Abstract
The concentrations of polycyclic aromatic hydrocarbons (PAHs) were determined in 25 surface sediment samples from five sites located at Oludeniz Lagoon of the Turkish Mediterranean coast. The total concentration of the PAHs (1.85 ± 1.39 mg/kg) was lower than the sediments from highly urbanized areas, while it was comparable with the sediments from similar locations. Acenaphthene and chrysene were dominant ones with the concentrations of 0.620 and 0.515 mg/kg, respectively. The isomeric ratios indicated that combustion is the predominant source of PAHs in the sediments. Factor analysis solution supports the same finding with three major factors accounting for 71.7 % of the variability in the data. Factor 1 with 43.4 % of the total variance identified as a pyrogenic source (coal combustion; 4 rings PAH and traffic related pollution; 5–6 rings PAHs). Factor 2 (explains 39 % of the total variance) represents PAHs originating from traffic, and factor 3 (explains 12 % of the total variance) represents petrogenic source. Our results suggest that combustion of fossil fuels affects most of the points, followed by combustion of biomass and human activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are one of the major categories of pollutants mainly originating from anthropogenic emissions like incomplete combustion and pyrolysis of organic matters including wood, fossil fuels, asphalt and industrial waste exposed to high temperature (WHO World Health Organization 1998; Yang et al. 1991). The PAHs originating from crude and refined petroleum are called as petogenic, and the ones produced as a result of combustion are also called as pyrogenic. Among all the contributing sources, stationary sources contribute about 80 % of the total emissions. As mobile sources, diesel- and gasoline-powered vehicles can be considered (Mostafa et al. 2009). In some countries like Turkey, biomass burning, forest fires and agricultural activities are also contributing significantly to the total PAH budget (Godoi et al. 2004).
Once produced, PAHs are transported either by atmospheric transport or stream pathways like oil spills, industrial discharges, municipal and urban runoff, and finally enter into the marine environment and accumulating in the sediments. Since PAHs are not very soluble in the water and hydrophobic, they are in the form of colloids, dissolved organic matter or suspended particles and eventually deposited in the sediment (Guo et al. 2009). Their semi-volatile nature makes them very mobile in the environment as a result of re-volatilization after deposition in different compartments of the environment. They are characterized by high toxicity, high stability in the environment and high lipophilicity, resulting in their transport through the trophic chain with final destination, the human organism (IARC 1983; Okay et al. 2000). Their occurrence in sediments is a major concern for human health, as several of them are known to be potential human carcinogens including benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[a]pyrene and benzo[ghi]perylene. Their adverse health effects on organisms are due to endocrine-disrupting activity (Kanaki et al. 2007; Zaghden et al. 2007)
Marine sediments consist an important source of information considering the human activities in the coastal area as well as the fate of xenobiotics during long-term time intervals. The scientific interest in the quality of marine sediments is quite recent and has increased especially during the last 10 years in relation to the application of the Water Framework Directive 2000/60/EC (Water Framework Directive 2000; Naddeo et al. 2005; Arsene et al. 2009; Demetriou 2004; Mylopoulos et al. 2008).Therefore, they have been included in the priority list of the Water Framework Directive 2000/60/EC, and also, 16 of them have been regulated by the US EPA as priority pollutants, and their distributions in the environment, sources and potential human health risks have become the focus of much attention (Anyakora et al. 2005). Although no relevant regulation has been established, the monitoring of PAHs in sediments can provide information for the assessment of the potential toxic effects and sources of these compounds as well as for the support of decision-making from management authorities.
The identification of precursors of PAHs that occur in the environment is a difficult process as the sources and the dynamics of these substances in the environment are very diverse (Venturini and Tommasi 2004). In spite of this difficulty, some PAHs show comparable thermodynamic stability and kinetics in the environment. Therefore, the ratio of the concentrations of some isomers has been used as an efficient method to assign the possible sources of PAHs observed in the sea sediments (Yunker et al. 1994; Budzinski et al. 1997; Dickhut et al. 2000; Yunker et al. 2002; Cortazar et al. 2008; Guo et al. 2009; Mostafa et al. 2009). In addition to isomeric ratios, statistical analysis method like principal component analysis (PCA) can be used for source identification.
Relatively, little study is conducted in the sea sediments of the Turkish costs. Their sources and composition in the sea sediments are almost unknown. Therefore, it is important to evaluate the PAH levels in sediment samples obtained from the Oludeniz Lagoon of Turkish Mediterranean coast where a huge number of tourists visit every year. This study will provide better understanding of the levels and origin of the PAH pollution observed in the Oludeniz Lagoon, a fragile region of the coast towards pollutions. Considering all the aspects stated above, the aims of this study were to determine the concentrations of PAHs in sediments of the Oludeniz Lagoon of Turkish Mediterranean coast as well as to assess their possible sources using isomeric ratios and the statistical analysis method like factor analysis (FA) which is a multivariate analyses that is used to reduce a large number of variables (measured PAH contents in sediment samples) into fewer numbers of factors. This technique extracts maximum common variance from all variables and puts them into a common score which means to simplify a complex data set by representing the set of variables in terms of a smaller number of underlying variables, known as factors (Hopke et al. 1967).
Experimental
Study area and sampling
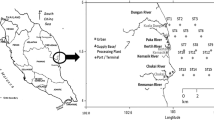
Ölüdeniz is one of the unique lagoons in the world, located in the intersection of the South Aegean and West Mediterranean coast of the Turkey (Fig. 1), and its depth ranges from 0.5 to 14 m. This site is vulnerable to several pollution sources due to huge number of tourists visiting the area, unplanned population and beach houses. Because of the above reasons, the Turkish Government has chosen this area as the natural heritage area. However, up to the time of this study, there was no scientific research dedicated to identify the serious sources of pollution from tourism and community activities. For this goal, sediment samples were collected at different locations of the studied site in February 2008. Sediment samples were measured at a total of five sampling points inside the lagoon. At each sampling points, five sediment samples were taken using grab sampler.
The top 0–1-cm surface layer was collected by using grab sediment sampler. Samples (100–250 g) were stored in a refrigerator at −20 °C in colored nylon bags in order to prevent any damage caused by the sunlight.
Materials and methods
All chemicals used during the study were of analytical reagent grade. The PAH-mix 68 (100 mg/L in cyclohexane) and internal standard-mix 25 (500 mg/L of acenaphtene-D10, perylene-D12, chrysene-D12, phenantherene-D10 in acetone) from Dr. Ehrenstorfer were used for the analysis of PAHs. Standards for the calibration curve were prepared by diluting them with dichloromethane (DCM, Merck, HPLC-grade). Standard reference materials, which were SRM 1941b (marine sediment) and 1597a (complex mixture of PAHs from coal tar prepared in DCM), were bought from National Institute of Standards and Technology. The surrogate solution (Surrogate Standard Base/Neutrals Mix 19) including three surrogates, nitrobenzene D5, p-terphenyl D14, 2-fluorobiphenyl, was bought from Dr. Ehrenstorfer. The concentration of surrogate was 1,000 μg/mL in DCM solution. The GC-MS surrogate solution mix which includes acenaphtene-D10 (Ace-D10), phenanthrene-D10 (Phe-D10), chrysene-D12 (Chr-D12) and perylene-D12 (Per-D12) (1,000 mg/L, in Acetone) was bought also from Dr. Ehrenstorfer. High purity nitrogen (99.999), helium (99.999), hydrogen (99.999) and dry air (99.999) were used for the analyses. The sodium sulphate (Na2SO4, Merck, Darmstadt, Germany) was used for the removal of the water from extracted samples. High purity glass wool was used during the extraction of sediment samples. Cellulose extraction thimbles (Schleicher & Schuell Microscience 603, 33 × 80 mm, ref. no. 10350240) were used for soxhlet apparatus. Sodium sulphate (anhydrous extra pure, Merck) and glass-wool were pre-cleaned by transferring them to a large glass column (1-L capacity or larger) and washing sequentially with hexane and DCM before use. Sodium sulphate was activated in an oven at 400 °C for 4 h. Glass-wool was conditioned overnight at 225 °C in an oven. Cellulose extraction thimbles were cleaned with proper solvents prior to the use with soxhlet extraction system. Alconox detergent powder (Supelco Cat. No. 1104) was used for the cleaning of all glassware used in laboratory. All glasswares were cleaned with detergent in hotwater and rinsed with deionized water. After rinsing with DCM/acetone, they were dried in an oven overnight.
All the PAH analysis was performed with Hewlett-Packard (HP) 6890 GC system equipped with a 5973 mass selective detector. Heidolph rotary evaporator (laborota 4000 efficient) and mini-vap evaporator with six ports (Catalog No. 22970, Cat. No. 22971) were used for the evaporation of solvents. Bransonic ultrasonic bath (Model B-2200 E4, 205 W, 220 V) was used for the sample extractions. Deionized water (ultra filtered type 1 water) was supplied by Barnstead nanopure ultrapure water system.
Analytical procedures
The following PAHs were analyzed: acenaphthene(Ace), acenaphthylene (Acy), anthracene (Ant), benzo[a]anthracene (BaA), chrysene (Chry), benzo[a]pyrene (BaP), benzo[b/k]fluoranthenes (Bb/kF), benzo[g,h,i]perylene (Bghi), dibenzo[a,h]anthracene(DahA), fluoranthene (Fluo), fluorene (Flu), indene[1,2,3-cd] pyrene (IP), naphthalene (Nap), phenanthrene (Phe) and pyrene (Pyr). All the PAH concentrations are reported on a dry weight (dw) sediment basis.
Ultrasonic bath extraction was optimized using chemo metrics, and optimized conditions were used for the extraction of 44 sediment samples collected from Ölüdeniz lagoon. For ultrasonic bath extraction, the most important three factors were the following: extraction time, solvent volume and amount of sediment. They were investigated at two levels which are low and high. Magnitude of the low and high values was determined by considering EPA procedures and literature studies (EPA METHODs 3540 C, 1996 and SW-846 3550C, 2007).
Surrogates were added to each sample before an extraction in order to observe extraction efficiency. Totally, four surrogates were used, and each surrogate was assigned to the different sets of PAHs according to number of rings. For example, acenaphtene-D10 (Ace-D10) and phenanthrene-D10 (Phe-D10) were used for the recovery calculations of three ring PAHs, chrysene-D12 (Chr-D12) for four rings PAHs and perylene-D12 (Per-D12) for the five to six ring PAHs. Calculated average recoveries (n = 26) of surrogates in sediment samples were 41, 60, 85 and 86 % for Ace D10, Phe D10, Chr D12 and Per D12 correspondingly
A strict procedure for quality control was maintained during the experiments. Surrogate standards were used throughout to compensate for losses from sample extraction and work-up. For all preparation methods, blank solutions were run parallel to the samples, and their results were taken into consideration. Sediment samples, spiked with surrogates, were analyzed with good precision ranging from 1 to 5 %. A strict QC/QA procedure was applied during the analyses. Used extraction and analyses methods were verified using NIST SRM 1597 as a reference for GC-MS. Table 1 shows the certified and found concentrations (as mg/L and with standard deviations) of PAHs in SRM 1597a, determined by using GC-MS. As can be seen from Table 1, percent recoveries of the PAHs are lying between 53.1 and 150. Details of analytical procedures are detailed in another publication by our group (Tuncel and Topal 2011, American Journal of Analytical Chemistry).
Results and discussion
PAHs in sediments
The mean and total concentrations for all the samples and for each individual sampling points for the 16 PAHs in sediment samples are presented in Fig. 2 and Table 2. There are five sampling stations with five samples each means a total of 25 samples. Each point is the average of five samples. Sampling point 4 had the highest concentration which was close to the mouth of the lagoon. Sampling points 1 and 3 were inside lagoon, and sediment samples had similar PAH concentrations. These sampling stations were nearest to the one only hotel around the lagoon. The sampling stations 2 and 5 were located just outside of the lagoon. The average total PAH (∑PAH) concentration in Ölüdeniz sediment samples was found as 1.85 ± 1.39 mg/kg (n = 25) as given in Table 2. Total PAH concentrations in sampling stations 2 and 4 are above the average value (showed as a line at x-axis, 1.85) and the rest are below the average value. Acenaphtene, chrysene, fluoranthene, phenanthrene and indeno(1,2,3–c,d) pyrene have the highest concentrations among the 16 PAHs analyzed.
Produced data were compared with literature and given in Table 3. As can be seen from Table 3, the total PAH concentration in Oludeniz lagoon is comparable with the values cited for sea sediments under urban influence like Palermo area in Italy, 60–1427 ng/g ( Gianguzza et al. 2006), Casco Bay in USA, 2,900 ng/g, (Kennicutt et al. 1994) and San Diego Bay again in USA 3,000 ng/g; (Anderson et al. 1996). But, Oludeniz sea sediments have lower concentrations as compared to the samples collected from harbors’, and higher concentration from open sea sediments. For example, Simpson et al. (1996) and Hong et al. (1995) reported 66,700 and 5,277 ng/g for Kitimat Harbor, Canada and Victoria Harbor, Hong Kong, China. In spite of these very high concentrations observed for harbors’, Zhou et al. (2000 and Maldonado et al. (1999) reported 367 and 137 ng/g for Western Baltic Sea and North western Black sea, respectively.
Based on the data in the literature for sediments, some researchers (Kim et al. 1999; Jiang et al. 2009; Hong et al. 1995) suggested classification criteria as an area is considered ‘highly contaminated’ when concentrations of total PAHs are higher than 500 ng/g, ‘moderately contaminated’ when concentrations range from 250 to 500 ng/g and ‘slightly contaminated’ when concentrations are lower than 250 ng/g. Based on this classification, station no. 4 is moderately, and others are slightly contaminated. Sampling station 4 with the highest total concentration was located to the mouth of the lagoon. Sampling stations 1 and 3 were inside lagoon and exhibited similar concentrations. They were near to the hotel around the lagoon. Therefore, hotel cannot be the source of contamination in the lagoon. However, motor boats which are close to mouth of the lagoon can be the source as can be understood from the sample 4 concentration. Sampling points of 2 and 5 were close to each other, and they were located just from the opening of the lagoon to the sea.
Sediment quality determinations for Ölüdeniz sediments
In order to determine the quality of the sediments collected from Oludeniz lagoon, the recommended sediment quality values for 18 PAHs were used (Table 4). There are four levels for the PAH pollution in Table 4. Both individual and the total PAH concentrations were considered in order to decide the level of pollution in sediment samples.
The consensus-based sediment quality guidelines (CBSQGs 2003) define three classes for the toxicity levels. These three levels are threshold effect concentration (TEC), lower concentration at which toxicity is unlikely; the probable effect concentration (PEC), upper concentration at which toxicity is probable; and finally the midpoint effect concentration (MEC) which is a concentration midway between the TEC and PEC concentrations (TEC + PEC/2 = MEC). There are four levels which are level 1 (≤TEC), level 2 (TEC < level 2 ≤ MEC), level 3 (MEC < level 3 ≤ PEC) and level 4 (>PEC).
To compare the study site concentrations with the table concentrations on a common basis, study site concentrations should be divided by the study site % total organic carbon (TOC) concentrations to yield a dry wt. normalized value. Using our TOC results, all PAH concentrations in Ölüdeniz sediment samples are classified as level 1, except Ace (level 4), Phe (level 2), BaA (level 2), Chr (level 2), IcP (level 2) and DaA (level 2).
Contributing sources
Studies conducted in different countries like UK (Stevens et al. 2003), Portugal (Perez et al. 2001) and France (Blanchard et al. 2004) indicted that the hydrolyses of organic matter originating from municipal sewage in wastewater treatment plants contribute to the desorption of the content of simpler structure PAHs with five and six aromatic rings are preferably adsorbed onto the particles forming the sludge. Among these simple PAHs, Phe, Fluo, Pyr and Nap can be counted. In total concentration from five sampling points, concentrations were about 42 % of the total. But, PAHs with five and six rings were in higher percent in the station no. 4. According to Yunker et al. (2002), high concentrations of these compounds are strongly linked to human activities. Predominant source of these PAHs with molar mass 276 and 278 is the oil products. Result of this study is consistent with this possibility as the station no. 4 is located at the mouth of the lagoon and exposed to the effluent from boating activities.
Considering the origin of the observed PAHs, the ones with MM 252 ( BbkF and BaP) are the most important in emissions produced by burning wood, coal fossil fuels (especially diesel) and vegetation. But, once released to the atmosphere, their concentrations decline rapidly due to depredation induced by sunlight (Yunker et al. 2002). Those of the PAHs from pyrolytic sources BaP are the major health concern because of its carcinogenic potential (WHO World Health Organization 1998). In our case, BaP is observed at moderate concentration ranging from 295 to 85 ng/g. This observation is also an indication of contribution of the combustion sources.
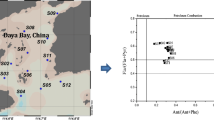
For identification of contributing sources, the ratio between the concentrations of the isomers has been used as an efficient method (Budzinski et al. 1997; Yunker et al. 2002). When human activities or combustion are the main sources of PAHs, the parent compound with low thermodynaic stability degrades faster compared to others with the same molecular mass. This causes a decrease of the ratio between a less stable parent PAHs and its more stable isomer, depending on the source. This ratio can be expressed as R 178 = [Ant]/[178], R 202 = [Flu]/[202] and R 228 = [BaA] /[228], where [178], [202] and [228] are the sum of the concentrations of isomers Ant and Phe (MM 178); Flu and Pyr (MM 202); and BaA and Chry (MM 228), respectively. Using these ratios, one can differentiate pyrogenic and anthropogenic sources of PAHs due to the high stability of these isomers related to other parent PAHs and also due to their great abundance in the environment (Yunker et al. 1994). When the ratio R178 is less than 0.10, it generally indicates that these compounds are from petrogenic sources. Diesel and lubricants are among the oil products that have this profile. If the ratio is greater than 0.10, it points to the dominance of combustion sources (Budzinski et al. 1997) mainly from coal and shale (Yunker et al. 2002). The pair of isomers Flu and Pyr shows the transition between the pyrogenic and petrogenic sources at 0.50 for R 202 and the combustion of kerosene, vegetation, coal and wood tend to have R 202 higher than 0.50. Emissions of gasoline, diesel and fuel oils by combustion and emissions from cars and diesel trucks usually have values lower than 0.50. The results of this research show (Fig. 3) PAHs in sediments with the R 178 ratio of anthracene to anthracene plus phenanthrene, Ant/(Ant + Phe) > 0.1, were typical of combustion sources (pyrogenic source). In addition, PAHs in sediments with the R 202 ratio of fluoranthene to fluoranthene plus pyrene, Fla/(Fla + Pyr) > 0.5, indicate that PAHs are mainly from combustion of grass, wood and coal. The value of 0.4 < Fla/(Fla + Pyr) [R 202] < 0.5 indicates the transition between oil and the products of combustion. Values lower than 0.40 indicate that the isomers BaA and Chry in the sediments are predominantly from combustion sources, except at station no. 2, presented an R 202 value of 0.08 representing the interface between combustion and mixed sources (the origin of parent PAHs can be oil as pyrogenic sources) (Mannino and Orecchio 2007). With this findings at hand, ratios at station no. 3 indicate mixed sources.
To make further analyses about contributing sources, factor analysis (FA) was performed using SPSS 17.0 program package. All factors with eigenvalues over 1 were extracted according to Bartlett’s test of sphericity and were rotated using the Varimax method. The variables in the same factor assumed to be originating from the same common source.
Having 25 samples is the limiting issue in our case, but still, we applied the analyses for further quantification of contributing sources. In spite of the limited number of samples, FA solution provided three factors which explain 95 % of total variance (Table 5). Result of three factors which we consider three different sources was in accordance with ratio analyses. The first factor explains that 43.94 % total variance was named as a pyrogenic source (coal combustion) as it contains mostly four ring PAHs. Among those were Fla, Pyr, BaA, Chr, Bbf and BkF. The second factor was named as traffic with 39 % of total variance as this factor contains 5,6 ring PAHs like Ace Fle Phe Ant Pyr BaA. The last one explains the lowest % total variance (12 %). We named this factor as petrogenic. It contains BkF with high loading.
Conclusions
Extraction and analysis methods validated by our group were used in this paper. Moderate level of concentrations of PAHs in sediments was obtained in Oludeniz sediment samples. Acenaphtene and chrysene were dominant ones among the sixteen PAHs analyzed. The predominance of Phe, Fluo and Pyr suggests the possibility of combustion and human activities as major contributing sources. Benzo[g,h,i]perylene, dibenzo[a,h]anthracene and indeno[1,2,3-cd]pyrene were predominant in sampling site nearby to hotel
Isomeric ratios indicated that combustion is the predominant source of PAHs. Further investigating the the pyrogenic sources, FA was performed to the data matrix which resulted with three factors as a pyrogenic source (coal combustion; four ring PAHs, a traffic related pollution; five to six ring PAHs) and a petrogenic source. Our results suggest that combustion of fossil fuels affects most of the points, followed by combustion of biomass and human activity.
This work provided important information about the occurrence, distribution and origin of PAHs in sediment from Oludeniz Lagoon, demonstrating that some water bodies of the Turkish Mediterranean coast already have moderate contamination by PAHs. This is a warning sign because of the touristic importance of the region.
References
Anderson B, Hunt J, Hester M, Phillips B (1996) Assessment of sediment toxicity at the sediment-water interface. In: Ostrander GK (ed) Techniques in aquatic toxicology. Lewis Publishers, Ann Arbor
Anyakora C, Ogbeche A, Palmer P, Coker H, Ukpo G, Ogah C (2005) GC/MS analysis of polynuclear aromatic hydrocarbons in sediment samples from the Niger Delta region. Chemosphere 60:990–997
Arsene C, Bougatioti A, Mihalopoulos N (2009) Sources and variability of non-methane hydrocarbons in the Eastern Mediterranean. http://www.srcosmos.gr/srcosmos/showpub.aspx?aa=11179
Baumard P, Budzinski H, Garrigues P, Dizer H, Hansen PD (1999) Polycyclic aromatic hydrocarbons in recent sediments and mussels (Mytilus edulis) from the Western Baltic Sea: occurrence, bioavailability and seasonal variations. Mar Environ Res 47:17–47
Blanchard M, Teil MJ, Ollivon D, Legenti L, Chevreuil M (2004) Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in wastewaters and sewage sludge’s from the Paris area (France). Environ Res 95:184–197
Budzinski H, Jones I, Bellocq J, Pi’erard C, Garrigues P (1997) Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde Estuary. Mar Chem 58(1–2):85–97
Consensus-based sediment quality guidelines; recommendations for use & application (2003) ,http://dnr.wi.gov/topic/brownfields/documents/cbsqg_interim_final.pdf
Cortazar E, Bartolom’e L, Arrasate S, Usobiaga A, Raposo JC, Zuloaga O (2008) Distribution and bioaccumulation of PAHs in the UNESCO protected natural reserve of Urdaibai, Bay of Biscay. Chemosphere 72:1467–1474
Demetriou J D (2004) Polluted Water Effluent in the Sea. http://journal.gnest.org/sites/default/files/Journal%20Papers/demetriou.pdf
Dickhut RM, Canuel EA, Gustafson KE, Liu K, Arzays KM, Walker SE (2000) Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region. Environ Sci Technol 34:4635–4640
Gianguzza A, Mannino MR, Olivo A, Orecchio S (2006) Occurrence and concentratıon of PAHs ın clams and sedıments of the marıne coastal lagoon of ganzırrı (Italy). extractıon, GC-MS analysıs, dıstrıbutıon and sources. Fresenius Environ Bull 15:1023–1030
Godoi AFL, Ravindra K, Godoi RHM, Andrade SJ, Santiago-Silva M, Vaeck LV (2004) Fast chromatographic determination of PAHs in aerosol samples from sugar caneburning. J Chromatogr A 1027:49–53
Guo W, He MC, Yang ZF, Lin CY, Quan XC, Men B (2009) Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in Daliao River water system in dryseason, China. J Hazard Mater 164:1379–1385
Hong HS, Xu L, Zhang L, Chen JC, Wong YS, Wan TSM (1995) Environmental fate and chemistry of organic pollutants in the sediment of Xiamen and Victoria Harbours. Mar Pollut Bull 31:229–236
Hopke PK, Gladney ES, Gordon GE, Zoller WH, Jones GA (1967) The use of multivariate analysis to identify sources of selected elements in the Boston urban aerosol. Atmos Environ 10:1015–1025
IARC (1983) Approaches to Classifying Chemical Carcinogens According to Mechanism of Action (IARC intern. tech. Rep. No. 83/001)
Jiang JJ, Lee CL, Fang MD, Liu JT (2009) Polycyclic aromatic hydrocarbons in coastal sediments of southwest Taiwan: an appraisal of diagnostic ratios in source recognition. Mar Pollut Bull 58:752–760
Johnson AC, Larsen PF, Gadbois DF, Humason AW (1985) The distribution of polycyclic aromatic hydrocarbons in the surficial sediments of Penobscot Bay (Maine, USA) in relation to possible sources and to other sites worldwide. Mar Environ Res 15:1–16
Kanaki M, Nikolaou A, Makri CA, Lekkas DF (2007) The occurrence of priority PAHs, nonylphenol and octylphenol in inland and coastal waters of Central Greece and the Island of Lesvos. Desalination 210(1–3):16–23
Kennicutt II, McDonald SJ, Mahlon C, Sericano J, Wade TL, Liu H, Safe SH (1994) Correlation between bioassay-derived P4501A1 induction activity and chemical analysis of clam (Laternula elliptica) extracts from McMurdo Sound, Antarctica. Chemosphere 28(12):2237–2248
Khim JS, Kannan K, Villeneuve DL, Koh CH, Giesy JP (1999) Characterization and distribution of trace organic contaminants in sediment from Masan Bay, Korea. Environ Sci Technol 33:4199–4205
Kim GB, Maruya KA, Lee RF, Lee JH, Koh CH, Tanabe S (1999) Distribution and sources of PAHs in Kyeonggi Bay, Korea. Mar Pollut Bull 38:7–15
Klamer HJC, Fomsgaard L (1993) Geographical distribution of chlorinated biphenyls (CBs) and polycyclic aromatic hydrocarbons (PAH) in surface sediments from the Humber Plume, North Sea. Mar Pollut Bull 26:201–206
Maldonado C, Boyana JM, Bodineau L (1999) Sources, distribution, and water column processes of aliphatic and polycyclic aromatic hydrocarbons in the Northwestern Black Sea water. Environ Sci Technol 33:2693–2702
Mannino RM, Orecchio S (2007) Polycyclic aromatic hydrocarbons (PAHs) in indoor dust matter of Palermo (Italy) area: Extraction, GC-MS analysis, distribution and sources. Atmos Environ 42:1801–1817
Mostafa AR, Hegazi AH, El-Gayar MS, Andersson JT (2009) Source characterization and the environmental impact of urban street dusts from Egypt based on hydrocarbon distributions. Fuel 88:95–104
Mylopoulos Y, Kolokythas E, Vagiona D, Kampragou E, Eleftheriadou E (2008) Hydrodiplomacy in practice: transboundary water management in Northern Greece. http://journal.gnest.org/sites/default/files/Journal%20Papers/287-294_451_MYLOPOULOS_10-3.pdf
Naddeo V, Zarra T, Belgiorno V (2005) European procedures to river quality assessment. http://journal.gnest.org/sites/default/files/Journal%20Papers/paper_11_NADDEO_383.pdf
Okay OS, Donkin P, Peters LD, Livingstone DR (2000) The role of algae (Isochrysis galbana) enrichment on the bioaccumulation of benzo[a]pyrene and its effects on the blue mussel Mytilus edulis. Environ Pollut 110(1):103–113
Pereira WE, Hostettler FD, Luoma SN, van Geen A, Fuller CC, Anima RJ (1999) Sedimentary record of anthropogenic and biogenic polycyclic aromatic hydrocarbons in San Francisco Bay, California. Mar Chem 64:99–113
Perez M, Romero LI, Sales D (2001) Organic Matter Degradation Kinetics in an Anaerobic Thermophilic Fluidised Bed Bioreactor. Anaerobe 7(1):25–35
Simpson CD, Mosi AA, Cullen WA, Reimer KJ (1996) Composition and distribution of polycyclic aromatic hydrocarbon contamination in surficial marine sediments from Kitimat Harbor, Canada. Sci Total Environ 181(3):265–278
Stevens LJ, Northcott GL, Stern GA, Tomy GT, Jones KC (2003) PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks and polychlorinated alkanes in U.K. sewage sludge: survey results and implications. Environ Sci Technol 37:462–467
Tuncel SG, Topal T (2011) Multifactorial optimization approach for determination of polycyclic aromatic hydrocarbons in sea sediments of Turkish Mediterranean coast. AJAC 2–7:783–794
Venturini N, Tommasi LR (2004) Polycyclic aromatic hydrocarbons and changes in the trophic structure of polychaete assemblages in sediments of Todos os Santos Bay, Northeastern, Brazil. Mar Pollut Bull 48:97–107
Water Framework Directive (2000) EC Directive of the European Parliament and of the Council 2000/60/EC establishing a framework for community action in the field of water policy, Official Journal C513 (2000), 23/10/2000
WHO (World Health Organization) (1998) Selected nonheterocyclic polycyclic aromatic hydrocarbons, Environmental Health Criteria 202. United Nations Environment Programme, International Labor Organization, Geneva
Yang SYN, Connell DW, Hawker DW, Kayal SI (1991) Polycyclic aromatic hydrocarbons in air, soil and vegetation in the vicinity of an urban roadway. Sci Total Environ 102:229–240
Yunker MB, Macdonald RW, Whitehouse BG (1994) Phase associations and lipid distributions in the seasonally ice-covered Arctic estuary of the Mackenzie Shelf. Org Geochem 22(3–5):651–669
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zaghden H, Kallel M, Elleuch B, Oudot J, Saliot A (2007) Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Sfax, Tunisia, Mediterranean Sea. Mar Chem 105(1–2):70–89
Zhou JL, Hong HS, Zhang ZL, Maskaoui K, Chen WQ (2000) Multi-phase distribution of organic micropollutants in Xiamen Harbour, China. Water Res 34:2132–2150
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Tuncel, S.G., Topal, T. Polycyclic aromatic hydrocarbons (PAHs) in sea sediments of the Turkish Mediterranean coast, composition and sources. Environ Sci Pollut Res 22, 4213–4221 (2015). https://doi.org/10.1007/s11356-014-3621-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3621-1