Abstract
The distribution of polycyclic aromatic hydrocarbons (PAHs) and n-alkanes was investigated in the surface sediment from the Shinano River in Niigata, Japan. The total concentrations of the PAHs and n-alkanes ranged from 102 to 10,450 ng/g dry weight and from 160 to 3,530 ng/g dry, respectively. Perylene (Per) in the sediment samples originated from both the same anthropogenic sources as benzo[a]pyrene and the natural sources; the anthropogenically derived Per was estimated at 0.4%–27% of the Per in the sediment. The carbon preference index values ranged from 0.77 to 2.48, suggesting the differences in the n-alkane sources at the investigated sites. The investigated sites were divided into three clusters by the cluster analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polycyclic aromatic hydrocarbons (PAHs) and n-alkanes distribute in the environment as ubiquitous contaminants (Cranwell and Koul 1989). The PAHs originate from the incomplete combustion of organic matter, the emissions of petrogenic products and the transformation of biogenic precursors. Although some PAHs, such as perylene, have biogenetic sources (Cranwell and Koul 1989; Kawata et al. 1997), most PAHs have anthropogenic sources. The anthropogenic PAHs mainly enter surface waters via atmospheric fallout, urban run-off, municipal effluents and oil spillage or leakage (Vrana et al. 2001). The n-alkanes originate from artificial sources, e.g., the biomass burning and the emissions of petroleum products, as well as natural sources, e.g., terrestrial vegetation and biogeneic. The PAHs and n-alkanes migrate from the surface water to the sediment because of their extremely low solubility in water. Therefore, sediment is the best media for evaluation of the impact of PAHs and n-alkanes on the water environment (Tareq et al. 2005). A number of studies have been performed on the distributions in river sediment of PAHs including the 16 PAHs listed as priority pollutants in Test Methods SW-846 by the US Environmental Protection Agency (Vrana et al. 2001); several papers have been published on the n-alkane distributions in river sediment (Ye et al. 2007).
The Shinano River is the longest river in Japan with a total length of 367 km. We have previously reported the distributions of four five-ring PAHs i.e., benzo[a]pyrene (BaP), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF) and perylene (Per), and 29 compounds with two to four rings, including naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flt) and pyrene (Pyr), in the surface sediment from rivers in Niigata including the Shinano River (Kawata et al. 1997, 2005). The objective of this study was to assess the recent distribution of the common PAHs and Per as well as n-alkanes in the surface sediment from the Shinano River, and to identify their compositions for their potential sources. The target PAHs were Nap, Acy, Ace, Flu, Phe, Ant, Flt, Pyr, benz[a]anthracene (BaA), chrysene (Chr), benzo[c]phenanthrene (BcP), BbF/ benzo[j]fluoranthene/BkF (BFs), BaP, Per, 3-methylcholanthrene (3Mc), dibenz[a,h]anthracene (DahA), indeno[1,2,3-c,d]pyrene (Inp) and benzo[g,h,i]perylene (BghiP). The target n-alkanes contain decane (C10) to dotriacontane (C32).
Materials and Methods
An ultrasonic processor VCX-130PD, 20 kHz and 130 W (Sonics & Materials, Newtown, CT), equipped with a titanium alloy probe (3 mm diameter), were used for extraction procedures. A Finnigan POLARIS Q gas chromatograph–ion trap mass spectrometer (Thermo Electron, Waltham, MA) equipped with a 30 m × 0.25 mm id (0.25 μm film thickness) fused-silica HPX-5 column (Agilent, Palo Alto, CA) was used for the quantitative analysis. The standard chemicals for the target compounds were purchased from Supelco (Bellefonte, PA). The other reagents were purchased from Wako (Osaka, Japan) and Kanto (Tokyo, Japan). Standard solutions, surrogate solutions and an internal standard solution (50 μg/mL) were prepared in acetone. A Varian Mega Bond Elut Si (Palo Alto, CA) was washed with 10 ml of dichloromethane prior to use.
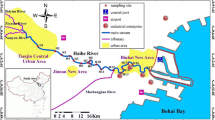
Sediment samples were collected at 14 sites from the Shinano River in Niigata Prefecture, Japan (Fig. 1) from November 2005 to April 2006. The sediment samples were stored at 5°C in the dark, and were extracted within 48 h after collection. The organic content (Table 1) was estimated as the loss upon ignition (550°C, 15 h) and calculated as percent dry weight (Kawata et al. 2005). The extraction procedure is as follows. Surrogate compounds (5 μg each) were added to 50 g of a sediment sample, and then the sample was added to a 50 mL portion of acetone. To the mixture was added 10 mL of dichloromethane and then ultrasonicated for 10 min. The slurry was next centrifuged at 3,000 rpm (1,700×g) for 10 min. This supernatant solvent phase was filtered through a glass-fiber filter. The extraction procedure was repeated twice. The extracts were combined and washed three times with 50 mL of purified water. After the organic layer was dried over anhydrous sodium sulfate, 100–250 mg of copper powder was added to the solution by shaking to remove the sulfur. The resulting solution was concentrated to about 5 mL by an evaporator and then to 1 mL under a purified nitrogen gas stream. The obtained solution was placed in a silica gel cartridge and eluted with 30 mL of dichloromethane (fraction 1), and subsequently with 50 mL of dichloromethane–acetone (95 + 5, v/v) solution (fraction 2). Each of eluate was condensed to 1 mL. A 1 μg internal standard solution was added to each of the concentrated solutions for GC/MS determination. The GC/MS conditions were as follows: column temperature programmed from 50°C (held for 2 min) to 290°C (held for 60 min) at 5°C/min, injector temperature 250°C, injection mode splitless, helium carrier gas flowrate 1.0 mL/min, MS transfer temperature 290°C, ion source temperature 250°C, ionization mode electron impact, ionization energy 70 eV. The quantification ions were 128 for Nap, 152 for Acy, 154 for Ace, 166 for Ful, 178 for Phe and Ant, 202 for Flt and Pyr, 228 for BaA and Chr, 228 for BcP, 252 for BFs, 252 for BaP and Per, 268 for 3Mc, 276 for Inp, 278 for DahA, 276 for BghiP, 57 and 71 for n-alkanes, 136 for naphthalene-d 8 (Nap-d 8 ), 188 for phenanthrene-d 10 (Phe-d 10 ), 212 for pyrene-d 10 (Pyr-d 10 ), 264 for perylene-d 12 (Per-d 12 ), and 243 for 9-bromoanthracene. The PAHs and n-alkanes were determined using the internal standard method. The PAH concentrations were adjusted for the recovery efficiencies of the surrogate compounds. The overall recoveries and relative standard deviations for the surrogate compounds were 32% and 22% for Nap-d 8 , 65% and 26% for Phe-d 10 , 65% and 17% for Pyr-d 10 , and 80% and 13% for Per-d 12 , respectively. The concentrations were calculated on a dry basis.
Results and Discussion
The PAH concentrations in the sediment are summarized in Table 1. The total concentrations of the PAHs (T-PAHs) ranged from 102 ng/g at site 4 to 10,450 ng/g at site 1. Site 1 is at a port located on the mouth of the river. Therefore, it was mainly contaminated by emissions from ships. Site 13, which had the second highest T-PAHs, is located at an urban and industrial area with textile factories and dye works factories; site 8, the third highest site, is located at 15 km downstream of an urban area and metalworking factories. Therefore, sites 13 and 8 were contaminated by emissions from the manufacturing plants. The detected compounds showed their characteristic compositions at each site. The four-ring PAHs were the major compounds at site 14; the five-ring PAHs except for Per and six-ring PAHs were the major compounds at sites 5, 6 and 13, and sites 1 and 12, respectively. Therefore, the sediments reflected the local contaminations around and upstream of the investigated sites (Kawata et al. 2005). Per was the major at sites 7, 9 and 10. As described later, Per in the sediment could be derived from natural sources as well as anthropogenic ones (Kawata et al. 1997).
Per was evaluated for its characteristic distribution by comparison with BaP, which originated from anthropogenic sources. The ratio of Per to BaP was compared to the ratio of BaP to the T-PAHs. The Per/BaP ratio in the sediment significantly decreased at p < 0.01 with the increasing BaP/T-PAHs (r = −0.793). Hence, the Per in the sediment samples originated not only from the same anthropogenic sources as BaP, but also from the natural sources as previously reported (Cranwell and Koul 1989). The anthropogenically derived Per in the sediment was estimated using the Per to BaP ratios in airborne particulate matter (Kawata et al. 1997). The anthropogenic Per in the sediment, PerANT, was calculated by the following Eq. (1):
where BaP is the BaP concentration in the sediment, and (Per/BaP)APM, 0.15, is the mean of the relative Per ratio to BaP in the airborne particulate matter in Niigata (Kawata et al. 1997). The naturally derived Per, PerNAT, was calculated by subtracting the PerANT from the Per concentration. The obtained PerANT and PerNAT values are listed in Table 1. The percent PerANT ratios were relatively high (25% and 27%) at sites 2 and 13, respectively, whereas those at the other sites were low (0.4% at site and 10%–12% at site 3). The mean values of the percentile PerANT in this study were 4.2% geometric and 7.5% arithmetic, which were lower than those (7.8% and 20%, respectively) of 80 sediments from the rivers and the coast of Niigata (Kawata et al. 1997).
The n-alkane concentrations in the sediments are summarized in Table 2. The total concentrations of the n-alkanes (T-Cs) ranged from 160 ng/g at site 6 to 3,530 ng/g at site 13. The T-PAHs at site 1 was 24 and 26 times the concentrations at sites 7 and 11, respectively (Table 1). In contrast, the T-Cs at site 1 was almost the same as that at site 11 and 83% of that at site 7. Moreover, the ratios of the T-PAHs at sites 3, 4 and 5, which are located within 2 km of each other, were 3.0:1.0:3.0 (Table 1), while the ratios of the T-Cs were 0.38:1.0:0.68. Therefore, the origins of the n-alkanes in the sediments could differ from those of the PAHs. The n-alkanes, except for C10, were classified into four groups according the carbon numbers of the n-alkanes, (C11–C19)ODD, (C12–C20)EVEN, (C21–C31)ODD and (C22–C32)EVEN, where (C i –C j )ODD and (C i –C j )EVEN are the concentrations of the n-alkane with odd and even carbon numbers, respectively, over the range i–j. The total concentrations of each group are listed in Table 2. Although the compositions of the four n-alkane groups were characteristic at each site, Σ(C21–C31)ODD was the major compounds except for sites 3, 4, and 14. Σ(C12–C20)EVEN was predominant at site 14, while Σ (C22–C32) EVEN was the major compounds at sites 3 and 4. The mean carbon number (MCN) of the n-alkanes in the sediment samples was calculated according to the following Eq. (2):
where [C i ] and [T-Cs] are the concentration of the n-alkane with carbon number i and that of the T-Cs, respectively. The MCN values (Table 2) at sites 5, 8 and 14 were 18–25, whereas those at the other sites were 27–29. The low MCN values could be ascribed to the high relative abundance of trees compared to grasses and herbs during the low flux of organic carbon (Tareq et al. 2005). The carbon preference index (CPI) has been used to estimate the source of the n-alkanes. The CPI values for the petroleum were reported to be 0.93–1.07 (Zdanaviciute et al. 2007). On the other hand, the CPI values for plants were reported to be 2.3–54.3 (Bi et al. 2005). Therefore, CPI values close to unity indicate the greater contribution of n-alkanes from artificial sources, e.g., biomass burning, and petroleum pollution; higher CPI values indicate a higher contribution from natural sources, e.g., terrestrial vegetation and biogeneic (Tareq et al. 2005). The CPI was calculated using the following Eq. (3):
where (C i –C j )ODD and (C i –C j )EVEN are the concentrations of the n-alkane with an odd carbon number and that with an even carbon number, respectively, over the range i–j. The calculated CPI values are given in Table 2. The CPI values at sites 9 and 13 were >2.0. Therefore, the natural sources could contribute to the n-alkanes at these sites. On the other hand, the CPI at sites 1, 3, 4, 7 and 10 were within 1.00 ± 0.20. Thus the artificial sources could predominantly contribute to the n-alkanes. Moreover, the CPI at site 14 was 0.77, which most approximates the CPI at site 4. However, the n-alkane compositions at these sites were markedly different, and the MCN at site 14 (18) was quite lower than that at site 4 (29). This indicates that the artificial sources at site 14 were different from those at site 4. In addition, Tareq et al. (2005) reported that the CPI correlated with the MCN in sediments from Indonesia. However, the MCN did not significantly correlate with the CPI in our study. This indicates the differences in the n-alkane sources at both investigated sites.
The similarity in the distribution patterns of the PAHs and n-alkanes from the sampling sites was compared using the cluster analysis. Among the PAHs, BaP and Per were selected, because the BaP concentration significantly correlated with those of the most anthropogenic PAHs, and Per has both anthropogenic and natural sources. Σ(C21–C31)ODD and Σ(C22–C32)EVEN were used for the cluster analysis, because the distributions of the n-alkanes were represented by the total concentrations of the n-alkanes with odd carbon numbers and those with even carbon numbers, and the n-alkanes with 21–31carbons were the predominant ones at most sites. To avoid the effect of the concentration levels, all the variables were divided by the total concentrations. Namely, BaP/T-PAH, Per/T-PAH, Σ(C21–C31)ODD/T-Cs and Σ(C22–C32)EVEN/T-Cs were used for the cluster analysis. Each variable was standardized to a mean of 0 and standard deviation of 1. Ward’s method was used as the algorithm of the analysis. The obtained dendrogram is shown in Fig. 2. Sites 1–14 were divided into three clusters (C1–C3). C1 contained seven sites with relatively low Per compositions, whereas C2 and C3 contained the sites with relative high Per compositions. Moreover, the percent PerANT ratios were 4.7%–27% and 0.4%–3.2% in C1 and the other clusters, respectively. Therefore, the sites in C1 could be affected by anthropogenic sources compared to the sites in C2 and C3; site 14 (C3) was a unique point with the lowest MCL and CPI of the investigated sites described above. C1 consisted of two semiclusters (C1a and C1b); the sites in C1a had higher BaP, Per and Σ(C22–C32)EVEN compositions, and lower CPI values than the sites in C1b. This suggests that the former sites could be affected by the anthropogenic sources more than the latter sites. Similarly, C2 consisted of two semiclusters (C2a and C2b); the sites in C2b had higher Σ(C22–C32)EVEN compositions and lower CPI values (close to unity) than the sites in C2a, suggesting that the former sites could be affected by the anthropogenic sources more than the latter sites. Consequently, the clusters could be characterized by the four variables.
References
Bi X, Sheng G, Liu X, Li C, Fu J (2005) Molecular and carbon and hydrogen isotopic composition of n-alkanes in plant leaf waxes. Org Geochem 36:1405–1417. doi:10.1016/j.orggeochem.2005.06.001
Cranwell PA, Koul VK (1989) Sedimentary record of polycyclic aromatic and aliphatic hydrocarbons in the Windemere catchment. Water Res 23:275–283. doi:10.1016/0043-1354(89)90092-4
Kawata K, Tanabe A, Mitobe H, Sakai M, Yasuhara A (1997) Distribution of perylene and five-ring polycyclic aromatic hydrocarbons in sediment and airborne particulate matter. Toxicol Environ Chem 63:97–106. doi:10.1080/02772249709358520
Kawata K, Tanabe A, Asada T, Oikawa K (2005) Distribution of semivolatile cyclic compounds in sediment from Niigata, Japan. Bull Environ Contam Toxicol 75:546–553. doi:10.1007/s00128-005-0786-9
Tareq SM, Tanoue E, Tsuji H, Tanaka N, Ohta K (2005) Hydrocarbon and elemental carbon signatures in a tropical wetland: biogeochemical evidence of forest fire and vegetation changes. Chemosphere 59:1655–1665. doi:10.1016/j.chemosphere.2005.02.019
Vrana B, Paschke A, Popp P (2001) Polyaromatic hydrocarbon concentrations and patterns in sediments and surface water of the Mansfeld region, Saxony-Anhalt, Germany. J Environ Monit 3:602–609. doi:10.1039/b104707h
Ye B, Zhang Z, Mao T (2007) Petroleum hydrocarbon in surficial sediment from rivers and canals in Tianjin, China. Chemosphere 68:140–149. doi:10.1016/j.chemosphere.2006.12.074
Zdanaviciute O, Dakhnova MV, Zheglova TP (2007) Geochemistry of oil and source rocks and petroleum potential of the western part of Baltic Syneclise. Abstract 15th Meet Assoc European Geolog Soc 82–83
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hori, T., Shiota, N., Asada, T. et al. Distribution of Polycyclic Aromatic Hydrocarbons and n-Alkanes in Surface Sediments from Shinano River, Japan. Bull Environ Contam Toxicol 83, 455–461 (2009). https://doi.org/10.1007/s00128-009-9743-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9743-3