1978) studied the toxicity of naphthalene and three methylated derivatives to the calanoid copepod Eurytemora affinis and registered LC50 between 2,000 and 30,000 nM after 24 h exposure. Also, they found significant effects of naphthalenes on life duration of E. affinis after chronic exposure (duration of their adult life) to concentrations of those PAHs of about 60–80 nM. Barata and co-workers registered Fluo LC10 and LC50 values of 313 and 500 nM for Tisbe battagliai, and 2,930, 970 and 760 nM (LC50) of Phe, Fluo and Py were reported for Oithona davisae (Barata et al. 2002, 2005). Narcotic effects, showed by the lack of motility, were also registered at lower concentrations than those causing death in O. davisae (Barata et al. 2005). Calculated EC50 of Phe, Fluo and Py for the narcotisation were 3,580, 660 and 530 nM, respectively. Thus, the acute 48-h LC50 values found for PAHs in the present study agree with values reported for copepods in the literature. But mortality may not be the most sensible response parameter for toxicity tests in zooplankton. The measurement of egg production rates of adult female copepods has been proposed as an alternative method for estimating pelagic secondary production, since it constitutes a measure of potential population recruitment rate (Poulet et al. 1995). Moreover, egg production is an important aspect of the adult biology of copepods, which integrates a number of metabolic processes and therefore has high ecological relevance (Berdugo et al. 1977). Thus, egg production of copepods has been previously shown to be a very sensitive biological response to several toxicants. For instance, Kusk and Petersen (1997) calculated an EC10 value of TBT on the egg production rate of A. tonsa of <10 ng l−1, based on data from Bushong et al. (1990). Furthermore, previous studies reported effects of pesticides on the egg production rate of copepods at lower concentrations than those affecting survival (Hutchinson et al. 1999; Willis and Ling 2003; Brown et al. 2003).
The present study shows some variability in the egg production rate for different batches of A. tonsa females. Such natural variability of A. tonsa egg production rate has been previously reported in literature. For instance, Hazzard and Kleppel (2003) reported variations in the egg production rate of A. tonsa populations with similar nutritional characteristics between 25 and 56 eggs female−1 day−1. Thor (2003) and Calliari et al. (2006) found variations in the egg production rate of A. tonsa between 20 and 35 eggs female−1 day−1. Those values are in agreement with the data obtained here (25–45 eggs female−1 day−1 in controls).
Studies on the effects of PAHs on sublethal response parameters like egg production rates of copepods are scarce. Berdugo et al. (1977) found effects on the fecundity of E. affinis exposed to 1 mg l−1 of naphthalene for 24 h. Also, in the above mentioned study, Ott et al. (1978) registered effects of naphthalene on the total numbers of nauplii produced and on the mean brood size of E. affinis at 380 times lower concentrations than those causing mortality, although the exposure period for the lethal response (24 h) was much lower than the exposure period for the sublethal responses (29 days). Barata et al. (2002) reported a reduction in the egg production of T. battagliai exposed to Fluo at concentrations below those affecting survival (EC50 = 66.9 μg l−1 ∼331 nM; LC50 = 101.1 μg l−1 ∼500 nM), which are similar to the levels registered here for Fluo in A. tonsa (EC50 = 433 nM; LC50 = 594 nM). Accordingly, our results showed lower EC50 on the egg production rate of A. tonsa than those causing mortality, at similar exposure times (48 h). On the other hand, Cowles and Remillard (1983) did not find a significant decrease in the egg production rate of Centropages hamatus exposed for 48–60 h to 80 ppb of crude oil water soluble fraction. In general, comparing the effective concentrations of the different PAHs tested in the present study, the lethal response was 2–8 times less sensitive than the egg production rate and the recruitment rate. This suggests that such short-term exposure in any estuarine situation might have long-term effects on A. tonsa population dynamics.
Several laboratory studies have demonstrated that UV light may significantly enhance PAHs toxicity to freshwater organisms (Arfsten et al. 1996; Steevens et al. 1999). However, few studies have been conducted on the interaction between PAHs and UV light with marine invertebrates (Spehar et al. 1999; Lyons et al. 2002; Steevens et al. 1999; Peachey 2005). Pelletier et al. (1997) reported 12 to >50,000 times higher toxicity of anthracene, Fluo and Py to larvae and juveniles of the bivalve Mulinia lateralis and the mysid Mysidopsis bahia under UV light than conventional toxicity. Spehar et al. (1999) reported that UV light increased the toxicity of Fluo toxicity to 21 freshwater and marine species. Lyons et al. (2002) reported significant increases in Py and benzo[a]pyrene toxicity to oyster (Crassostrea gigas) embryos in the presence of UV light. Peachey (2005) found significant higher toxicity of Fluo and Py to larvae of three crustaceans (Libnia dubia, Menippe adina and Panopeus herbstii) under UV light. In the present work, the toxicity of Py on the egg production rate and survival of A. tonsa was increased between 4 and >12 times under UV light exposure, whilst no increase in toxicity on hatching success of A. tonsa eggs was detected. Also, Py exposure in darkness yielded an EC10 value of 134 nM for the potential recruitment rate of A. tonsa, whereas EC10 was 16 times lower (8.2 nM) under UV light, although a significant reduction in the recruitment rate was observed under UV light exposure. The studies mentioned above showed much higher increase in PAHs toxicity in the presence of UV light, but it has to be borne in mind that the UV light regimes used here are much lower than levels used in those studies (UVA: 350–3,000 μW cm−2; UVB: 6–600 μW cm−2). Nevertheless, the low—and naturally relevant—light intensity used in our study still induced increased toxicity effects, and photo-induced toxicity of PAHs in the marine environment should be taken into account in standard water and sediment toxicity tests. Standard tests have until now been conducted in darkness or under fluorescent light which typically contains no UV thereby resulting in severe underestimation of toxicity.
The effect concentrations reported here are well above PAH concentrations usually found in coastal waters. Such high concentrations might only be expected to occur in the vicinity of oil-slicks or in areas affected by an oil-spill (e.g. Berdugo et al. 1977; Laffon et al. 2006; González et al. 2006). For instance, González et al. (2006) registered 0.09–4.84 μg l−1 chrysene equivalents in seawater samples from the Galician coast (NW Spain) 1 month after the Prestige oil spill and Laffon et al. (2006) measured 1.4 μg l−1 dissolved PAHs 1 year after the spill. However, although toxic effects of Py reported here (EC10 = 134 nM = 27 μg l−1) occurred at higher concentrations than those found for open oceanic waters, similar concentrations to those reported for photo-induced toxicity of Py under UV light (EC10 = 8.2 nM, 1.7 μg l−1), have been detected in polluted coastal and estuarine waters (Law et al. 1997; Maskaoui et al. 2002; Zhou and Maskaoui 2003). Thus, the level of risk associated with the occurrence of Fluo, Phe and Py may be estimated by comparing the reported environmental concentrations of those compounds with the toxicity threshold to A. tonsa obtained here (Newman 2001). Environmental concentrations presented in Table 3 represent maximum values (C max) measured in industrialised estuaries. The environmental risk may be calculated as risk quotients, RQ = C max/PNEC, where PNEC (Predicted No Effect Concentration) was estimated as the EC10 applying an assessment factor (AF) of 10 (PNEC = EC10/AF) (OECD 1992). RQ values greater than 1 indicate that adverse effects for the exposed organisms are already taking place. RQ of 0.3–0.7, 0.04–0.2, 0.6–1.2 and 1.3–15.6 were obtained for Fluo, Phe, Py and Py + UV, respectively (Table 3). Therefore, environmental levels of Py in industrialised estuaries are already causing deleterious effects on A. tonsa populations whereas RQ for Fluo are approaching 1 indicating that the risk for adverse effects is high.
In conclusion, based on our EC10 and EC50 values, Py was 1–2 times more toxic than Fluo and 3–8 times more toxic than Phe, whereas UV light increased the toxicity of Py for egg production rate, recruitment rate and survival of females by 3–16 fold. It is therefore proposed that the additional risk of phototoxicity of PAHs should be considered in the toxicity evaluation of PAHs. Results from this study also support that egg production rate and recruitment rate are more sensitive to toxicants than measurement of mortality rate. Moreover, these sublethal endpoints showed less variability within treatments than mortality rate, making them more suitable as measurement variables. Concentrations of Fluo, Phe and Py found in coastal waters are lower than the toxic concentrations reported here. However, environmental worst-case concentrations in polluted estuaries may have a direct impact on copepod population dynamics.
References
Albers PH (1995) Petroleum and individual polycyclic aromatic hydrocarbons. In: Hoffman DJ, Rattner BA, Burton CA, Cairns J Jr (eds) Handbook of ecotoxicology. Lewis Publishers, Boca Raton
Andersen HR, Halling-Sorensen B, Kusk KO (1999) A parameter for detecting estrogenic exposure in the copepod Acartia tonsa. Ecotoxicol Environ Saf 44:56–61
Arfsten DP, Schaeffer DJ, Mulveny DC (1996) The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: a review. Ecotoxicol Environ Saf 33:1–24
Barata C, Baird DJ, Medina M, Albalat A, Soares AMVM (2002) Determining the ecotoxicological mode of action of toxic chemicals in meiobenthic marine organisms: stage-specific short tests with Tisbe battagliai. Mar Ecol Prog Ser 230:183–194
Barata C, Calbet A, Saiz E, Ortiz L, Bayona JM (2005) Predicting single and mixture toxicity of petrogenic polycyclic aromatic hydrocarbons to the copepod Oithona davisae. Environ Toxicol Chem 24:2992–2999
Berdugo V, Harris RP, O’Hara SCM (1977) The effect of petroleum hydrocarbons on reproduction of an estuarine planktonic copepod in laboratory cultures. Mar Pollut Bull 8(6):138–143
Berggreen U, Hansen B, Kiørboe T (1988) Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: implications for determination of copepod production. Mar Biol 99:341–352
Besiktepe S, Dam HG (2002) Coupling of ingestion and defecation as a function of diet in the calanoid copepod Acartia tonsa. Mar Ecol Prog Ser 229:151–164
Brown RJ, Rundle SD, Hutchinson TH, Williams TD, Jones MB (2003) A copepod life-cycle test and growth model for interpreting the effects of lindane. Aquat Toxicol 63:1–11
Bushong SJ, Ziegenfuss MC, Unger MA, Hall LW Jr (1990) Chronic tributyltin toxicity experiments with the Chesapeake Bay copepod, Acartia tonsa. Environ Toxicol Chem 9:359–366
Calliari D, Andersen CM, Thor P, Gorokhova E, Tiselius P (2006) Salinity modulates the energy balance and reproductive success of co-occurring copepods Acartia tonsa and A. clausi in different ways. Mar Ecol Prog Ser 312:177–188
Christoffersen K, Hansen BW, Johansson LS, Krog E (2003) Influence of LAS on marine calanoid copepod population dynamics and potential reproduction. Aquat Toxicol 63:405–416
Corner EDS, Harris RP, Kilvington CC, O’Hara SCM (1976) Petroleum compounds in the marine food web: short-term experiments on the fate of naphthalene in Calanus. J Mar Biol Assoc UK 56:121–133
Cowles TJ, Remillard JF (1983) Effects of exposure to sublethal concentrations of crude oil on the copepod Centropages hamatus. Mar Biol 78:45–51
Durbin AG, Durbin EG, Wlodarczyk E (1990) Diel feeding behavior in the marine copepod Acartia tonsa in relation to food availability. Mar Ecol Prog Ser 68:23–45
Emmens CW (1948) Principles of biological assay. Chapman & Hall Ltd, London
González JJ, Viñas L, Franco MA, Fumega J, Soriano JA, Grueiro G, Muniategui S, López-Mahía P, Prada D, Bayona JM, Alzaga R, Albaigés J (2006) Spatial and temporal distribution of dissolved/dispersed aromatic hydrocarbons in seawater in the area affected by the Prestige oil spill. Mar Pollut Bull 53(5–7):250–259
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol 8:229–239
Hayes WJ Jr (1991) Dosage and other factors influencing toxicity. In: Hayes WJ Jr, Laws ER Jr (eds) Handbook of pesticide toxicology, vol 1. General principles. Academic, San Diego, pp 39–105
Hazzard SE, Kleppel GS (2003) Egg production of the copepod Acartia tonsa in Florida Bay: role of fatty acids in the nutritional composition of the food environment. Mar Ecol Prog Ser 252:199–206
His E, Beiras R, Seaman MNL (1999) The assessment of marine pollution—bioassays with bivalve embryos and larvae. In: Southeward AI, Tyler PA, Young CM (eds) Advances in marine biology, vol 37. Academic, London, p 178
Hutchinson TH, Williams TD, Eales GJ (1994) Toxicity of cadmium, hexavalent chromium and copper to marine fish larvae (Cyprinodon variegatus) and copepods (Tisbe battagliai). Mar Environ Res 38(4):275–290
Hutchinson TH, Pounds NA, Hampel M, Williams TD (1999) Impact of natural and synthetic steroids on the survival, development and reproduction of marine copepods (Tisbe battagliai). Sci Total Environ 233:167–179
ISO (1999) Determination of acute lethal toxicity to marine copepods (Copepoda, Crustacea), vol 14669. International Organisation for Standardization, ISO, Geneva, Switzerland
Jones RH, Flynn KJ, Anderson TR (2002) Effect of food quality on carbon and nitrogen growth efficiency in the copepod Acartia tonsa. Mar Ecol Prog Ser 235:147–156
Kennish MJ (1992) Ecology of estuaries: anthropogenic effects. CRC, Boca Raton
Kiørboe T, Møhlenberg F, Hamburger K (1985) Bioenergetics of the planktonic copepod Acartia tonsa: relation between feeding, egg production and respiration, and composition of specific dynamic action. Mar Ecol Prog Ser 26:85–97
Kleppel GS, Burkart CA (1995) Egg-production and the nutritional environment of Acartia tonsa—the role of food quality in copepod nutrition. ICES J Mar Sci 52:297–304
Kusk KO, Petersen S (1997) Acute and chronic toxicity of tributyltin and linear alkylbenzene sulfonate to the marine copepod Acartia tonsa. Environ Toxicol Chem 16(8):1629–1633
Laffon B, Rábade T, Pásaro E, Méndez J (2006) Monitoring of the impact of Prestige oil spill on Mytilus galloprovincialis from Galician coast. Environ Int 32:342–348
Law RJ, Dawes VJ, Woodhead RJ, Matthiessen P (1997) Polycyclic aromatic hydrocarbons (PAH) in seawater around England and Wales. Mar Pollut Bull 34(5):306–322
Lotufo GR (1997) Toxicity of sediment-associated PAHs to an estuarine copepod: effects on survival, feeding, reproduction and behavior. Mar Environ Res 44(2):149–166
Lyons BP, Pascoe CK, McFadzen IRB (2002) Phototoxicity of pyrene and benzo(a)pyrene to embryo-larval stages of the pacific oyster Crassostrea gigas. Mar Environ Res 54:627–631
Manahan SE (2001) Fundamentals of environmental chemistry, 2nd edn. Lewis Publishers, Boca Raton
Maskaoui K, Zhou JL, Hong HS, Zhang ZL (2002) Contamination by polycyclic aromatic hydrocarbons in the Jiulong River Estuary and Western Xiamen Sea, China. Environ Pollut 118:109–122
Medina M, Barata C (2004) Static-renewal culture of Acartia tonsa (Copepoda: Calanoida) for ecotoxicological testing. Aquaculture 229(1–4):203–213
Medina M, Barata C, Telfer T, Baird DJ (2002) Age- and sex-related variation in sensitivity to the pyrethroid cypermethrin in the marine copepod Acartia tonsa. Arch Environ Contam Toxicol 42(1):17–22
Mullin MM, Sloan PR, Eppley RW (1966) Relationship between carbon content, cell volume, and area in phytoplankton. Limnol Oceanogr 11:307–309
Nagpal NK (1993) Ambient water quality criteria for polycyclic aromatic hydrocarbons. Water Quality Branch, Water Management Division, Ministry of Environment, Lands and Parks, Victoria http://www.env.gov.bc.ca/wat/wq/BCguidelines/pahs/index.html#TopOfPage
Newman MC (1995) Quantitative methods in aquatic ecotoxicology. Advances in trace substances research. Lewis Publishers, Boca Raton
Newman MC (2001) Fundamentals of ecotoxicology. Lewis Publishers, Boca Raton
OECD (Organisation for Economic Co-operation and Development) (1992) Report on the OECD workshop on the extrapolation of laboratory aquatic toxicity data to the real environment. Environment Monograph No. 59
OECD (Organisation for Economic Cooperation and Development) (2004) Proposal for a new guideline: calanoid copepod development and reproduction test with Acartia tonsa. OECD Draft Guidelines for Testing of Chemicals, Paris, 39pp
Ott FS, Harris RP, O’Hara SCM (1978) Acute and sublethal toxicity of naphthalene and three methylated derivatives to the estuarine copepod, Eurytemora affinis. Mar Environ Res 1(1):49–58
Pane L, Boccardo S, Bonfiglioli F, Mariottini GL, Priano F, Conio O (2005) Polycyclic aromatic hydrocarbons in water, seston and copepods in a harbour area in the Western Mediterranean (Ligurian Sea). Mar Ecol 26:89–99
Peachey RBJ (2005) The synergism between hydrocarbons pollutants and UV radiation: a potential link between coastal pollution and larval mortality. J Exp Mar Biol Ecol 315:103–114
Pelletier MC, Burguess RM, Ho KT, Kuhn A, McKinney RA, Ryba SA (1997) Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ Toxicol Chem 16(10):2190–2199
Poulet SA, Ianora A, Laabir M, Klein Breteler WCM (1995) Towards the measurement of secondary production and recruitment in copepods. ICES J Mar Sci 52:359–368
Reeve MR, Walter MA (1977) Observations on the existence of lower threshold and upper critical food concentrations for the copepod Acartia tonsa Dana. J Exp Mar Biol Ecol 29:211–221
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research, 3rd edn. Freeman WH, New York
Spehar RL, Poucher S, Brooke LT, Hansen DJ, Champlin D, Cox DA (1999) Comparative toxicity of fluoranthene to freshwater and saltwater species under fluorescent and ultraviolet light. Arch Environ Contam Toxicol 37:496–502
Steevens JA, Slattery M, Schlenk D, Aryl A, Benson WH (1999) Effects of ultraviolet-B light and polyaromatic hydrocarbon exposure on sea urchin development and bacterial bioluminiscence. Mar Environ Res 48:439–457
Thor P (2003) Elevated respiration rates of the neritic copepod Acartia tonsa during recovery from starvation. J Exp Mar Biol Ecol 283:133–143
Walker CH, Hopkin SP, Sibly RM, Peakall DB (2001) Principles of ecotoxicology, 2nd edn. Taylor & Francis, London
Willis K, Ling N (2003) The toxicity of emamectin benzoate, an aquaculture pesticide, to planktonic marine copepods. Aquaculture 221:289–297
Wright DA, Welbourn P (2002) Environmental toxicology. Cambridge University Press, Cambridge
Zhou JL, Maskaoui K (2003) Distribution of polycyclic aromatic hydrocarbons in water and surface sediments from Daya Bay, China. Environ Pollut 121:269–281
Acknowledgements
We are indebted to Rodrigo J. Gonçalves for his help during UV light experiments. This study was supported by a Juan de la Cierva Contract from the Spanish Government (Ministerio de Educación y Ciencia) to Juan Bellas and grant no. 24.4/2004-0200 from The Swedish Research Council for Environment, Agricultural sciences and Spatial Planning to Peter Thor. Research was funded by the project VEM2003-20068-C05-02 from the Spanish Government (Ministerio de Ciencia y Tecnología).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bellas, J., Thor, P. Effects of selected PAHs on reproduction and survival of the calanoid copepod Acartia tonsa . Ecotoxicology 16, 465–474 (2007). https://doi.org/10.1007/s10646-007-0152-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-007-0152-2