Abstract
The levels of Cd, Cu, Pb and Zn in surface sediment, soft tissue and shell of the oyster Saccostrea cucullata collected from three locations, in the intertidal zones of Lengeh Port, northern part of Persian Gulf were measured. Results indicated that there were a positive correlation across Zn (r = 0.58, p = 0.025), Cd (r = 0.74, p < 0.01) and Cu (r = 0.80, p < 0.01) levels in the soft tissue of oyster and sediment which supported this fact that the soft tissue of S. cucullata can be considered as biomonitoring agent for Cd, Zn and Cu in the Lengeh Port.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Metal pollution of aquatic environments has become a global issue in both the developing and developed world (Boran and Altinok 2010; Shariati et al. 2011). The presence of metals in aquatic ecosystems is the result of two main sources of contamination: natural processes or natural occurring deposits, and anthropogenic activities. During the last few decades, urban and industrial developments along the coastal areas, rivers and estuaries have contributed to the major part of the anthropogenic metal load of the sea and have directly influenced the coastal ecosystems (Azarbad et al. 2010; Yuzereroglu et al. 2010). Metals are a group of the most important pollutants which cause environmental degradation in coastal areas (Raposo et al. 2009). High levels of metals in aquatic ecosystems are regarded as serious pollutants because they can be toxic and get incorporated into the food chain (Alyahya et al. 2011).
Among marine organisms, oysters are a valuable source for protein and vitamin and they are valued as food, and have and important commercial value. Besides, they are an important source for supplying essential elements (Garcia-Rico et al. 2010). They have been employed as biomonitors of environmental pollution by metals around the world (Hamed and Emara 2006; Pourang et al. 2010). One of the attributes, that had let to the use of marine mussels as a biomonitoring agent for metals is that they are commercially important seafood species world wide, other attributes, due to which mussels are often chosen for biomonitoring studies, are that they are sedentary organisms, long lived, easily identified and sampled, reasonably abundant and available throughout the year, tolerant of natural environmental fluctuations and pollution (Bartolome et al. 2010). This study was aimed to recognize relationship between metals (Cd, Cu, Pb and Zn) in surface sediment and their concentrations in soft tissue of Saccostrea cucullata from the intertidal zones of Lengeh Port (Persian Gulf) and to investigate whether soft tissue of S. cucullata can be considered as a biomonitoring material for metal pollution.
Materials and Methods
The Persian Gulf is a shallow basin with an average depth of 35–40 m and a total area of around 240 km2. It joins free international waters through the Strait of Hormuz. The turnover and flushing time have been estimated to be in the range of 3–5 year indicating that pollutants are likely to reside in the Persian Gulf for a considerable time (Pourang et al. 2005). In terms of pollution, the water quality of the Persian Gulf is influenced by various industrial outputs, shipping, fishing and collecting, mining, power plants, and discharging their wastewater directly to the sea or via rivers (Agah et al. 2009).
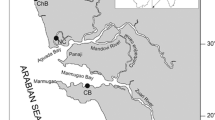
48 oysters (S. cucullata) and 15 surface sediment samples were collected from the Coastal environment (inter-tidal areas) of Lengeh Port, northern part of Persian Gulf at the same time on October 2010 (Fig. 1). About 16 S. cucullata samples of similar sizes were collected from three sampling sites, namely Kong Port, Koohin Area and Bostaneh Port to avoid environmental variations and/or sample bias. Details of the oyster samples which include number of oysters analyzed, shell lengths and site descriptions are given in Table 1.
The sampling sites included areas of different environmental backgrounds like fishing boats activities, urban areas, ports, ship repairing operations, industries and shipyard. The top 0–3 cm of surface sediments were collected near the oyster habitats. Five replicates of sediments were sampled from each site. The sediment samples were placed in tightly sealed solvent-rinsed stainless steel containers and oyster samples were placed in a plastic zip bag and transported to the laboratory in ice. The samples were then stored at −20°C until further analysis (Yap et al. 2002). Frozen oysters were scattered on the paper towel and thawed partially at room temperature before opening. In the laboratory, the soft tissues (ST) of oyster were carefully separated from the shell. Then shell length of each individual was measured by caliper. In the next stage all the oysters and sediments samples were dried in an oven at 105°C for 72 h until constant dry weights (dw). Also, the sediment samples were defrosted at room temperature, and then the samples were transferred to an oven and dried at a temperature of 105°C for 24 h. The metal analyses were performed in the 63 μm fraction, because the metals are usually strongly associated with small and fine grain sediments (Yap et al. 2002). About 1 g of each dried sample was weighed and placed in a hot block digester and digested with concentrated HNO3 (AnalaR grade, BDH 69 %) first at low temperature for 1 h and then at high temperature (140°C) for at least 3 h. The digested samples were then diluted to a certain volume with distilled deionized water (DDW). After filtration through Whatman No 1 filter paper, the levels of Cd, Cu, Pb and Zn in prepared samples were determined using a flame atomic absorption spectrophotometer Model 67OG. The data are presented in micrograms per gram dry weight basis. To avoid possible contamination, all glassware and equipment used were acid-washed. To check for contamination, procedural blanks were analyzed in every five samples. The accuracy of the analytical technique was verified by the analysis of the NIST standard reference materials oyster tissue (SRM-1566b) and estuarine sediment (SRM-1646a). The results show good precision for the SRM with an overall 95 % confidence level. In addition, the accuracy of the results was good with respect to the certified values. The recoveries were above 90 % for all trace metals measured except for Pb (83 %).
An analysis of variance (ANOVA) was performed to determine any significant differences in metal levels in the ST. Differences were considered significant only when p values were lower than 0.05. Prior to analysis data were inspected for homogeneity of variance (Levene test). All statistical calculations were carried out using statistic 5.0 for windows and SPSS statistical software version 17.0.
Results and Discussion
Concentration of metals in surface sediments is presented in Table 2. Among the three stations, levels of Cd, Cu, Pb and Zn ranged from 1.10 to 1.57 μg g−1 dw, 3.45 to 7.50 μg g−1 dw, 96.71 to 195.90 μg g−1 dw and 14.40 to 85.05 μg g−1 dw, respectively. This indicated that the sediment can accumulate these metals in the following order of abundance: Pb > Zn > Cu > Cd.
Analysis of variance (ANOVA), followed by the least significant different (LSD) test, was carried out to compare the metal levels in the oysters from different sites (Table 3).
Based on the results of the one-way ANOVA, concerning the three sampling sites statistically significant differences were observed in the levels of Pb (F = 71.6; df = 2, 45; p < 0.01) and Cd (F = 6.35; df = 2, 45; p < 0.01). On the other hands, there was no significant variation in the Cu (F = 0.92; df = 2, 45; p > 0.05) and zinc (F = 0.44; df = 2, 45; p > 0.05) levels among the sampling sites. LSD test showed a significant difference (p < 0.05) at the 95 % confidence level in the Pb between different sampling sites, and level of Cd also revealed a significant variation (p < 0.05) among stations 1 and 2, however, the values were not significantly different what was observed at site 3 (Table 3).
Between the different locations (Tables 2 and 3), it can be concluded that samples from site 3 showed the highest levels of Pb (195.90 μg g−1) and Cd (1.57 μg g−1), whereas the lowest level of Pb was observed in the sampling site 1 (96.71 μg g−1) and also for Cd at sampling sites 1 and 2 (1.10 μg g−1). High levels of Pb and Cd in site 3, could be due to various port activities like dumping of cargo ships, fishing boats, shipyard and other coastal activities (peer et al. 2010). Maximum level of Zn were recorded in the samples from site 2 (85.05 μg g−1), as there are no industrial sources nearby this site. However, it is assumed that probably these arise from the weathering of local metal bearing rocks and lateral offshore transport of suspended material or from ship repairing operations in this area (Zulkifli et al. 2010). While the highest sediment Cu content was recorded at site 1 (7.50 μg g−1), the high copper concentration observed are probably caused by the proximity to shipyards and harbors where copper-based, antifouling paints are used and also discharges of waste water in contact with metal surfaces, such as tanks and pipelines (Frias-Espericueta et al. 2009). Meanwhile, the lowest levels of Zn and Cu at the station 3 could probably due to very little contamination by these metals due to land base activities. The comparison between metal levels in the samples from the three sites illustrated that all metal levels differed significantly in sediment samples. The average values of Cd, Cu, Pb and Zn levels in soft tissue (ST) and shell (SH) of S. cucullata collected from the sampling sites are listed in Table 4. Metal levels in the ST of S. cucullata were compared to the mussel shell, and the ratios of soft tissue/shell (ST/SH) were calculated and results are presented in Table 4.
The results revealed that the mean levels (μg g−1 dw) of Cd, Cu, Pb and Zn in the oyster ST from 3 sites in ranged from 10.28 to 12.03 and 294.10 to 345.80, 20.64 to 58.23, and 735.60 to 760.40, respectively. Average levels of in the oyster SH ranged from 3.50 to 6.55 and 138.50 to 306.90, 117.10 to 130.60, 114 to 199.10, respectively. This illustrated that the soft tissue of S. cucullata can accumulate these metals in the following order of abundance: Zn > Cu > Pb > Cd. However, this pattern of metal concentration differed from that observed in the shell which had the following order of abundance: Cu > Zn > Pb > Cd. These results indicated that the accumulation of Cd, Cu, Pb and Zn in the ST and the SH of S. cucullata was slightly different. Applying the t test (%5 significant level) for comparing the means of two categories (ST and SH of oysters), it was concluded that levels of Cd, Cu, Pb and Zn showed significant differences (t = 20.4, df = 94, p < 0.01) for Cd, (t = 5.69, df = 94, p < 0.01) for Cu, (t = 27.02, df = 94, p < 0.01) for Pb and (t = 45.3, df = 94, p < 0.01) for Zn. Based on the results mentioned above, the Cd and Cu levels were found about 2 times higher in the ST than those in the SH of oysters. The Zn levels in the ST were 3–6 times higher than its levels in the SH of oysters. The Pb levels in the SH were 3–5 times higher than those in the ST of S. cucullata. These results indicated that the soft tissue had higher affinities for Cd, Cu and Zn than the shell of S. cucullata. The high concentration of Cd, Cu, and Zn found in the oyster soft tissue could be attributed to the Cd, Cu, and Zn in mollusks binds to metalothionein, proteins of low molecular weight that decrease metal toxicity; however, they would not reduce the potential risk for consumption (Cadena-Cardenas et al. 2009). Cd is non-essential element, and not needed for oyster growth and may even be deleterious (Huanxin et al. 2000), some animals such as bivalve mollusks are well known for their ability to accumulate metals and other substances in their tissues. For example, oysters and mussels can accumulated in their tissues Cd levels up to 100,000 times higher than the levels observed in the water where they live (Avelar et al. 2000). Conversely, it is relevant that Zn and Cu are essential elements and play important roles in growth, cell metabolism and survival of most animals including bivalves and they are greatly enriched in oyster tissue over the levels in water or even sediment without apparent detrimental effects (Priya et al. 2011; Vázquez-Sauceda et al. 2011). On the other hand, the shells of S. cucullata accumulated more Pb than the soft tissues. The high levels of Pb found in the oyster shell could be probably due to crystalline structures of the shell matrix which have a higher capacity for incorporation of this metal than the soft tissue (Yap et al. 2008). Therefore, it seems that oyster has no tendency to accumulate the high level of Pb in its body. Our results are consistent with the results found by Peer et al. (2010) and Yap et al. (2003) who found the SH of oyster was a concentrating organ of lead contamination in coastal waters.
Correlations between the average metal levels in ST of S. cucullata with respect to elemental levels in sediment are illustrated in Fig. 2. The significant positive correlation (r = 0.80, p < 0.01) was observed in Cu level between the ST of oysters and sediments (Fig. 2a). This result was in agreement with that reported byYap et al. (2002) who found significant correlations between Cu in total soft tissues of P. viridis and sediment. Figure 2c revealed that Zn in total soft tissues of S. cucullata was significantly correlated with the total Zn in the sediment (r = 0.58, p = 0.025). Noorhaidah et al. (2010) reported significant (p < 0.05) correlations between Zn levels in different soft tissues of T. telescopium and some geochemical fractions of Zn in the sediment. Also, correlation between the ST of S. cucullata and sediment through positive, was slightly low (r = 0.51, p = 0.048) for Pb (Fig. 2d). Apeti et al. (2005) found that there was low relationship (r = 0.20) between total Pb in sediment and Pb in total soft tissue of C. virginica. In addition, significant positive correlation (r = 0.74, p < 0.01) was observed among Cd levels in sediment and soft tissue of oyster (Fig. 2b). Similar results have been observed for other marine bivalves where Cd levels in soft tissues are related to those in sediments (Vázquez-Sauceda et al. 2011). This is due to the ingestion of sediment particles by filter feeding mollusks, which is an important mechanism for incorporation of cadmium into the oysters. Based on the results mentioned above, the ST of S. cucullata can be a useful biomonitoring agent for Zn, Cd and Cu.
Based on the results obtained above, high levels of zinc, cadmium and copper in the soft tissues of S. cucullata relative to those in the shells elucidate S. cucullata concentrates zinc, cadmium and copper in the soft tissue, while the shells of S. cucullata accumulated more Pb than the soft tissues of S. cucullata. Also, results of this study revealed that there are several reasons for the prevalent use of the soft tissue of S. cucullata as a biomonitoring agent for Zn, Cd and Cu levels in the Persian Gulf. The reasons being the observed: the observed higher concentration of Zn, Cd and Cu in the oyster ST than the SH of S. cucullata and also the highest correlation in Zn, Cd and Cu accumulations among oysters ST and sediments.
References
Agah H, Leermakers M, Elskens M, Fatemi S, Baeyens W (2009) Accumulation of trace metals in the muscle and liver tissues of five fish species from the Persian Gulf. Environ Monit Assess 157:499–514
Alyahya H, El-Gendy AH, Al Farraj S, El-Hedeny M (2011) Evaluation of heavy metal pollution in the Persian Gulf using the clam Meretrix meretrix Linnaeus, 1758. Water Air Soil Pollut 214:499–507
Apeti DA, Robinson L, Johnson E (2005) Relationships between heavy metal concentrations in the American oyster (Crassostrea virginica) and metal levels in the water column and sediment in Apalachicola Bay Florida. American. J Environ Sci 1(3):179–186
Avelar WEP, Mantelatto FLM, Tomazelli AC, Silva DML, Shuhama T, Lopes JLC (2000) The marine mussel Perna perna (mollusca, bivalvia, mytilidae) as an indicator of contamination by heavy metals in the Ubatuba Bay, Sao Paulo, Brazil. Water Air Soil Pollut 118:65–72
Azarbad H, Javanshir KhoiA, Mirvaghefi A, Danekar A, Shapoori M (2010) Biosorption and bioaccumulation of heavy metals by rock oyster Saccostrea cucullata in the Persian Gulf. Int Aqua Res 2:61–69
Bartolome L, Navarro P, Raposo JC, Arana G, Zuloaga O, Etxebarria N, Soto M (2010) Occurrence and distribution of metals in mussels from the Cantabrian Coast. Arch Environ Contam Toxicol 59(2):235–243
Boran M, Altinok I (2010) A review of heavy metals in water, sediment and living organisms in the Black Sea Turkish. J Fish Aquat Sci 10(1–2):565–572
Cadena-Cardenas L, Mendez-Rodriguez L, Zenteno-Savin T, Garcia-Hernandez J, Acosta-Vargas B (2009) Heavy metal levels in marine mollusks from areas with, or without, mining activities along the Gulf of California Mexico. Arch Environ Contam Toxicol 57(1):96–102
Frias-Espericueta MG, Osuna-Lopez I, Banuelos-Vargas I, Lopez–Lopez G, Muy-Rangel MD, Izaguirre-Fierro G, Rubio-Carrasco W, Meza-Guerrero PC, Voltolina D (2009) Cadmium, copper, lead, and zinc contents of of the mangrove oyster Crassostrea corteziensis of seven coastal Lagoons of NW Mexico. Bull Environ Contam Toxicol 83:95–99
Garcia-Rico L, Tejeda-Valenzuela L, Burgos-Hernandez A (2010) Seasonal variations in the concentrations of metals in Crassostrea corteziensis from Sonora, Mexico. Bull Environ Contam Toxicol 85(2):209–213
Hamed MA, Emara AM (2006) Marine molluscs as biomonitors for heavy metal levels in the Gulf of Suez Red Sea. J Mar Syst 60(3–4):220–234
Huanxin W, Lejun Z, Presley BJ (2000) Bioaccumulation of heavy metals in oyster (Crassostrea virginica) tissue and shell. Environ Geol 39(11):1216–1225
Ml Vázquez-Sauceda, Aguirre-Guzmán G, Sánchez-Martínez J, Pérez-Castañeda R (2011) Cadmium, Lead and zinc concentrations in water, sediment and oyster (Crassostrea virginica) of San Andres Lagoon Mexico. Bull Environ Contam Toxicol 86(4):410–414
Noorhaidah A, Yap CK (2010) Correlations between speciation of Zn in sediment and Zn concentrations in different soft tissues of the gastropod mollusc Telescopium telescopium Collected from Intertidal Areas of Peninsular Malaysia Pertanika. J Trop Agric Sci 33(1):79–90
Peer FE, Safahieh A, Sohrab AD, Tochaii SP (2010) Heavy metal concentrations in rock oyster Saccostrea cucullata from Iranian coasts of the Oman Sea Trakia. J Sci 8:79–86
Pourang N, Nikouyayn A, Dennis S (2005) Trace element concentration in fish, sediments and water from northern part of the Persian Gulf. Environ Monit Assess 109: 293–316
Pourang N, Richardson CA, Mortazavi MS (2010) Heavy metal concentrations in the soft tissues of swan mussel (Anodonta cygnea) and surficial sediments from Anzali Wetland Iran. Environ Monit Assess 163(1–4):195–213
Priya SL, Senthilkumar B, Hariharan G, Selvam AP, Purvaja R, Ramesh R (2011) Bioaccumulation of heavy metals in mullet (Mugil cephalus) and oyster (Crassostrea madrasensis) from Pulicat Lake, south east coast of India. Toxicol Ind Health 27(2):117–126
Raposo JC, Bartolome M, Cortazar E, Arana G, Zabaljauregui M, De Deigo A, Zuloaga O, Madariaga JM, Etxebarria N (2009) Trace metals in oysters, Crassotrea sps., from UNESCO Protected Natural Reserve of Urdaibai: space–time observations and source identification. Bull Environ Contam Toxicol 83:223–229
Shariati F, Esaili Sari A, Mashinchian A, Pourkazemi M (2011) Metallothionein as potential biomarker of cadmium exposure in persian sturgeon (Acipenser persicus). Biol Trace Element Res 143(1):281–291
Yap CK, Ismail A, Tan SG, Omar H (2002) Correlations between speciation of Cd, Cu, Pb and Zn in sediment and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia. Environ Int 28:117–126
Yap CK, Ismail A, Tan SG, Abdul Rahim I (2003) Can the shell of the green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia be a potential biomonitoring material for Cd, Pb and Zn? Estuar Coast Shelf Sci 57:623–630
Yap CK, Hatta Y, Edward FB, Tan SG (2008) Distribution of heavy metal concentrations (Cd, Cu, Ni, Fe and Zn) in the different soft tissues and shells of wild mussels Perna viridis collected from BAGAN TIANG and KUALA KEDAH Malaysian. Appl Biol 37(2):1–10
Yuzereroglu TA, Gok G, Cogun HY, Firat O, Aslanyavrusu S, Maruldali O, Kargin F (2010) Heavy metals in Patella caerulea (mollusca, gastropoda) in polluted and non-polluted areas from the Iskenderun Gulf (Mediterranean Turkey). Environ Monit Assess 167(1–4):257–264
Zulkifli SZ, Yussuff FM, Arai T, Ismail A, Miyazaki N (2010) An assessment of selected trace elements in intertidal surface sediments collected from the Peninsular Malaysia. Environ Monit Assess 169(1–4):457–472
Acknowledgments
We are grateful to Mr. Argangi for his valuable help in conduction of sampling operations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaharlang, B.H., Bakhtiari, A.R. & Yavari, V. Assessment of Cadmium, Copper, Lead and Zinc Contamination Using Oysters (Saccostrea cucullata) as Biomonitors on the Coast of the Persian Gulf, Iran. Bull Environ Contam Toxicol 88, 956–961 (2012). https://doi.org/10.1007/s00128-012-0591-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0591-1