Abstract
The major beds of oyster along the central-west coast of India are exposed to different anthropogenic activities and are severely exploited for human consumption. In this viewpoint, tissues of oyster Crassostrea madrasensis, C. gryphoides and Saccostrea cucullata were analyzed for Cu, Ni, Cd and Pb concentrations (dry weight) from Chicalim Bay, Nerul Creek and Chapora Bay in pre-monsoon, monsoon and post-monsoon seasons. A higher concentration of Cu (134.4–2167.9 mg kg−1) and Cd (7.1–88.5 mg kg−1) was found, which is greater than the recommended limits in all the three species (and sites). Moreover, significant (p < 0.05) variations were observed for all the metals concentrations among the species, seasons and sites. The high concentrations of Cd and Cu in tissues of edible oyster pose a threat to human health. Therefore, continuous monitoring, people awareness and a stringent government policy should be implemented to mitigate the metal pollution along the studied sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Metal pollution in aquatic ecosystems, due to increasing levels of contaminants, represents a serious and growing problem worldwide. Metals, especially heavy metals, are serious pollutants due to their toxicity (at high concentration), persistence and bioaccumulation in aquatic organisms (Rainbow 2002). Marine bivalves such as oysters, mussels and clams have been widely used in biomonitoring programs. For example “Mussel Watch” programs use bivalves due to their ability to accumulate and tolerate high concentrations of heavy metals compared to other marine organisms (UNEP 1993). Among the bivalves, oysters (filter feeder) are well known as a universal sentinel accumulator of both essential and non-essential elements from the ambient environment (Amiard et al. 2008). Consequently, oysters from contaminated sites serve a potential risk to human health. Nevertheless, worldwide, oysters are a highly esteemed nutritious seafood, as they constitute rich source of proteins and a number of essential elements (Asha et al. 2014).
India is fortunate to have large resources of oysters. In 2008, 2400 tonnes of oyster production (http://www.fao.org/fishery/countrysector/naso_india/en) was recorded from the Indian coast. Crassostrea and Saccostrea are the major species occurring along the coastal waters of India (Asha et al. 2014). Edible oysters such as C. madrasensis (Preston 1916), C. gryphoides (Schlotheim 1813), and S. cucullata (Born 1778) are under severe exploitation along the Goa coast. Since estuaries of Goa receives numerous deposits of heavy metals from its Fe-Mn ores industries, barge building activities and waste disposals from the adjacent human settlements, high concentrations of heavy metals have been reported from marine components (sediment, suspended particulate matter and seawater) of Goa (Alagarsamy 2006; Kessarkar et al. 2013; Veerasingam et al. 2015; Prajith et al. 2016).
Although heavy metals concentrations in waters of Goa is known, no such study has been conducted on oyster species. Therefore, it is necessary to carry out a study concerning heavy metals levels in commercially important oyster species that are harvested regularly for human consumption. Keeping this aspect in forefront the present study has been undertaken with the following objectives (1) to determine levels of essential metals: copper (Cu), nickel (Ni) and non-essential metals: cadmium (Cd) and lead (Pb) in the tissue of three oyster species (C. madrasensis, C. gryphoides, and S. cucullata) and (2) to find out whether the oysters occurring along the coastal waters of Goa are safe for human consumption.
Materials and Methods
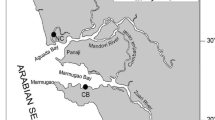
Three sites including a reference site (where oysters are harvested on regular basis for human consumption) were chosen from the Goa coast, India (Fig. 1) to determine the heavy metals concentrations in oyster species. The details of selected sites are as follows:
Chicalim Bay (CB) (15°24′3.52″ N, 73°51′14.24″ E) is located on the southern bank towards confluence of Zuari. This site hosts various ship and barge building industries, yards, workshops, anthropogenic activities and iron ores transportations. Nerul Creek (NC) (15°30′37.70″ N, 73°46′48.75″E) opens into the Aguada Bay of Mandovi Estuary, extends inside the land in U-shape up to a length of about ~8.5 km. This site is under the influence of fishing and other tourism activities. Also, this creek opens into the Mandovi River which is used for iron ores transportation from mines located upstream. Chapora Bay (ChB) (15°36′30.43″N, 73°44′7.19″E) a reference site located far from the main city. Unlike the other two sites, this site is not influenced by mining activity and has no ship building activities.
During the low tide, sampling was carried out in the intertidal region of the above mentioned sites in monsoon (July 2013), post-monsoon (November 2013) and pre-monsoon (March 2014). Surface water temperature (°C) and pH were measured on site using a calibrated thermometer and a portable pH meter, respectively. Salinity (psu) was determined by a refractometer (ATAGO, S/Milli-E). Dissolved oxygen (DO) of surface water was analyzed using Wrinkler’s method and expressed in mg l−1 (Parson et al. 1984). Oysters (n = 20 as one pool, 45–50 mm length) of each species from each site and season were collected and brought to the laboratory. Whole soft tissues of oyster were cleaned with deionized water to remove impurities. Then, tissues were dried at 60°C and digested with 65 % HNO3 (suprapure grade) as per the method described by Cheung and Wong (1992) with minor modification. Fish protein certified reference material for trace metals (DORM-4) was used to ensure the quality of the results. Accuracy of Cu, Ni, Cd and Pb analyses, are expressed in recovery percentage 87.23 %, 87.83 %, 98.03 % and 71.46 %, respectively. The precision measured as relative standard deviation (%RSD) of triplicate sample values were <10 %. The detection limits for Cu, Ni, Cd and Pb were 100, 100, 10 and 50 µg kg−1, respectively. Utmost care was taken at every step of sample processing to avoid contamination. The concentrations of metals (Cu, Ni, Cd and Pb) in all the samples were determined using a Graphite Furnace Atomic Absorption Spectrometer (Perkin Elmer, PinAAcle 900T).
The statistical analysis of data was conducted using PRIMER 6 (Primer-E Ltd., Plymouth, UK) software. Mean and standard deviation (SD) were calculated on all measured values of heavy metal concentrations. Data was also checked for normality (Shapiro-Wilks test) and homogeneity of variance (Levene’s test). However, when data failed to meet normality, permutational MANOVA (PERMANOVA) test was performed on untransformed data to find out the significance level in spatio-temporal variation of metals concentrations. Finally, principle component analysis (PCA) was adopted to know the spatio-temporal relationship in metal concentrations measured in oyster tissue. Further, scatterplot on PCA scores of first two significant PCs were plotted across the axes to know the seasonal influence of a particular metal on the analyzed samples.
Results and Discussion
Metal uptake and accumulation in bivalves depend on several factors including size, sex, reproductive state, changes in tissue composition, bioavailability of metals, seasons and hydrodynamics of the environment (Boyden and Phillips 1981). In this perspective, physico-chemical properties such as temperature, pH, salinity and DO of surface water were measured at all the three sampling sites (Table 1). Considerably, low values of surface water temperature, pH and salinity and high values of DO were recorded in the monsoon period than in non-monsoon (pre-monsoon and post-monsoon). Dilution of seawater by heavy rainfall and influx of oxygen-rich riverine water together with cloud cover and low incoming solar radiation during monsoon could be the reasons for the observed lower surface water temperature, salinity and higher DO.
Table 2 summarizes the mean concentrations of Cu, Ni, Cd and Pb in the tissues of three oyster species from the three selected sites during three different seasons. The hierarchy of measured heavy metals content in the tissues of oysters was in the following order: Cu > Cd > Ni > Pb. The differences in heavy metals’ burden in oysters may be due to the different affinity of metals to oyster tissues, different uptake, deposition and excretion rates. Moreover, results of PERMANOVA showed a significant variation (p < 0.05) in metals concentrations across all the seasons, sites and species as well as the interactions among these three main effects (Table 3). Although metals concentrations between study sites (CB and NC) and the reference site (ChB) showed significant (p < 0.05) variation, it is noteworthy that metal values observed at ChB was similar in range with CB and NC. This suggests that the reference site is also impacted by anthropogenic activities probably over the nearby fish landing jetty and sewage disposal from land inhabitants along the bank of the river Chapora. Similar hydrological parameters observed at all the three sites further supports the above statement. Metal concentrations in tissues of aquatic invertebrates living in the same habitat vary within closely related taxa and species within the same genus due to the species-specific differences for metal uptake and accumulation (Rainbow 2002 and references therein).
To understand the spatio-temporal relationship in the metal contents recorded in three different oyster species, a PCA analysis was performed (Fig. 2). Based on the eigenvalues (>1), the first two principal components (PCs) were retained as they explained 60.5 % of the total variability. Loading values described the relationship between metal levels and the principal components. PC1 showed strong loadings of both Ni and Pb, while PC2 presented strong loadings of Cu and moderately high loading for Cd. This sets the stage for the seasonal groupings on the PCA scatterplot (PC1 vs. PC2). Three groups were formed which apparent with an emphasis on impacts among three different seasons irrespective of sites and oyster species. Notably, Group A (pre-monsoon) and Group C (monsoon) are separated largely on the basis of PC1. Group A was formed of six samples directed towards the upper right side of the plot showed signature of Ni and Cu. Group C composed of 5 samples located at the upper left side of a scatterplot showed less concentration of Pb and enrichment of Cd. On the basis of PC2, Group B (post-monsoon) comprised of eight samples, formed distinct group from Group A and C mainly due to low concentration of Cu and/or Cd. These results clearly demonstrate that different seasons and local conditions have more impact on accumulation of metals in oysters.
The concentrations of Cu in oyster tissues varied from 134.4 to 2167.9 mg kg−1 (Table 2), which is higher than the recommended limit (32 mg kg−1) set by FAO Guideline (1983). The maximal levels of Cu in oyster tissues observed in pre-monsoon season is also supported by the Group A in the PCA scatterplot. The higher accumulation might be attributed to the increase in surrounding surface water temperature during pre-monsoon season. The rise in surrounding temperature increases the metabolic activities, resulting in higher filtration rate, larger collection of suspended matter and higher uptake of heavy metals (Belivermis et al. 2015). Another plausible reason could be due to the higher availability of Cu in the ambient environment. Since the studied sites are a famous destination for tourism and fishing, Cu-rich antifouling paint residues coming from barge building, fishing activities and tourism boats contaminates the estuarine environment (Alagarsamy 2006). Similarly, high values of Cu (170–610 mg kg−1) were reported in tissue of S. cucullata from Deltaic Sundarbans (Sarkar et al. 1994). Since high concentrations of Cu (2100–4400 mg kg−1 dry wt.) cause oyster mortality (Hung and Han 1990), the excessive concentration of Cu noticed in the current study indicates a higher risk to oysters in the Goa coast. Moreover, consumption of oyster tissue as seafood with such a high concentration of Cu can lead to stunted growth, cirrhosis of the liver and jaundice in humans (Gorman 1993).
The Ni concentrations in oysters varied between 0.70 and 5.61 mg kg−1 (Table 2). The maximum Ni value (5.61 mg kg−1) was obtained in C. madrasensis tissue from ChB in pre-monsoon, while the minimum value (0.70 mg kg−1) was measured in S. cucullata from NC in post-monsoon. The concentrations of Ni detected in this study were found well below the acceptable limit (70–80 mg kg−1) (USFDA 1993). This shows that Ni does not have strong binding affinity in oyster tissue. Though the Ni concentration is far below the permissible limit, measured levels of Ni were comparatively high in pre-monsoon which was also statistically represented in Group A of the PCA scatterplot (Fig. 2). This tendency is hypothesized to be due to increase in filtration activity of oyster in non-monsoon period as discussed earlier in case of Cu. A similar Ni level (5.67 mg kg−1) was recorded in oyster C. madrasensis from Pulicat Lake (India) (Laxmi Priya et al. 2010).
The concentrations of Cd in oyster tissues ranged between 7.1 and 88.5 mg kg−1. Since the concentration of Cd at Chicalim Bay (CB) was unexpectedly very high (154.6 mg kg−1), it has been excluded from the range values. These values were observed to be much more elevated than the tolerable limit of 0.5 mg kg−1 (FAO 1983). Among four studied metals, Cd showed relatively high accumulation in monsoon than the non-monsoon period. These observations are coinciding with Group C of the PCA scatterplot. This could be attributed to three different reasons. First, although heavy rainfall during the monsoon period dilutes the metal concentrations in the aquatic system, a decrease in salinity (i.e. less chloride concentration) during the monsoon period increases the availability of free Cd ions to filter feeders (Engels and Fowler 1979) and thus higher metal uptake by oysters. Second, Cd mimics calcium (Ca) ions (which is abundant in seawater) due to its similar geochemical properties, particularly ionic radius (cf. Ca 9.7 and Cd 9.8 nm) (Huanxin et al. 2000). Third, Cd also has strong affinity towards the metallothionein (MT) proteins and sequestered metal in detoxified form like Cu (Rainbow 2002). Results of the present study is further corroborated by previous studies where authors have found high concentrations of Cd in clam Paphia malabarica (1.4–8.4 mg kg−1) along the Goa coast (Kumari et al. 2006) and in oyster S. cucullata (10–40 mg kg−1) along Deltaic Sunderbans (Sarkar et al. 1994). It has been found that Cd intakes of 0.43–0.71 µg kg−1 day−1 cause a toxic effect on consumers mainly to high risk groups, including women with low iron stores, people with renal impairment, smokers and children (Cheng and Gobas 2007). Therefore, consumption of oysters from the studied sites should be limited.
The highest value of Pb (1.70 mg kg−1) was observed in S. cucullata in post-monsoon whereas, lowest value (0.10 mg kg−1) was obtained in pre-monsoon season at CB (Table 2). Although the average concentration of Pb was below the recommended limit of 1.0 mg kg−1 (EU 2001), a few samples exhibited values >1.0 mg kg−1 which indicates that oysters from the study regions are contaminated by Pb to some extent. Heavy traffic load of motor vehicles, boats, ships, combustion of fossil fuel, organic waste discharge in the vicinity of sampling sites as reported by Veerasingam et al. (2015) could be the reasons for the Pb concentrations in some oyster samples.
Most metal toxicants could easily interchange and disperse through aquatic ecosystems. For example, re-suspension of surface sediment releases particulate matter along with metals into the overlying waters (Zvinowanda et al. 2009). Since oysters are filter feeders, it is necessary to further investigate the concentrations of bioavailable metals from sources like surficial sediment, particulate (suspended particulate matter) and dissolved metals from the oyster beds ambience.
Based on the results of our study we can conclude that waters along the Goa coast are highly contaminated with heavy metals. Furthermore, concentrations of Cu and Cd in oysters are above the limits recommended by international authorities for safe consumption by humans. Therefore, consumption of oysters from the studied sites should be avoided. It is important to identify source(s) of these metals and measure should be taken to reduce the metal pollution in seafood. The present work calls for continuous monitoring, people awareness and a stringent government policy to control metal pollution in the coastal waters along the Goa coast.
References
Alagarsamy R (2006) Distribution and seasonal variation of trace metals in the surface sediments of the Mandovi Estuary, West coast of India. Estuar Coast Shelf Sci 67:333–339
Amiard JC, Amiard-Triquet C, Charbonnier L, Mesnil A, Rainbow PS, Wang WX (2008) Bioaccessibility of essential and non-essential metals in commercial shellfish from Western Europe and Asia. Food Chem Toxicol 46:2010–2022
Asha KK, Anandan R, Mathew S, Lakshmanan PT (2014) Biochemistry Biochemical profile of oyster Crassostrea madrasensis and its nutritional attributes. Egypt J Aquat Res 40:35–41
Belivermis M, Warnau M, Metian M, Oberhansli F, Teyssie JL, Lacoue-Labarthe T (2015) Limited effects of increased CO 2 and temperature on metal and radionuclide bioaccumulation in a sessile invertebrate, the oyster Crassostrea gigas. ICES J Mar Sci. doi:10.1093/icesjms/fsv236
Boyden CR, Phillips DJH (1981) Seasonal and inherent variability of trace elements in oysters and their implications for indicator studies. Mar Ecol Prog Ser 5:29–40
Cheng WW, Gobas FA (2007) Assessment of human health risks of consumption of cadmium contaminated cultured oysters. Human Ecol Risk Assess 13(2):1–13
Cheung YH, Wong MH (1992) Trace metal contents of the pacific oyster (Crassostrea gigas) purchased from markets in Hong Kong. Environ Manag 16(6):753–761
Engel DW, Fowler BA (1979) Factors influencing cadmium accumulation and its toxicity to marine organisms. Environ Health Perspect 28:81–88
European Union (EU) (2001) Commission Regulation as regards heavy metals. Directive 2001/22/EC, No. 466/2001
FAO (1983) Compilation of legal limits for hazardous substances in fish and fishery products. Food and Agriculture Organization of the United Nations, Rome, FAO Fishery Circular No. 464, pp 5–100
Gorman M (1993) Environmental hazards: marine pollution. ABC-CLIO, Santa Barbara
Huanxin W, Lejun Z, Presley BJ (2000) Bioaccumulation of heavy metals in oyster (Crassostrea virginica) tissue and shell. Environ Geol 39(11):1216–1226
Hung TC, Han BC (1990) Copper availability and assimilative capacity in sea water along the charting coastal area. Counc Agric Fish Ser 23:221–229
Kessarkar PM, Shynu R, Rao VP, Chong F, Narvekar T, Zhang J (2013) Geochemistry of the suspended sediment in the estuaries of the Mandovi and Zuari Rivers, Central west coast of India. Environ Monit Assess 185:4461–4480. doi:10.1007/s10661-012-2883-7
Kumari LK, Kaisary S, Rodrigues V (2006) Bioaccumulation of some trace metals in the short-neck clam Paphia malabarica from Mandovi estuary, Goa. Environ Int 32:229–234
Laxmi Priya S, Senthilkumar B, Hariharan G, Paneer Selvam A, Purvaja R, Ramesh R (2010) Bioaccumulation of heavy metals in mullet (Mugil cephalus) and oyster (Crassostrea madrasensis) from Pulicat Lake, south east coast of India. Toxicol Ind Health 27(2):117–126. doi:10.1177/0748233710381892
National Aquaculture Sector Overview-India, Food and Agriculture Organization of the United Nations. http://www.fao.org/fishery/countrysector/naso_india/en. Accessed 2 March 2016
Parson TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pregamon press, Oxford
Prajith A, Rao VP, Chakraborty P (2016) Distribution, provenance and early diagenesis of major and trace metals in sediment cores from the Mandovi estuary, western India. Estuar Coast Shelf Sci 170:173–185
Rainbow PS (2002) Trace metal concentrations in aquatic invertebrates: why and so what? Environ Pollut 120:497–507
Sarkar SK, Bhattacharya B, Debnath S (1994) The suitability of tropical marine bivalves as biomonitors of heavy metals in deltaic sundarbans, north-east India. Chemosphere 29(4):759–770
UNEP (1993) United National Environment Program: Environmental Data Report. UNEP, Oxford
USFDA (1993) Food and drug administration, Guidance document for nickel in shell fish. DHHS/PHS/FDA/CFSAN/office of seafood, USFDA Washington
Veerasingam S, Vethamony P, Mani Murali R, Fernandes B (2015) Depositional record of trace metals and degree of contamination in core sediments from the Mandovi estuarine mangrove ecosystem, west coast of India. Mar Pollut Bull 91:362–367
Zvinowanda CM, Okonkwo JO, Shabalala PN, Agyei NM (2009) A novel adsorbent for heavy metal remediation in aqueous environments. Int J Environ Sci Technol 6(3):425–434
Acknowledgments
The authors are thankful to S.W.A. Naqvi, Director, CSIR-NIO for his encouragement and providing all facilities to carry out this work. We are also grateful to Dr. B. N. Nath for providing chemicals and laboratory facilities for sample analysis. The first author would like to acknowledge Council of Scientific and Industrial Research (CSIR), India for providing the fellowship to carry out the Ph.D. research. We thank the anonymous reviewers for their insightful comments on the manuscript. This is NIO contribution no. 5954.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shenai-Tirodkar, P.S., Gauns, M.U. & Ansari, Z.A. Concentrations of Heavy Metals in Commercially Important Oysters from Goa, Central-West Coast of India. Bull Environ Contam Toxicol 97, 813–819 (2016). https://doi.org/10.1007/s00128-016-1956-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1956-7