Abstract
A sensitive and simple method for simultaneous analysis of flubendiamide and its metabolite desiodo flubendiamide in cabbage, tomato and pigeon pea has been developed. The residues were extracted with QuEChERS method followed by dispersive solid-phase extraction with primary secondary amine sorbent to remove co extractives, prior to analysis by HPLC coupled with UV–Vis detector. The recoveries of flubendiamide and desiodo flubendiamide were ranged from 85.1 to 98.5% and 85.9 to 97.1% respectively with relative standard deviations (RSD) less than 5% and sensitivity of 0.01 μg g−1. The method offers a less expensive and safer alternative to the existing residue analysis methods for vegetables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flubendiamide (N′-[1,1-dimethyl-2-(methylsulfonyl) ethyl]-3-iodo-N-{4-[2, 2,2-tetrafluoro-1-(trifluoromethyl) ethyl]-0-tolyl} phthalimide) is a novel insecticide belongs to class benzene dicarboxamide with ryanodine receptor modulator type biochemical action in lepidopterous insect pests (Tohnishi et al. 2005; Masaki et al. 2006). Figure 1 illustrates the chemical structures of them. Flubendiamide acts through a new biochemical mode of action in lepidopterous insect pests (Tohnishi et al. 2005; Masaki et al. 2006). It shows excellent activity against adults and larvae, fast acting properties, and extended residual activity, mainly by ingestion. Flubendiamide has excellent fast-acting and residual activity against a broad spectrum of lepidopterous insect pests, such as Helicoverpa spp., Heliothis spp., Spodoptera spp., Plutella spp., Pseudoplusia spp., Trichoplusia spp., Agrotis spp., loopers, cutworms, fruitworms, armyworms, corn borers, including resistant strains as well on Brassica leafy vegetable, corn (field, sweet), rice, pigeon pea, cotton, cucurbit vegetable group, fruiting vegetable group, grape, leafy vegetable group, pome fruit group, stone fruit group and tree nut group (Tohnishi et al. 2005; Masaki et al. 2006).
Analysis of Flubendiamide in different food matrices was reported by HPLC–DAD (Battu et al. 2008), HPLC–UV (Mohapatra et al. 2010; Sahoo et al. 2009; Gopal and Mishra 2008), LCMS/MS with electrospray ionization (ESI) (Billian 2007) and Liquid Chromatography-Tandem Mass Spectrometric Ion-Switching (Caboni et al. 2008). There is, however, a little information available on the methods for the simultaneous analysis of these insecticides. The LCMS instruments are costly and are not commonly available in India and reported HPLC analytical methods are also expensive and time-consuming.
We adopted the principal of the QuEChERS method (Quick, Easy, Cheap, Effective, Rugged and Safe) (Anastassiades et al. 2003), which can provide high-quality results with a minimum number of steps and low solvent and glassware consumptions. This multi-pesticide residues method used an acetonitrile extraction and ‘‘dispersive SPE’’ to replace many complicated cleanup steps which were usually employed in traditional methods (Anastassiades et al. 2003).
This paper presents a simple and rapid method based on HPLC–UV coupled to dispersive solid phase extraction cleanup for simultaneous determination of flubendiamide and its metabolite desiodo flubendiamide residues in cabbage, tomato and pigeon pea by employing QuEChERS method.
Materials and Methods
Analytical reference standards of flubendiamide (96.7% purity) and desiodo flubendiamide (99.3% purity) were supplied by Rallis India Ltd, Bangalore. All the other chemicals and solvents were from J. T. Baker, Mumbai (India) and were of analytical grade. Primary secondary amine (PSA, 40 μm, Bondesil) were purchased from Varian (Palo Alto, CA, USA). Stock solutions (1,000 μg mL−1) were prepared by dissolving reference standards in acetonitrile. Mixture standard solutions were prepared by mixing diluting the individual standard stock solutions. Working standard solutions (0.01, 0.05, 0.2, 0.5 and 1.0 μg mL−1), used for sample spiking and for preparation of standard curve, were obtained from stock solution by volumetric serial dilution and stored at −4°C.
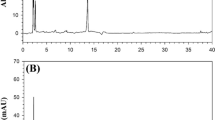
Analysis of flubendiamide and desiodo flubendiamide residues was carried out using a High Performance Liquid Chromatography (HPLC) with the following parameters: Agilent Technologies (1200 series) with a UV–Vis detector. A reversed-phase Hypersil BDS C18 column (25 cm long, 4 mm i.d) at ambient temperature and Chemstation software system was employed to acquire and process chromatographic data was used throughout the entire experiment. The program was set for an isocratic elution with flow rate of 1 mL min−1, wavelength, 210 nm and the aliquots of 20 μL of the samples were injected. All the solvents were filtered with a 0.45 mm membrane filter. With these operating parameters the retention time of the desiodo flubendiamide was at 7.66 min and flubendiamide was 9.77 min (Fig. 2).
The blank samples of cabbage, tomato fruit, and pigeon pea (grain, shell and straw) used in experiments were free from pesticide and collected from Horticultural Research Station, Mondouri, BCKV, West Bengal, India.
Fresh and healthy 500 g of each sample were drawn and immediately chopped and homogenized. From this, a representative subsample of 10 g was transferred to 50 mL screw capped polypropylene tube followed by addition of 10 mL of acetonitrile. The polypropylene tubes were vortexed for 2 min. This was followed by salting out by addition of 1.0 g NaCl, and 4.0 g MgSO4 were added, and the vortexing process was repeated for 2 min followed by centrifugation at 10,000 rpm for 10 min. Known (6 mL) amount of supernatant was transferred to a polypropylene centrifuge tube which contains following sorbents: 0, 10, 25, 50, 75 and 100 mg PSA, 150 mg anhydrous magnesium sulfate, after vortex-mixing for 1 min, the mixtures were centrifuged at 5,000 rpm for 5 min and the cleaned extract 2 mL was transferred into a turbovap tube and evaporated to dryness under a gentle stream of nitrogen by using the Turbovap LV (Caliper Life Sciences, Russelsheim, Germany) set at 40°C and 7.5 psi Nitrogen flow and the residues were reconstituted in 0.5 mL of mobile phase and filtered through a 0.2 mm filter prior to HPLC analysis. In order to study the performance of the method, recovery study was carried out at 0.01, 0.05 and 0.1 μg g−1, for flubendiamide and desiodo flubendiamide and processed according to the above procedure.
Results and Discussion
The choice of mobile phase is crucial for the separation of the pesticide from co-extractives. Among various mobile phase tested, acetonitrile: water (60:40 vv−1) showed excellent separation, resolution, sensitivity and repeatability at 210 nm. The linear dynamic response in the range of 0.01–1.0 μg g−1 with a correlation coefficient of 0.999 signifies the optimization of HPLC. Retention time and peak area were checked for repeatability by injecting test mixture standard solution into the HPLC system over three runs. The within-day relative standard deviation (RSD) of the retention time of test mixture standard solution was less than 0.1% and for the obtained peak area less than 5%. The limits of detection (LOD) were established by considering a value of three times the background noise of the blank sample at the retention time of each pesticide. The limits of quantification (LOQ) were calculated by considering a value of 10 times that of background noise. The LOQ of flubendiamide and desiodo flubendiamide were 0.01 μg g−1 (Table 1).
In this study, we found that sample cleaned-up was the most important step. In order to optimize the cleanup, the PSA different amounts (0, 10, 25, 50, 75 and 100 mg) of PSA have been studied. The result showed that with increasing amounts, there was no obviously improvement of recovery. In our study, acetonitrile was chosen as the extracting solvent. In fact, we found that 10 mg of PSA and 150 mg of MgSO4 were added to effectively remove the interferences in the tomato fruit, pigeon grain extract and 25 mg of PSA and 150 mg of MgSO4 were added to effectively remove the interferences in the cabbage head, pigeon pea shell and pigeon pea straw extracts.
The reproducibility of the chromatographic method was established by performing the analysis of selected sample fortified at 0.05 μg g−1. The sample was injected 5 times. A good reproducibility was expressed as relative standard deviations (RSD, in %), which was obtained for the peak areas within 4%. Moreover, the reproducibility of the complete analytical method, expressed as RSD, was in the range from 0.38 to 3.22% for all the studied compounds and replicate analysis of a fortified sample for 5 times (Fig. 3).
The present method was validated with standardized amount of PSA in selected substrates by conducting recovery experiments at the level of LOQ (0.01 μg g−1), five times LOQ (0.05 μg g−1) and ten times of LOQ (0.10 μg g−1). The results from fortification studies were statistically analyzed using MS-Excel to calculate recovery percentage and relative standard deviation (RSD). Three replications were developed for each level. The recoveries achieved following the proposed method are shown in Tables 2 and 3.
The recoveries of flubendiamide and desiodo flubendiamide which were obtained with the described procedure. The individual mean recovery rates for flubendiamide ranged from 85.1 to 98.5% at fortification levels of 0.01, 0.05 and 0.10 μg g−1 (overall mean recoveries: 87.0–95.3%, overall RSDs = 1.83–4.13%). The individual mean recovery rates for desiodo flubendiamide ranged from 85.7 to 96.4% at fortification levels of 0.01, 0.05 and 0.10 μg g−1 (overall mean recoveries: 89.2–95.0%, overall RSDs = 2.61–4.06%).
As evidenced this optimized analytical method could be used to monitor flubendiamide and desiodo flubendiamide residue in cabbage head, tomato fruit, and pigeon pea. The QuEChERS method analysis represents a rapid, simple, and highly sensitive method to serve the quality control purposes.
References
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and ‘‘dispersive solid-phase extraction’’ for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Battu RS, Singh B, Kooner R, Singh B (2008) Simple and efficient method for the estimation of residues of flubendiamide and Its metabolite desiodo flubendiamide. J Agric Food Chem 56(7):2299–2302
Billian P (2007) Residue analytical method for the determination of residues of flubendiamide and its metabolites in plant and animal materials by HPLC with electrospray MS/MS-detection. Pflanzenschutz-Nachrichten Bayer 60:263–296
Caboni P, Sarais G, Angioni A, Vargiu S, Pagnozzi D, Cabras P, Casida JE (2008) Liquid chromatography-tandem mass spectrometric ion-switching determination of chlorantraniliprole and flubendiamide in fruits and vegetables. J Agric Food Chem 56(17):7696–7699
Gopal M, Mishra E (2008) Analytical method for estimation of a new insecticide flubendiamide and its safety evaluation for usage in rice crop. Bull Environ Contam Toxicol 81:360–364
Masaki T, Yasokawa N, Tohnishi M, Nishimatsu T, Tsubata K, Inoue K, Motoba K, Hirooka T (2006) Flubendiamide, a novel Ca2+ channel modulator, reveals evidence for functional cooperation between Ca2+ pumps and Ca2+ release. Mol Pharma 69(5):1733–1739
Mohapatra S, Ahuja AK, Deepa M, Sharma D, Jagadish GK, Rashmi N (2010) Persistence and dissipation of flubendiamide and des-iodo flubendiamide in cabbage (Brassica oleracea Linne) and soil. Bull Environ Contam Toxicol 85(3):352–356
Sahoo SK, Sharma RK, Battu RS, Singh B (2009) Dissipation kinetics of flubendiamide on chili and soil. Bull Environ Contam Toxicol 83(3):384–387
Tohnishi M, Nakao H, Furuya T, Seo A, Kodama H, Tsubata K, Fujioka S, Kodama H, Hirooka T, Nishimatsu T (2005) Flubnediamide, a Novel insecticide highly active against lepidopterous insect pests. J Pest Sci 30(4):354–360
Acknowledgments
The authors are indebted to Department of Agricultural Chemicals for providing infrastructural facilities, Bidhan Chandra Krishi Viswavidyalaya, Kalyani, India for conduct the experiment and M/S Rallis India Ltd for providing the necessary financial support to accomplish this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paramasivam, M., Banerjee, H. Simultaneous Determination of Flubendiamide its Metabolite Desiodo Flubendiamide Residues in Cabbage, Tomato and Pigeon Pea by HPLC. Bull Environ Contam Toxicol 87, 452–456 (2011). https://doi.org/10.1007/s00128-011-0389-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0389-6