Abstract
Average initial deposits of flubendiamide on chili were found to be 1.06 and 2.00 mg kg−1, respectively, following two applications of flubendiamide 480SC at 60 and 120 g a.i. ha−1 at 10 days interval. More than 80% of flubendiamide residues dissipated just after 3 days of the last application at both the dosages. Residues of flubendiamide dissipated below detectable level of 0.01 mg kg−1 in 7 and 10 days at single and double dosages, respectively. Half-life (t 1/2) of flubendiamide on chili was observed to be 0.96 and 0.91 days, respectively, at single and double dosages. Desiodo flubendiamide was not detected at 0.01 mg kg−1 level in chili samples collected at different time intervals. Red chili and soil samples collected after 20 days did not reveal the presence of flubendiamide or its metabolite desiodo flubendiamide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Chili (Capsicum annuum L.; Capsicum frutescene L.), also called red pepper’, is an important cash crop in India and is grown for its pungent fruits, which are used both green and ripe (the latter in the dried form) to impart pungency to the food. As a condiment, it has become indispensable in every Indian home. It is also used medicinally, and in chutnies and pickles. The pungency is due to the active principle `capsicin’ contained in the skin and the septa of the fruit. The world consumption of chilies and paprika is going up due to the increasing popularity of ethnic foods. The increased availability of oleoresins and spice oils of chili has also enhanced its consumption in various food preparations. India is the largest producer of chilies in the world but its production pattern is highly erratic.
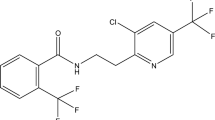
Increasing awareness of the potential impact of persistent crop protection agents has led to the development of eco-friendly new molecules to ensure minimum risk to man and environment. Flubendiamide, N′-[1,1-dimethyl-2-(methylsulfonyl)ethyl]-3-iodo-N-{4-[2,2,2tetrafluoro-1-(trifluoro methyl) ethyl]-0-tolyl} phthalimide belongs to a new chemical class, the phthalic acid diamides and is widely used against lepidopteran pests on a variety of annual and perennial crops (Fig. 1a, b). It provides superior plant protection against a broad range of economically important lepidopterous pests including Helicoverpa spp, Heliothis spp, Spodoptera spp, Plutella spp, Trichoplusia spp and Hyrotis spp. Experiments in North America have shown flubendiamide to be hydrolytically stable, relatively immobile in soil, practically non-detectable is key rotated crops. Flubendiamide has a favourable ecological, ecotoxicological and environmental profile with low mammalian toxicity and no genotoxic, mutagenic or oncogenic properties noted (Shane 2006). Flubendiamide has been recently introduced in India by Bayer Crop Science Limited and is presently under consideration of approval of use on major crops. However, no information is available on persistence of flubendiamide on chili. Therefore, the present study was carried out to investigate the persistence and dissipation kinetics of flubendiamide residues in samples of chili and soil.
Materials and Methods
The working standards of flubendiamide (purity 93.6%) along with reference standard of desiodo flubendiamide (purity 100%) were supplied by M/s Bayer Crop Science Limited, Mumbai. All the solvents used were of laboratory grade. These were redistilled in all glass apparatus and suitability of solvents was ensured by running reagent blank along with actual analysis. Acetonitrile was of HPLC grade. The stock solutions of flubendiamide was prepared at 1,000 μg mL−1 in acetonitrile of HPLC grade. These solutions were diluted to obtain concentrations of 100.0, 10.0, 1.0, 0.1, 0.01 μg mL−1.
Chili (var. CH-1) was raised and transplanted during Rabi 2007 according to recommended agronomic practices at Entomological Research Farm, Punjab Agricultural University, Ludhiana, India using randomized block design (RBD). The first application of Flubendiamide 480 SC at 60 and 120 g a.i. ha−1 was made at fruit development stage using Aspee Knapsack sprayer fitted with hollow cone nozzle. Subsequently the 2nd application was made at 10 days interval. Each treatment was replicated thrice and size of each plot was 50 m2. In control plots, only water was sprayed.
About 500 g samples of chili was collected from each treated and control plots at 0 (2 h), 1, 3, 5, 7, 10 and 15 days after the last application of the insecticide. Red chili and soil samples were collected after 20 days of the last application. Samples were extracted immediately after sampling.
The extraction and cleanup of chili and soil samples for residues of flubendiamide, its metabolite desiodo flubendiamide were carried out as per procedure reported by Battu et al. (2008). Samples of chili fruits were chopped and finely blended and a representative 50 g sample was placed overnight into 100 mL acetonitrile in an erlenmayer flask. The extract was filtered into 1 L separatory funnel along with rinsings of acetonitrile. The filtrate in the separatory funnel was diluted with 600 mL brine solution and partitioned the contents three times into 100, 50 and 50 mL chloroform. The chloroform fractions were combined, dried over anhydrous sodium sulfate.
Soil samples were dried, ground and sieved. A representative sample of 50 g was extracted as per the procedure mentioned for chili fruits.
The chloroform fractions of both the chili and soil samples were treated with 500 mg activated charcoal powder for about 2–3 h at room temperature. The clear extract so obtained was filtered through Whatman filter paper No.1, concentrated to near dryness and added about 20 mL HPLC grade acetonitrile and again concentrated using rotary vacuum evaporator at 30°C. Repeated the process to completely evaporate chloroform and the final volume was reconstituted to about 5 mL using HPLC grade acetonitrile.
The residues of flubendiamide and its metabolite desiodo flubendiamide were estimated on HPLC by employing Phenomenex Luna C18 column at 254 λ (wavelength) and using acetonitrile: water (60:40, v/v) mixture as mobile phase at 1.2 mL min−1. Under these operating conditions the retention time of flubendiamide and desiodo flubendiamide were found to be 12.13 and 8.62 min, respectively. Residues were estimated by comparison of peak height/peak area of the standards with that of the unknown or spiked samples run under identical conditions. Half-scale deflection was obtained for 10 ng flubendiamide and limit of quantification (LOQ) was found to be 0.01 mg kg−1.
Chili and soil samples were spiked with flubendiamide and desiodo flubendiamide metabolite at different levels and analyzed as per the methodology described above. Per cent recovery of flubendiamide and desiodo flubendiamide in chili and soil found to be consistent and more than 80% (Table 1).
The persistence of flubendiamide has generally been expressed in terms of DT50 i.e. time for disappearance of pesticide to 50% of its initial concentration. The DT50 of flubendiamide was calculated using Hoskins (1961) formula.
Results and Discussion
The results of dissipation of flubendiamide and its metabolite desiodo flubendiamide in chili and soil are presented in Table 2. Average initial deposits of flubendiamide on chili were found to be 1.06 and 2.00 mg kg−1, respectively, following two applications of flubendiamide 480 SC at 60 and 120 g a.i. ha−1 at 10 days interval. More than 80 per cent of flubendiamide residues dissipated just after 3 days of the last application at both the dosages. Residues of flubendiamide dissipated below LOQ of 0.01 mg kg−1 in 7 and 10 days at single and double dosages, respectively. The DT50 flubendiamide was found to be 0.96 and 0.91 days at single and double dosages, respectively. Desiodo flubendiamide was not detected at 0.01 mg kg−1 level in chili samples collected at different time intervals. Red chili and soil samples collected at 20 days after last spray also did not reveal the presence of flubendiamide and its metabolite desiodo flubendiamide.
The residues of flubendiamide were confirmed by high performance thin layer chromatography (HPTLC). This technique was able to identify and quantify 50 ng of flubendiamide. Cleaned up sample extracts of different substrates were spotted on pre coated silica gel 60F on aluminium sheets along with reference standards of flubendiamide, visualized through scanner (TLC Scanner 3, D2 lamp with wavelength range of 190–400 nm) and confirmed by relative front (Rf) of the sample with that of reference standards under similar conditions.
References
Battu RS, Singh B, Kooner R, Singh B (2008) Simple and efficient method for the estimation of residues of flubendiamide and its metabolite desiodo flubendiamide. J Agric Food Chem 56(7):2299–2302. doi:10.1021/jf073081s
Hoskins WM (1961) Mathematical treatment of the rate of loss of pesticide residues. FAO Plant Prot Bull 9:163–168
Shane H (2006) Flubendiamide: the next generation in Lepidopteran pest management. Paper presented at the Annual Meeting of the Entomological Society of America (ESA) held at Research Triangle Park, NC, 10–13 Dec 2006
Acknowledgments
The authors are thankful to Indian Council of Agricultural Research, India for sponsoring the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahoo, S.K., Sharma, R.K., Battu, R.S. et al. Dissipation Kinetics of Flubendiamide on Chili and Soil. Bull Environ Contam Toxicol 83, 384–387 (2009). https://doi.org/10.1007/s00128-009-9782-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9782-9