Abstract
Mercury (Hg) speciation and bioavailability were studied in surface water, surface sediment and freshwater fish samples collected upstream and downstream of the Medupi (currently under construction) and Matimba power stations in the Waterberg area, Limpopo Province. The initial survey was conducted in May 2010 and continued periodically/seasonally until October 2014. This study was designed to provide an overall description of the levels of Hg in areas potentially impacted by emissions from the coal-fired power station and provide the necessary information to enhance the understanding of the factors regulating the fate and transport of Hg in the environment. Percent loss on ignition (LOI) and ancillary water quality measurements were also carried out. In this study, the total mercury (TotHg) concentrations ranged between 0.92 and 29.13 ng/L, and 0.13 and 8.00 ng/L for methylmercury (MeHg) in water. Total Hg concentrations ranged between 0.50 and 28.60 ng/g, while the MeHg concentration ranged between 0.08 and 2.22 ng/g in sediments. Mercury concentrations in fish ranged between 40 and 1200 ng/g for TotHg, and 13.42 and 600 ng/g for MeHg. Methylmercury concentrations in freshwater fish sampled exceeded the United States Environmental Protection Agency criteria (300 ng/g) in 10% of the total fish sampled and 5% exceeded the WHO guideline (500 ng/g).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There has been growing concern over the release of mercury (Hg) into the atmosphere and its potential hazardous impact on human health as well as the receiving environment (Hylander and Meili 2003). Mercury is regarded as a neurotoxin with demonstrated adverse human health impacts. Anthropogenic sources are estimated to account for 50–75% of the annual input of Hg to the global atmosphere (Meili 1991; USEPA 1997; Seigneur et al. 2004). Coal combustion represents the largest industrial contributor to global Hg emissions (Pacyna and Pacyna 2001), and although it is present in trace amounts in coal, it can still contribute significantly to the Hg load. South Africa is considered as one of the world’s largest coal producers and relies on coal for more than 90% of its electricity generation (DME 2005). Coal production in South Africa has traditionally been concentrated in the Highveld region of Mpumalanga Province. Historical estimates of mercury have reported that during 2010, 19.8 t of mercury was emitted by Eskom’s coal-fired power stations, estimated at 77% of total mercury emissions in the country (Roos 2011). Mercury emissions from coal-fired power stations are estimated to account for approximately 75% of anthropogenic sources in South Africa (Scott 2011).
Eskom currently operates 13 coal-fired power stations (including Matimba), most of which are located in the Mpumalanga and Limpopo provinces, with two new coal-fired power stations (Medupi and Kusile) currently under construction. Emissions from coal-fired power stations are regarded as the major anthropogenic Hg source, contributing in excess of 50% of the overall anthropogenic Hg emissions. Previous studies have stated that Hg emissions from sources in South Africa, mostly coal combustion, contribute more than 10% to global Hg emissions (Pacyna et al. 2006). However, in a later study, Pirrone et al. (2010) reported much lower values (<4%). The Matimba power station is fuelled by the open cast Grootegeluk coal mine on the Waterberg Coalfield and is also contracted to supply the Medupi power station. The Waterberg and watershed areas represent significant spatial components to conduct surface water, sediment and biological monitoring. The historic and economic relevance of the areas supports the need to monitor Hg in these water resources. The bioaccumulation potential of Hg within these areas has previously been poorly quantified.
Several processes affect the fate, transport and speciation of Hg in aquatic ecosystems. These processes have previously been highlighted in similar areas in South Africa (Somerset et al. 2011). In aquatic ecosystems, methylmercury (MeHg) is found in elevated concentrations in top predators. The process by which Hg is accumulated in the lower trophic levels of aquatic food webs is still unclear (Wiener et al. 2003). Although diet has been demonstrated to be the most dominant mechanism of MeHg uptake in fish (Hall et al. 1997), other factors such as size, age and feeding habits are also important in the ultimate MeHg sequestration in fish tissue. The biomagnification and concomitant toxicity of MeHg through the aquatic food chain is thus of particular concern. Wet deposition is thought to be the primary mechanism by which Hg emitted to the atmosphere is transported to surface waters and land, although dry deposition may also contribute substantially (Braune et al. 2005).
Concerns for human and ecosystem health have provided a basis for the increased attention on studying Hg in the environment. Mercury emissions and guideline values for Hg levels in the environment are regulated by several agencies worldwide. These include the World Health Organization (WHO) and United States Environmental Protection Agency (USEPA) (Table 1). In South Africa, current legislation has set quality guidelines for the presence of Hg in aquatic resources (40 ng/g). However, there is no legislation governing Hg emissions. Possible future control limits for atmospheric emission of mercury in South Africa may result in a need to design and implement a programme to monitor ecosystem responses to changes. This project was initiated by Eskom to establish a baseline of mercury levels in the vicinity of these stations and to implement a long-term monitoring plan, as part of a sustainable approach, to establish any changes that could be attributed to the increase in combustion of coal in the area when Medupi comes online. The results obtained from this report could aid in the development of policies on a regional and national scale.
2 Methodology
2.1 Study Area
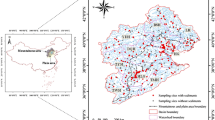
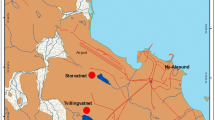
The Waterberg area is located in the Limpopo Water Management Area (WMA) of the Limpopo province (Fig. 1; Table 1). It contains four main drainage rivers, namely the Lephalala, Matlabas, Mogalakwena and Mokolo Rivers. The Limpopo River serves as a border between South Africa, Botswana, Zimbabwe and Mozambique and flows in an easterly direction exiting into the Indian Ocean. The Mokolo River runs through the Mokolo Dam and confluences in the north with the Limpopo River. The region is characterised by dry winters and wet summers and has an average annual rainfall of approximately 485 mm, of which 420 mm falls between October and March. The area between one of its major cities Lephalala and the Limpopo River contains South Africa’s richest remaining coalfield. Two of the 13 coal-fired power stations are found in the Waterberg area. These are the Medupi (currently under construction) and Matimba power stations.
2.2 Sample Collection and Processing
Standard protocols for collecting samples for total mercury (TotHg) and MeHg analysis were employed throughout sample collection (USEPA 1996; Mason and Sullivan 1998). The collection protocol utilises acid-cleaned Teflon® bottles and preservation with HCl as per USEPA recommendations. Acid cleaning consists of an initial soaking in 25% HNO3 for 2 weeks, triple rinsing with a MilliQ water and a further 2-week soak in 10% HCl followed by a triple rinse in MilliQ water before drying completely in a laminar flow hood. A triple rinse in MilliQ water is also performed when transferring bottles between acid baths. Teflon® bottles are then double-bagged in ziplock bags until needed. Unfiltered water samples were collected by submerging a 500-mL acid-cleaned Teflon bottle approximately 0.5 m below the water surface (without making contact with the sediment bed). Sample bottles were firmly sealed following acidification with a 0.5% v/v HCl solution. The samples were then labelled, double-bagged and transported on ice to the analytical laboratory for analysis.
Surface sediment cores (up to 6 cm in depth) were extruded using an acid-cleaned polypropylene cylindrical corer and sectioned at 2-cm intervals, after which each section was individually transferred into clean 50-mL centrifuge tubes, labelled, double-bagged and transported on dry ice to the analytical laboratory. Surface water and sediment samples for TotHg and MeHg determination were collected at selected sites in May 2010 (wet season), August 2010 (dry season), March 2011 (wet season), May 2011 (dry season), August 2011 (dry season), January 2012 (wet season), April 2012 (wet season), July 2012 (dry season), October 2012 (wet season), January 2013 (wet season), April 2013 (wet season), June 2013 (dry season), September 2013 (wet season), January 2014 (wet season), April 2014 (wet season), July 2014 (dry season) and October 2014 (wet season) (Table 1; Fig. 1). Not all sites were sampled during each sampling period. For example, Site MM was not included during the initial sampling phase (May 2010). From the April 2013 sampling phase, freshwater fish samples were also collected to be included in the monitoring process. Sample collection was undertaken over a period of 17 sampling phases which allowed for both spatial and seasonal comparisons to be made.
2.3 Field Analysis
Field measurements of surface water temperature (°C), pH, TDS (g/L), SP conductivity (mS/cm) and dissolved oxygen (DO; mg/L) were collected at each sampling site during each sampling event. All measurements were conducted using a YSI Model 556 Multi-parameter instrument. At each site, water samples were also collected for chemical analysis at Eskom’s Research Testing and Development (RT & D) laboratories using accredited methods. Analyses included ammonia, chlorides, magnesium, nitrates, dissolved oxygen, phosphates and sulphates.
2.4 Sediment Loss on Ignition
Loss on ignition (LOI) was determined in each sediment layer by drying wet sediment at 105 °C and heating dried sediment samples at 550 °C.
2.5 Mercury Analysis
2.5.1 Total Mercury
Total Hg in surface water followed the USEPA Method 1631 (USEPA 2002). This protocol included the oxidation of Hg with bromine monochloride (BrCl), pre-reduction with hydroxylamine hydrochloride (NH2OH · HCl) and further reduction with stannous chloride (SnCl2). The TotHg analysis for water samples was performed by Cold Vapour Atomic Fluorescence Spectrometry (CVAFS) using a Tekran Model 2500 CVAFS Mercury Detector and HP 3396 Integrator.
TotHg concentrations in surface sediment and freshwater fish samples were determined following USEPA protocols (Method 7473; USEPA 2007). The TotHg in solid samples, i.e. sediment and freeze-dried fish, were measured using a DMA-80 Solid Phase Direct Mercury Analyzer (Milestone Inc., Monroe, CT, USA).
2.5.2 Methylmercury
Methylmercury concentrations in surface water, surface sediment and freshwater fish samples were determined following US Environmental Protection Agency (USEPA) protocols (Method 1630; USEPA 2001a). For surface water and surface sediment, samples were distilled in 20% KCl and 50% H2SO4. For freshwater fish, samples were freeze-dried and digested in 25% KOH in methanol. The MeHg analysis for all samples was performed by CVAFS using a Tekran Model 2500 CVAFS Mercury Detector and HP 3396 Integrator.
2.6 Statistical Analysis
Statistical analysis was performed using Microsoft Excel XLSTAT. The mean standard deviation was obtained for all replicate samples and was statistically compared. A one-way analysis of variance (ANOVA) was undertaken to determine any significant differences. The criterion for significance was set at <0.05 (p < 0.05).
2.7 Quality Assurance
Reported values (unless otherwise stated) are averages of concentrations of replicates. A standard calibration curve with a regression coefficient (R 2) of at least 0.998 was obtained daily during analysis. Routine quality assurance and quality control (QA/QC) was carried out on each group of ten samples, which included matrix spikes and matrix spike duplicates, matrix blanks, replicate samples and certified reference material (CRM) of known Hg content (TORT-2, lobster hepatopancreas; 270.0 ng/g ± 60 for TotHg and 152.0 ng/g ± 13 for MeHg). The limit of detection (LOD), expressed as three standard deviations of the blanks, was 0.05 ng/L for TotHg and 0.07 ng/L for MeHg. The instrument LOD for the Tekran 2500 is 0.02 ng/L, and for the DMA-80, it is 0.003 ng Hg. Replicate samples yielded no significant differences, while all sample reference materials (SRMs) analysed fell within the specified certified ranges.
3 Results and Discussion
3.1 Physico-chemical Field Measurements
Table 2 presents the average physico-chemical data for surface water collected on-site at each sampling site and for all sampling trips. In general, the four sampling stations were similar regarding environmental characterisation.
3.2 Mercury in the Aqueous Phase
The concentrations of TotHg in natural unpolluted waters are generally between 0.2 and 15 ng/L and most often below 5 ng/L (Stein et al. 1996), although the USEPA dictates a maximum guideline of 12 ng/g which may result in chronic effects to aquatic life (USEPA 1992). The TotHg and MeHg concentrations in surface water in this study are shown in Fig. 2. The lowest average TotHg and MeHg concentrations were 1.87 ng/L at CM (2012 wet season) and 0.25 ng/L at MD (2013 wet season) and the highest were 29.13 ng/L at MM (2013 dry season) and 8.01 ng/L at CM (2014 wet season). Approximately 70% of TotHg concentrations exceeded the global average of 5 ng/L (Mason et al. 1994), while 35% of TotHg exceeded the 12 ng/L concentration indicated by the USEPA (USEPA 1992). The aqueous TotHg concentrations reported in this study are similar to previous studies conducted at sampling sites with impacts from coal-fired power stations (Williams et al. 2010; Walters et al. 2011), while generally higher MeHg concentrations are reported in the current study. Although MeHg concentrations in aqueous samples were overall low, chronic exposure to MeHg can become a concern for biota, if exposed over extended durations (Williams et al. 2010). The average percentage of MeHg in relation to TotHg was 25.38%. This is above the proportion (1–5%) observed by Mason et al. (1994), for water resources in the USA. It is likely that a significant amount of methylation occurs in sediment, suggesting that significant fluxes of MeHg from sediment to the overlying water column occur (Hall et al. 2008).
Spatial distributions were observed. Aqueous Hg (i.e. TotHg and MeHg) was generally higher at the downstream sites (i.e. MM and CM), while sediment Hg (TotHg and MeHg) was highest at the most downstream site (i.e. BT) (see below). This suggests that most of the Hg is suspended and transported downstream from the power stations, and the local hydrological regimes and dynamic of sediment transport can explain the lower aqueous and higher sediment concentrations at the downstream site (i.e. BT). This could also serve as a potential storage basin for Hg. Because water is transient, any MeHg present upstream of the power stations will be transported downstream (or deposited in sediment), similar to what has been reported previously (Williams et al. 2010; Walters et al. 2011). Understanding the atmospheric sources of Hg deposited to ecosystems is important for determining the local and regional impacts of anthropogenic Hg sources. Wet and dry deposition represents an important source of Hg to both terrestrial and aquatic ecosystems. Stack emissions from coal-fired power stations include both vapour- and particle-bound phases. Matimba has a stack height of approximately 250 m. The wind rose using hourly data between 2010 and 2014 in Fig. 3 provides a means of predicting the potential regional sources of Hg by plotting the percentages of time when the wind blew from each of the 16 directions. The prevailing wind direction for the study period was from the east-northeast and east directions. As evidenced by the wind rose, the overall major potential source locations lie to the west and west northwest, thereby suggesting other potential regional sources.
Evidence of seasonal variation was observed in the Hg concentrations of water samples. Higher TotHg concentrations were generally reported during the wet season. An increase in aqueous Hg concentrations during the wet season suggests wet deposition of Hg to the aquatic environment during storm events. In addition, periods of high rainfall promote the physical remobilisation of fine-grained sediments and resuspension of Hg from sediment to the water column, similar to what has been reported by others (Churchill et al. 2004; Williams et al. 2010). In terms of aqueous MeHg, concentrations were negatively correlated with sediment MeHg during the wet season at all sites with the exception of MM, while during the dry season, aqueous MeHg was positively correlated with sediment MeHg at all sites.
3.3 Mercury in Sediments
In the aquatic environment, Hg accumulates in sediments where methylation and demethylation processes occur. Sediments are also regarded as a potential source of metal contamination (which includes Hg) for the surrounding water column. As such, sediments are regarded as a major repository of anthropogenic pollutants such as Hg. It is estimated that >90% of trace metals present in the aquatic environment are associated with suspended particles and sediments (Calmano et al. 1993; Wang et al. 2009). The TotHg and MeHg concentrations in surface sediments are shown in Fig. 4. In this study, the lowest average TotHg and MeHg concentrations were 0.50 ng/g (BT) and 0.08 ng/g (MD), respectively, while the highest TotHg and MeHg were 28.60 ng/g (BT) and 2.22 ng/g (MM), respectively. All TotHg concentrations were below the USEPA sediment quality guideline of 200 ng/g (USEPA 2000). Total Hg concentrations in sediment samples were highest at the most upstream site (i.e. BT). Unlike to the distribution pattern of TotHg, the highest MeHg concentrations were noted at MM (2.22 ng/g). Compared with other recent studies conducted at similar sampling sites with similar impacts, the TotHg concentrations in river sediment reported in this study were generally lower than those previously reported for sediments adjacent to coal-fired power stations (Williams et al. 2010). In this study, the MeHg/TotHg ratio ranged between 0.71 and 92.62%. Generally, %MeHg in surface sediments can be used as a proxy for the rate of methylation (Drott et al. 2007) and accounts for approximately 1–3% of TotHg in sediment (Revis et al. 1990). The elevated %MeHg values reported suggest either that enhanced methylation has occurred (Conway et al. 2003).
Under anaerobic conditions, Hg2+ has a high affinity for sulphides, resulting in the formation of insoluble HgS, which is deposited in the sediment and is unavailable for methylation (Andersson et al. 1990). Similarly, Hg has a high affinity for organic matter (Hintelmann et al. 1995), as such their toxicity and bioavailability in sediment is largely dependent on their interactions within the matrix (Pinedo-Hernandez et al. 2015). The relation of Hg concentration in sediments to the corresponding organic carbon content (i.e. loss of ignition (LOI)) was examined. While both Hg species were not correlated with organic carbon content, site-specific correlations were found between TotHg and MeHg concentrations (MD: R 2 = 0.43, MM: R 2 = 0.90). This indicates that at these sites, Hg is available for methylation under specific conditions.
3.4 Mercury in Fish
Methylmercury is the most toxic form of organic Hg, produced by the conversion of metallic, inorganic, or organic Hg by sulphate- reducing microorganisms present in sediments (Benoit et al. 1998). It is easily and rapidly taken up by living organisms and biomagnified through the aquatic food chain and, consequently, represents a hazard to higher trophic level organisms, including humans that accumulate Hg through the consumption of fish (Bourdineaud et al. 2011; Nøstbakken et al. 2012). As such, fish play a significant role in the distribution of Hg between biotic compartments in aquatic environments. The TotHg and MeHg concentrations of freshwater fish samples which were collected seasonally from April 2013 are presented in Table 3. Total Hg concentrations varied from 37.30 ng/g dry weight (d.w.) for Barbus species collected at BT in April 2013 to 1291.95 ng/g d.w. for Aplocheilichthus johnstoni collected from MD in January 2014 (average TotHg concentration = 267.78 ng/g d.w.). Methylmercury concentrations varied from 13.42 ng/g d.w. for B. spp. collected at BT in April 2013 to 656.02 ng/g dw for A. johnstoni collected from MD in January 2014 (average MeHg concentration = 158.76 ng/g d.w.). The Hg concentrations reported in this study are higher than those reported in previous studies with sampling areas having similar anthropogenic impacts (Williams et al. 2010). Interestingly, the highest TotHg and MeHg concentrations are reported at MD.
The observed variations in fish muscle Hg (i.e. TotHg and MeHg) concentrations can be explained by several factors including biological variability associated with the fish species (i.e. age, size, diet), geological influences (i.e. sediment), chemical variability (i.e. ancillary water quality) and other environmental factors. A principal component analysis (PCA) was used to explain any potential correlations between aqueous and sedimentary Hg concentrations with fish Hg collected from their corresponding locations. As shown in Fig. 5, the PCA explained 73.42, 70.01, 80 and 84.32% of the total variability at MD, MM, CM and BT, respectively. Site-specific associations with Hg variables are evident in the PCA plots. These indicated that Hg concentrations in fish were largely correlated with sediment Hg concentrations, suggesting that sediment-associated Hg is readily available for uptake by fish (Williams et al. 2010), reinforcing the role that sediment plays in being a source of Hg to fish (Gilmour et al. 1992). Similarly, Hg in the water column is readily absorbed into muscle tissues of aquatic organisms, particularly via digestion (USEPA 1997; NRC 2000; Leaner and Mason 2002; Gilbertson and Carpenter 2004; Kontas 2006; Drott et al. 2007) as evidence in the PCA diagrams. In this study, fish Hg concentrations were largely associated with aqueous Hg (MD, CM, BT) and sediment Hg (MD, MM, CM). Mercury in sediments can be bioaccumulated in aquatic organisms and transferred along the food chain, resulting in a threat to humans (Díez 2009; Wang et al. 2013). Subsistence fishing forms part of the daily livelihoods of the communities living near the water resources sampled in this study. Although less than 10% of samples exceeded the USEPA of 300 ng/g (USEPA 2001b), and less than 5% the WHO safety limit of 500 ng/g and the Joint FAO-WHO Food Standards Programme CODEX Committee on Contaminants in Foods (CODEX Alimentarius Commission 2011).
4 Conclusions
Mercury is a hazardous contaminant occurring naturally and anthropogenically in the aquatic environment. Its speciation, fate and transport in the aquatic environment are controlled by severally physical, chemical and biological factors, which decide its toxicity in the environment. This study investigated the distribution of Hg species (TotHg and MeHg) in environmental compartments from four sampling sites located in the vicinity of a coal-fired power plant in the Waterberg area (Limpopo province). Several factors are known to affect TotHg and MeHg concentrations in aquatic environments. Point sources of Hg pollution as well as long-range atmospheric transport and deposition from global sources can be a large contributor of Hg to water resources (Mason et al. 1994). In this study, the highest aqueous TotHg and MeHg concentrations were measured at downstream sampling sites and could likely be attributed to the immediate deposition of atmospheric Hg and the subsequent downstream movement of suspended Hg. Total Hg concentrations in sediment were generally highest at BT, while the highest MeHg concentration was measured immediately downstream of the power stations (i.e. MM). Declines in Hg concentrations were evident during the wet season indicating that Hg is flushed to the downstream surface waters’ possible dilution effects, reinforcing the influence of subsurface transport and/or production of MeHg. The results also indicated that Hg bioaccumulation and risks to ecosystems are highly variable. Total Hg concentrations in freshwater fish ranged over several orders of magnitude. The lowest concentration measure was less than 40 ng/g (BT), while the highest concentration exceeded 1200 ng/g (MD). For MeHg, the lowest concentration measured 13.42 ng/g (BT) and the highest exceeding 600 ng/g (MD). Methylmercury concentrations in freshwater fish sampled exceeded the USEPA criteria (300 ng/g) in less than 10% of the total fish sampled and less than 5% exceeded the WHO guideline (500 ng/g). Consumption of elevated MeHg content in fish are the primary route of exposure to humans, and is also the primary cause for fish consumption advisories worldwide. The presence of relatively high TotHg concentrations (close to and exceeding 300 and 500 ng/g) are of concern due to the fact that (1) MeHg often accounts for 90% of TotHg, (2) the fish caught in this study were small and likely juveniles and (3) that Hg levels in fish are known to increase with age and trophic position. In this study, fish Hg concentrations frequently exceeded guideline limits for fish consumption (USEPA and WHO guidelines). Fish consumption is the main exposure pathway of Hg to humans (NRC 2000). This is especially true in rural areas where populations rely on subsistence fishing.
References
CODEX Alimentarius Commision (2011) Joint FAO/WHO Food Standards Programme CODEX Committee on Contaminants in Foods. Fifth Session, The Hague, The Netherlands.
Andersson, I., Parkman, H., & Jernelov, A. (1990). The role of sediments as sink or source for environmental contaminants: a case study of mercury and chlorinated organic compounds. Limnologica, 20, 347–359.
Benoit, J. M., Gilmour, C. C., Mason, R. P., Riedel, G. S., & Riedel, G. F. (1998). Behavior of mercury in the Patuxent River estuary. Biogeochemistry, 40(2), 249–265.
Bourdineaud, J. P., Fujimura, M., Laclau, M., Sawada, M., & Yasutake, A. (2011). Deleterious effects in mice of fish-associated methylmercury contained in a diet mimicking the Western populations’ average fish consumption. Environment International, 37(2), 303–313.
Braune, B. M., Outridge, P. M., Fisk, A. T., Muir, D. C. G., Helm, P. A., Hobbs, K., Hoekstrad, P. F., Kuzyf, Z. A., Kwan, M., Letcher, R. J., Lockhart, W. L., Norstrom, R. J., Stern, G. A., & Stirling, I. (2005). Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: an overview of spatial and temporal trends. Science of the Total Environment, 351–352, 4–56.
Calmano, W., Hong, J. and Förstner, U. (1993). Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. In: Proceedings of the First International Specialized Conference, pp. 223–235.
Churchill, R. C., Maethrel, C. E., & Suter, P. J. (2004). A retrospective assessment of gold mining in the Reedy Creek sub-catchment, northeast Victoria, Australia: residual mercury contamination 100 years later. Environmental Pollution, 132(2), 355–363.
Conway, C. H., Squire, S., Mason, R. P., & Flegal, A. R. (2003). Mercury speciation in the San Francisco Bay estuary. Marine Chemistry, 80(2–3), 199–225.
Díez, S. (2009). Human health effects of methylmercury exposure. Reviews of Environmental Contamination and Toxicology, 198, 111–132.
DME (Department of Minerals and Energy). (2005). Digest of South African Energy Statistics Department of Minerals and Energy, Pretoria.
Drott, A., Lambertsson, L., Bjorn, E., & Skyllberg, U. (2007). Effects of oxic and anoxic filtration on determining methyl mercury concentrations in sediment pore waters. Marine Chemistry, 103(1–2), 76–83.
Gilbertson, M., & Carpenter, D. O. (2004). An ecosystem approach to the health effects of mercury in the Great Lakes basin ecosystem. Environmental Research, 95(3), 240–246.
Gilmour, C. C., Henry, E. A., & Mitchell, R. (1992). Sulfate stimulation of mercury methylation in freshwater sediments. Environmental Science and Technology, 26(11), 2281–2287.
Hall, B. D., Bodaly, R. A., Fudge, R. J. P., Rudd, J. W. M., & Rosenberg, D. M. (1997). Food as the dominant pathway of methylmercury uptake by fish. Water, Air, and Soil Pollution, 100(1), 13–24.
Hall, B. D., Aiken, G. R., Krabbenhoft, D. P., Marvin-DiPasquale, M., & Swarzenski, C. M. (2008). Wetlands as principal zones of methylmercury production in southern Louisiana and the Gulf of Mexico region. Environmental Pollution, 154(1), 124–134.
Hintelmann, H., Welbourn, P. M., & Evans, R. D. (1995). Binding of methylmercury compounds by humic and fulvic acid. Water, Soil and Air Pollution, 80(1), 1031–1034.
Hylander, L. D., & Meili, M. (2003). 500 years of mercury production: global annual inventory by region until 2000 and associated emissions. The Science of the Total Environment, 304, 13–27.
Kontas, A. (2006). Mercury in the Izmir basin: an assessment of contamination. Journal of Marine Systems, 61(1–2), 67–78.
Leaner, J. J., & Mason, R. P. (2002). Methylmercury accumulation and fluxes across the intestine of channel catfish, Ictalurus punctatus. Comparative Biochemistry and Physiology - Part C, 132(2), 247–259.
Mason, R. P., Fitzgerald, W. F., & Morel, F. M. M. (1994). The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochimica et Cosmochimica Acta, 58(15), 3191–3198.
Mason, R.P., & Sullivan, K.A. (1998). Mercury and methylmercury transport through an urban watershed. Water Resources, 32, 321–330.
Meili, M. (1991). The coupling of mercury and organic matter in the biogeochemical cycle—towards a mechanistic model for the boreal forest zone. Water, Air, and Soil Pollution, 56(1), 333–347.
National Research Council (NRC). (2000).Toxicological effects of methylmercury. National Academy Press, 344pp, Washington, DC.
Nøstbakken, O. J., Martin, S. A., Cash, P., Torstensen, B. E., Amlund, H., & Olsvik, P. A. (2012). Dietary methylmercury alters the proteome in Atlantic salmon (Salmo salar) kidney. Aquatic Toxicology, 108, 70–77.
Pacyna, J. M., & Pacyna, E. G. (2001). An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environmental Reviews, 9(4), 269–298.
Pacyna, E. G., Pacyna, J. M., Steenhuisen, F., & Wilson, S. (2006). Global anthropogenic mercury emission inventory for 2000. Atmospheric Environment, 40(22), 4048–4063.
Pinedo-Hernandez, J., Marrugo-Negrete, J., & Dize, S. (2015). Speciation and bioavailability of mercury in sediments impacted by gold mining in Columbia. Chemopshere, 119, 1289–1295.
Pirrone, N., Cinnirella, S., Feng, X., Finkelman, R. B., Firedli, H. R., Leaner, J., Mason, R., Mukherjee, A. B., Stracher, G. B., Streeet, D. G., & Telmer, K. (2010). Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmospheric Chemistry and Physics, 10, 5951–5964.
Revis, N. W., Osborne, T. R., Holdsworth, G., & Hadden, C. (1990). Mercury in soil: a method for assessing acceptable limits. Archives of Environmental Contamination and Toxicology, 19, 221–6.
Roos, B.L. (2011). Mercury emissions from coal-fired power stations in South Africa. MSc Dissertation. University of Johannesburg, p93.
Scott, G. (2011). Emission testing at two South African power stations, using the US-EPA’s mercury toolkit. Presentation at the International Mercury Emissions from Coal Meeting (MEC), Kruger Park Hotel, 18 May 2011.
Seigneur, C., Vijayaraghavan, K., Lohman, K., Karamchandani, P., & Scott, C. (2004). Global source attribution for mercury deposition in the United States. Environmental Science and Technology, 38(2), 555–569.
Somerset, V., Williams, C., Van der Horst, C., Silwana, B., Le Roux, S., Iwuoha, E. (2011). Survey and analysis of the Pb, Zn, Hg, Cd, Ni, Al, Fe, Pd, Pt and Rh levels in specific river catchments of the North West and Limpopo Provinces. CSIR-NRE Internal Report, February 2011. CSIR, Stellenbosch, Report No. CSIR/NRE/WR/ER/2011/0005/B.
Stein, E. D., Cohen, Y., & Winer, A. M. (1996). Environmental distribution and transformation of mercury compounds. Critical Reviews in Environmental Science and Technology, 26(1), 1–43.
US EPA (United States Environmental Protection Agency). (1992) Water quality standards; establishment of numeric criteria for priority toxic pollutants; states’ compliance; final rule. Federal Register, 40 CFR Part 131, v 57; 246(60) 847 – 860, 916.
US EPA (United States Environmental Protection Agency). (1997) Mercury study report to Congress, Vol. 3—Fate and transport of mercury in the environment: Washington, D.C., Office of Air Quality Planning and Standards and Office of Research and Development, EPA–452/R–97–005, 376 p, Washington DC, USA.
US EPA (United States Environmental Protection Agency). (2000) Guidance for assessing chemical contamination data for use in fish-advisories. Vol 1: field sampling and analysis, Third Ed. EPA publication no. EPA-823-B-00-007. Washington DC, USA.
US EPA (United States Environmental Protection Agency). (2001a) Method 1630: Methylmercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS. EPA Publication No. 821-R-01-020. Washington DC, USA.
US EPA (United States Environmental Protection Agency). (2001b) Mercury update: impact on fish advisories. EPA-823-F-01-012. US EPA, Washington DC.
US EPA (United States Environmental Protection Agency). (2002) Mercury in water by oxidation, purge and trap, and cold water atomic fluorescence spectrometry. EPA Publication No.: EPA-821-R-02-019. Washington DC, USA.
USEPA (2007) Method 7473: mercury in solids and solutions by thermal decomposition amalgamation, and atomic absorption spectrophotometry. EPA SW-846, Washington DC, USA.
Walters, C. R., Somerset, V. S., Leaner, J. J., & Nel, J. M. (2011). A review of mercury pollution in South Africa: current status. Journal of Environmental Science and Health, Part A, 46(10), 1129–1137.
Wang, T., Wang, J., Tang, Y., Shi, H., & Ladwig, K. (2009). Leaching characteristics of arsenic and selenium from coal fly ash: role of calcium. Energy and Fuels, 23(6), 2959–66.
Wang, H. S., Xu, W. F., Chen, Z. J., Cheng, Z., Ge, L. C., Man, Y. B., Giesy, J. P., Du, J., Wong, C. K. C., & Wong, M. H. (2013). In vitro estimation of exposure of Hong Kong residents to mercury and methylmercury via consumption of market fishes. Journal of Hazardous Materials, 248–249, 387–393.
Wiener, J. G., Krabbenhoft, D. P., Heinz, G. H., & Scheuhammer, A. M. (2003). Ecotoxicology of mercury. In D. J. Hoffman, B. A. Rattner, G. A. Burton Jr., & J. Cairns Jr. (Eds.), Handbook of ecotoxicology (2dth ed., pp. 409–463). New York: Lewis.
Williams, C. R., Leaner, J. J., Nel, J. M., & Somerset, V. S. (2010). Mercury concentrations in water resources potentially impacted by coal-fired power stations and artisanal gold mining in Mpumalanga, South Africa. Journal of Environmental Science and Health, Part A, 45(11), 1363–1373.
Acknowledgements
This work is supported by the Eskom’s Research Testing and Development Department (Sustainability Division). The authors wish to thank the editors and anonymous reviewers for giving us many constructive comments that significantly improved the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walters, C., Couto, M., McClurg, N. et al. Baseline Monitoring of Mercury Levels in Environmental Matrices in the Limpopo Province. Water Air Soil Pollut 228, 57 (2017). https://doi.org/10.1007/s11270-016-3230-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3230-3