Abstract
We characterized 16 polycyclic aromatic hydrocarbons (PAHs) in surface sediments of natural mangrove habitats in China, as well as assessed their sources and the risks they pose. Our results indicate that the total concentrations of the 16 PAHs ranged from 3.16 to 464.05 ng g−1 dw (mean value of 72.80 ng g−1 dw), which were generally lower than those in other coastal environments in China and in other countries. The compositional patterns were dominated by four-ring PAHs, including fluoranthene, pyrene, benzo[a]anthracene and chrysene. Petrogenic sources, specifically, petroleum spills, were the dominant sources of PAHs in the surficial sediments of mangroves in China. Selected ratios of PAHs from two-tailed Pearson correlation analysis and principal-component analysis for different sites also indicate pyrolytic sources of PAHs. Results of the ecological risk assessment show little negative effect of most of the PAHs in the surface sediments. Overall, the data obtained in this study reveal relative low PAHs pollution in the mangrove swamps of China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the recent increase in population, food production, as well as industrial and urban development along coastlines, threats to coastal environments due to pollutants such as polycyclic aromatic hydrocarbons (PAHs) have been increasing. Because of their toxic, bioaccumulative, persistent, mutagenic, and carcinogenic properties, PAHs have become an increasing concern for public health (Chen et al. 2011; Deng 2013; Guo et al. 2012; Wu et al. 2003). Sixteen PAHs (Table 1) have been identified as priority pollutants by the United States Environmental Protection Agency (US EPA). PAHs comprise a group of organic pollutants containing two or more fused aromatic rings of carbon and hydrogen atoms. They are found in many aquatic environments around the world, including marine swamps (Bouloubassi et al. 2012; Bragato et al. 2012; Callén et al. 2013; Carver et al. 1986; Kaivosoja et al. 2012; Lea-Langton et al. 2013; Readman et al. 2002).
Mangrove wetlands, one of the most important coastal swamps in the world, consist of woody trees and shrubs that occur in a saline intertidal environment along tropical and subtropical coasts. Mangroves have important roles in coastal protection, supporting their own area and adjacent coastal fisheries, as well as providing timber and a host of other natural products (Ong et al. 2004). Mangroves are known to show high primary productivity, having abundant detritus, rich organic carbon, anoxic/reduced conditions, and the capacity to efficiently trap suspended material from the water column. Consequently, mangrove wetlands are sites of intense material processing with a potentially high impact on the cycling of pollutants, including PAHs (Li et al. 2015; Yuan et al. 2001). Because they are located at the interface of terrestrial and marine ecosystems, mangroves are susceptible to anthropogenic contamination with PAHs through tidal water, river water, and land-based sources. Many mangrove swamps have been found to be contaminated by PAHs, with the total concentration of PAHs in some areas being higher than that in marine sediments (Li et al. 2014a, b; Zhang et al. 2004). This finding suggests that high levels of PAHs in mangroves are an important risk posed on living organisms (Payne et al. 2003).
In China, several mangroves are sites of intensive anthropogenic activity. Contamination of mangrove swamps by PAHs has become increasingly serious (Zhang et al. 2014). In the last decade, several investigations have been carried out to characterize their composition, transport, and accumulation, as well as to elucidate their sources and to assess the potential risk they pose on the China coast. Limited information exists on the accumulation of PAHs in Chinese mangroves. Previous studies suggest that PAH contamination has significant spatial and temporal variations and that greater understanding of PAH contamination of Chinese mangroves is necessary. The present work, therefore, aimed to investigate the extent, spatial distribution, environmental risk, and major possible sources of PAHs in contaminated mangrove sediments collected from five provinces in China.
Materials and methods
Sample collection
Surface sediments were sampled from natural mangrove swamps in Zhejiang, Fujian, Guangdong, Guangxi, and Hainan (Fig. 1). We collected 112 samples of surface sediments (0–5 cm) using a metal scoop and then immediately transferred them into clean aluminum bags. Sampling details were list in Table 2. Each sediment sample was a composite of five random samples in the same location. The samples were transported to the laboratory as soon as possible and kept in a freezer at −20 °C for >12 h before they were freeze-dried.
Sample extraction and cleanup
The analysis of all the samples was conducted in Marine Geology Experiment Testing Center of China Geological Survey, Qingdao, China. For PAH analysis, the samples were freeze-dried and ground into a fine powder (<0.25 mm). Extraction of PAH was carried out according to the method reported by Nicola et al. (2005). Sieved samples (10 g) were spiked with a mixture of perdeuterated internal standards ([d-10]phenanthrene, [d-10]pyrene, [d-12]chrysene, [d-12]perylene, and [d-12]benzo[g,h,i] perylene), and each sample was subsequently mixed with equal quantities of anhydrous sodium sulfate in 60 mL of dichloromethane/acetone (1:1, v/v) solution for 20 min in a sonicator thrice. The extract was concentrated, solvent-exchanged with n-hexane, and further reduced to approximately 1 mL under gentle nitrogen flow. The resulting extract was loaded into a 1:2 alumina/silica gel glass column for fractionation and cleanup. The first fraction, which contained aliphatic hydrocarbons, was eluted with 15 mL of n-hexane. The second, which contained PAHs, was collected by elution with 5 mL of n-hexane and 70 mL of methylene chloride/n-hexane (30:70) solution. The PAH fractions were concentrated to 0.4 mL under a gentle N2 stream. Known quantities of internal standard were added to the sample prior to instrumental analysis. Consecutively, the dried residues were dissolved in 1 mL of n-hexane.
Instrumental analysis
PAH concentrations were measured by gas chromatography using an HP 5890 GC equipped with an HP-5MS capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness) coupled to an HP 5975 mass spectrometer with a mass-selective detector. The carrier gas, helium, was introduced at a flow rate of 1.0 mL min−1. The oven temperature was initially set at 70 °C and then increased at a rate of 20 °C min−1 to 280 °C and held for 24 min. Quantitation was conducted by using primary ions for each compound; two to three secondary ions were used for qualitative confirmation.
Quality assurance and quality control
Glassware and sodium sulfate were solvent-rinsed and heated for 4 h at 450 °C prior to use. For quality assurance and control, a spiked blank, a procedural blank, matrix spikes, and a duplicate sample were analyzed in the same way as the sample for each batch of ten samples. No quantifiable targets were detected in these blanks. Analysis of a reagent blank demonstrated that the analytical system and glassware were free of contamination. The recovery for the spiked blanks was 75.0–120.0 %. Results reported in this work were not corrected for recoveries. The relative standard deviation for parallel samples is <15 % (n = 3). The detection limit for the analytical procedure ranged from 0.5 to 8 ng g−1 d w, depending on each analysed PAH.
Statistical analyses
The ratio of PHE/ANT, FLU/PYR, ANT/(ANT + PHE), and FLU/(FLU + PYR) was calculated to determine the source of PAHs. It can be categorized into different groups (Yunker et al. 2002): PHE/ANT > 10: petrogenic pollution; PHE/ANT < 10: pyrolytic pollution; ANT/(ANT + PHE) < 0.1, and FLU/(FLU + PYR) < 0.4: petroleum pollution, 0.1 < ANT/(ANT + PHE) < 0.5, and 0.4 < FLU/(FLU + PYR) < 0.5: petroleum combustion; ANT/(ANT + PHE) > 0.5, and 0.1 < FLU/(FLU + PYR) < 0.5: grass, wood and charcoal combustion. Pearson correlation analysis was used to determine the correlation between Total organic carbon (TOC) and total concentrations and the different compounds of 16 PAHs. The principal component analysis with multivariate linear regression was conducted to identify the source contributions of PAHs and performed using IBM SPSS Statistics v20 for Windows.
Analysis of total organic carbon
Total organic carbon in the sediments was analyzed by using an EA3000 element analyzer. The freeze-dried and homogenized sediments were first acidified with 10 % (v/v) HCl solution overnight to remove carbonate and then freeze-dried and analyzed for TOC (Hedges and Stren 1984).
Results and discussion
Level and spatial distribution of PAHs in the mangrove surficial sediments of China
Among the mangrove swamps, those in Chengmai had the highest PAH concentration and those in Techeng Island were the least contaminated (Fig. 2). The sequence of mean values of PAH congeners according to study areas is Chengmai > Fuding > Shenzhen > Qinzhou Bay > Dongzhaigang > Xiamen > Longhai > Nansha > Yingluo Bay > Yueqing Bay > Zhanjiang > Beilun River > Dachongkou > Zhenzhu Bay > Yunxiao > Techeng Island. Total concentrations of 16 PAHs categorized as priority pollutants by the US EPA in the mangrove sediments ranged from 3.16 to 464.05 ng g−1 dw and had a mean value of 72.80 ng g−1 dw. Site DZ18 had the highest value (464.05 ng g−1 dw) followed by sites CM1 (395.76 ng g−1 dw) and SZ1 (379.70 ng g−1 dw), while the lowest concentration was at site BL6 (3.16 ng g−1 dw), which contained only eight PAHs. The tourism industry, which is dependent on mangroves, is a major contributor to local economies in Dongzhaigang in Hainan. Exhaust from diesel engines in land and ship traffic contributes to the increase in PAH concentration. All 16 PAHs were detected in site ZZW2 at Zhenzhu Bay, site NS4 at Nansha, and site ZJ5 at Zhanjiang. We detected PAHs in all sediment samples, but the contributions of individual compounds differed. ANY was found in 5.36 % of the sediment samples, and other PAH congeners were found in over 50 % of the sample sites. PYR accounted for over 14.79 % of the concentrations of ∑16PAHs, while ANT, ANA, and ANY accounted for <1.0 %.
The PAH concentrations in the mangrove areas were generally higher than those of other coastal environments in previous study (Bernard et al. 1996; Tam et al. 2001; Nagelkerken et al. 2008). However, different mangrove swamps had different characteristics. In our study, Chengmai had the highest mean concentration (222.56 ng g−1 dw), which is less than that for other mangrove areas in China, such as those in Hong Kong (138–2135 ng g−1 dw; Ke et al. 2002). It is similar to the recently reported concentrations in mangrove surficial sediments of Beibu Gulf (3.01–388 ng g−1 mean value of 95.5 ng g−1 dw; Li et al. 2015) and lower than those in some coastal mangrove swamps of China in the previous study (113–728 ng g−1 dw, mean value of 378 ng g−1 dw; Zhang 2006) and in mangrove surficial sediments of Deep Bay (238–726 ng g−1 dw; Zhang et al. 2004). This difference may be due to the strictly enforced environmental controls for mangrove protection in recent years. The increasing storm frequency and intensity associated with climate change and sea-level rise highlight the importance of coastal protection, as mangrove forests provide many goods and services. Concentrations were also markedly lower than those in other coastal areas of China such as Xiamen Western Harbor (425.3–1522.4 ng g−1 dw; Yuan et al. 2001) and the Pearl River Estuary (144–1289 ng g−1 dw, mean value of 430 ± 216 ng g−1 dw; Yuan et al. 2015), and slightly lower than those in the intertidal zones of Bohai Bay (Huang et al. 2011) and Hangzhou Bay (Chen et al. 2006). Mangrove ecosystems are important intertidal estuarine wetlands along coastlines of tropical and subtropical regions that have abundant microorganisms. Many isolated bacterial and fungal species in mangroves have been reported to be capable of biodegrading petroleum hydrocarbons and even polynuclear aromatic hydrocarbons (Marquez-Rocha et al. 2005). Results of two-tailed Pearson correlation analyses show no correlation between ∑PAHs and gross domestic product (GDP) of cities in mangrove areas. However, cities near areas of low PAH concentrations also had low GDP. This difference may be due to the long distances of these areas from areas with intensive human activity, the economies of which are dependent on tourism, as well as due to the absence of significant pollutant resources in these areas. The results of Futian in Shenzhen were much lower than the previous study in Shenzhen (1649–7925 ng g−1 dw, mean value of 4480 ng g−1 dw; Li et al. 2014a, b), that might result from the different position of the sample sites. And many polluted factories have been moved from Futian to other districts in Shenzhen in recent years to better conserve the mangrove wetland in Futian National Nature Reserve. Except for that in the mangrove area of Chengmai (222.56 ng g−1 dw), the average concentrations of the 16 PAHs in the mangrove sediments in China were lower than those in mangrove sediments from other parts of the world, including the exclusive economic zone of Qatar in the Arabian Gulf (2.6–1025.1 ng g−1 dw, mean value of 117.3 ± 255.9 ng g−1 dw; Soliman et al. 2014), Guadeloupe in France (49–1065 ng g−1 dw, mean value of 188.0 ng g−1 dw; Ramdine et al. 2012), the coasts of Coco’ River (3.04–2234.76 ng g−1 dw) and Ceara’ River (3.34–1859.21 ng g−1 dw) in Fortaleza (Cavalcante et al. 2009), and Todos os Santos Bay (8–4163 ng g−1 dw) in Brazil (Venturini et al. 2004). However, concentrations in most of the surficial sediments of mangrove areas in China are higher than those in similar sediments in the Malaysian Peninsula (20–112 ng g−1 dw; Raza et al. 2013). PAH concentrations in mangrove sediments from China were lower than those in wetland soils for various land uses in the coast of the Pearl River estuary: industrial sites of Panyu (mean value of 1019 ± 289 ng g−1 dw), wharf sites (mean value of 842 ± 203 ng g−1 dw), cropland sites (mean value of 690 ± 173 ng g−1 dw), milldam sites (mean value of 617 ± 92 ng g−1 dw), and natural wetland sites (mean value of 427 ± 135 ng g−1 dw) (Xiao et al. 2014). They are also lower than those in Jiaozhou Bay wetland soils (176.1–563.3 ng g−1 dw, mean value of 345.3 ng g−1 dw; Lang et al. 2015), in reed wetland soils of Liaohe Estuary (235–374 ng g−1 dw; Li et al. 2014a, b), in Kiawah Island Retention Pond in Carolina (mean value of 122.5 ± 93.7 ng g−1 dw; Weinsteina et al. 2010), in Poyang Lake in China (105–513 ng g−1 dw; Zhao et al. 2014), in Taihu Lake in China (209–1003 ng g−1 dw; Zhang et al. 2012), in a mangrove swamp following an oil spill in Hong Kong (138–2135 ng g−1 dw; Ke et al. 2002), in mudflat sediments (180–830 ng g−1 dw), and in mangrove area sediments (630–960 ng g−1 dw) in Hong Kong (Zheng et al. 2002). They are similar to those in the old Yellow River Estuary in China (100.4–197.3 ng g−1 dw, mean value of 167.5 ng g−1 dw) (Yuan et al. 2014), Luan River Estuary in China (Zhang et al. 2016) but higher than those in Chongming wetland in China (38.7–136.2 ng g−1 dw; Wang et al. 2012), in agricultural soils in Poland (1–55.1 ng g−1 dw; Maliszewska-Kordybach et al. 2009), in continental shelf sediments of a discharge area near the Coatzacoalcos River in the Gulf of Mexico (5.9–83.6 ng g−1 dw, mean value of 39.9 ng g−1 dw; Ruiz-Fernandez et al. 2016), and in the Elizabeth River wetlands (1.2–22.2 ng g−1 dw; Kimbrough and Dickhut 2006). Various anthropogenic activities, such as continuously discharges of domestic sewage from households, effluents from industrial processes, construction of highways and heavy traffic are responsible for the high concentrations of PAHs in the surfical sediments of Chengmai mangrove. Therefore, the Chinese government should control the pollution to protect the mangrove areas while enabling the economy to develop.
Composition patterns of PAHs in sediments from Chinese mangroves are shown in Fig. 3. Mean concentrations of two-, three-, four-, five-, and six-ring PAHs in the areas under study were 6.31, 12.76, 28.34, 20.90, and 11.14 ng g−1 dw, respectively. Compositional patterns were dominated by four-ring PAHs (FLU, PYR, BaA, and CHR). Percentages of four-ring PAHs to total PAHs ranged from 27.67 to 53.12 %. Those for two- and three-ring PAHs (NAP, ANA, ANY, FLR, PHE, and ANT) ranged from 12.30 to 36.76 %, and those for five- and six-ring PAHs (BbF, BkF, BaP, DBA, BPE, and IPY) ranged from 23.44 to 57.61 %. Mean percentages of PAHs in the areas follow the trend four-ring > five-ring > three-ring > six-ring > two-ring. Because of major differences in mineralogy, grain size, organic matter, water content, and sources of anthropogenic inputs, the distribution of hydrocarbons in the environment can vary greatly from one area to another. PAHs with few aromatic rings are less thermodynamically stable and can easily be degraded by microbes in mangrove sediments. Rankings of PAH congeners according to number of rings in the areas are as follows. Dachongkou in Guangxi and Longhai in Fujian: four-ring > three-ring > five-ring > two-ring > six-ring. Beilun River and Qinzhou Bay in Guangxi, Xiamen in Fujian: four-ring > three-ring > five-ring > six-ring > two-ring. Dongzhaigang in Hainan, Zhenzhu Bay in Guangxi, and Yunxiao in Fujian: four-ring > five-ring > three-ring > two-ring > six-ring. Fuding in Fujian and Chengmai in Hainan: five-ring > four-ring > six-ring > three-ring > two-ring. Yingluo Bay in Guangxi: four-ring > five-ring > six-ring > two-ring > three-ring. Nansha in Guangdong: four-ring > five-ring > six-ring > three-ring > two-ring. Techeng Island in Guangdong: four-ring > two-ring > five-ring > three-ring > six-ring. Zhanjiang in Guangdong: two-ring > four-ring > six-ring > five-ring > three-ring. Yueqing Bay in Zhejiang: five-ring > four-ring > three-ring > six-ring > two-ring. Futian in Guangdong: four-ring > five-ring > three-ring > six-ring > two-ring.
Correlation of PAHs with TOC
Kim et al. (1999) demonstrated that sediment properties such as organic matter content influence the distribution and concentration of PAHs. Results of two-tailed Pearson correlation analysis show a significant positive correlation (n = 112, p < 0.01) between total PAH content and TOC in the study (Fig. 4).
Sources of PAHs in the mangrove surficial sediments of China
PAHs are distributed in the environment throughout the world and are generated by many pathways. Therefore, recognition of PAH sources is necessary for the control of their distribution and for allocation of responsibility for remedial actions. In modern ecosystems, the two main sources of PAHs are classified as pyrogenic or petrogenic. Different sources produce different compounds and lead to different concentrations in the environment. Studies have shown the practicability of using isomeric ratios and two-tailed Pearson correlation analyses for PAH concentrations (Budzinski et al. 1997; Magi et al. 2002; Sicre et al. 1987; Soclo et al. 2000). Therefore, we used these to assess further the sources of PAHs in surficial sediments in China’s mangrove areas.
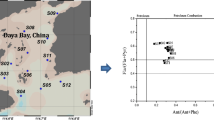
PHE/ANT, FLU/PYR, ANT/(ANT + PHE), and FLU/(FLU + PYR) ratios have been proven to be useful in the identification of PAH sources (Fang et al. 2007; Guo et al. 2006). Figure 5 showed the ratios and sources of PAHs in surficial sediments of mangrove areas in China. PHE is more thermodynamically stable between the two structural isomers. A higher PHE/ANT ratio (>10) was observed with petrogenic pollution, and a lower ratio (<10) with pyrolytic pollution. We also assumed that petroleum usually contains compounds that are more thermodynamically stable, such as NAP, FLU, PHE, and CHR. FLU and PYR are usually the most abundant compounds in pyrolytic PAH sources such as combustion of petroleum, grass, wood, and coal (Magi et al. 2002; Sicre et al. 1987; Zhang et al. 2004). Therefore, FLU/PYR ratios of >1 indicate pyrolytic origin, and values of <1 indicate petrogenic sources (Magi et al. 2002). PHE/ANT ratios for sediments from BL4 in Beilun River in Guangxi are <10, and FLU/PYR ratios are >1, suggesting that the source of the site was pyrolytic. Sites DZ9, DZ16, DZ17, and DZ18 in Dongzhai; CM1 and CM2 in Chengmai; SK2, SK3, SK6, SK8, SK10, SK11, SK12, and SK14 of Yingluo Bay; BL3, BL5, BL7, BL9, BL10, BL11, and BL12 in Beilun River; DCK1 in Dachongkou; all sites in Zhenzhu Bay, Qinzhou Bay and Xiamen, except site XM4; all sites in Yunxiao; LH1, LH3, LH4, and LH6 in Longhai in Fujian; all sites of Nansha and Shenzhen; sites TCD1, TCD3, and TCD5 on Techeng Island; ZJ5 in Zhanjiang; sites FD1, FD2, and FD3 in Fuding; all sites in Yueqing Bay were petrogenic sources. PAH sources in other sites were a mixture of pyrogenic and pyrolytic sources.
ANT/(ANT + PHE) and FLU/(FLU + PYR) ratios indicate that both combustion and petroleum were the sources in sites BL1 and BL13 on Beilun River, in DCK1 in Zhenzhu Bay, in QZ3 in Qinzhou Bay, in sites SK4 and SK7 in Yingluo Bay (Guangxi), in LH2 in Longhai, in XM4 in Xiamen, in FD1 and FD3 in Fuding in Fujian, in all sites in Yueqing (Zhejiang), in TCD4 on Techeng Island (Guangdong), as well as in DZ3, DZ4, DZ7, DZ8, DZ10, DZ11, DZ12, and DZ13 in Dongzhaigang (Hainan). The ANT/(ANT + PHE) ratio was >1 and the FLU/(FLU + PYR) ratio was >0.5 in site BL4 in Beilun River (Guangxi), as well as in sites FD4 and FD5 in Fuding in Fujian only, indicating that their source was combustion of grasses, wood, and coal. Only site FD2 in Fuding in Fujian and other sites had a petroleum combustion source.
We performed two-tailed Pearson correlation analyses to assess further the source of PAHs in the areas. ANY shows no correlation with other compounds, indicating that they may have different sources. A positive correlation between NAP, FLR, PHE, ANT, FLU, PYR, BaP, IPY, DBA, and BPE, IPY (n = 112, p < 0.01) was observed. Similar results were also obtained with high molecular weight (HMW) compounds (four-, five-, and six-ring compounds). Low molecular weight (LMW) PAHs (two- and three-ring PAHs) are usually generated by release of petroleum and by diagenetic processes, and HMW PAHs (four-, five-, and six-ring PAHs) are generated by combustion of organic matter at very high temperature.
Principal-component analysis was performed to understand further the relationship between PAH compositions and possible chemical sources for each factor. Loading plots of the four principal components are showing in Fig. 6. Most of the variance (83.59 %) of the normalized dataset may be explained by the first four factors.
Principal-component 1 (PC1) explains 54.25 % of the total variance. High positive loadings of NAP, FLR, PHE, ANT, FLU, PYR, BaA, CHR, BbF, BkF, BaP, DBA, BPE, and IPY are listed in Fig. 6. Except for FLR, PHE, and ANT, the compounds have high molecular weight. PAHs with low molecular weight are abundant in petrogenic sources mainly caused by petroleum spills (Dobbins et al. 2006; Marr et al. 1999), whereas those with high molecular weight are abundant in pyrolytic sources. Samples with high positive loadings mainly contained four- to six-ring PAHs. BPE has been identified as a tracer for automobile emissions because it has been found to be enriched along with BaP in a traffic tunnel (Harrison et al. 1996; Larsen and Baker 2003). BkF and its isomers such as BbF and BaP are dominant compounds in particulate samples of roadside air (Boonyatumanond et al. 2007). Therefore, samples with high positive score mainly contained five- and six-ring PAHs, and vehicular traffic pollution was a major contributor to PAH contamination for this component. PC2 explains 16.42 % of the total variance. It is positively dominated by FLR and PHE. NAP, ANA, FLR, and PHE which were mainly from petrogenic sources, but the negative compounds were mainly high-molecular-weight PAHs such as BaA, CHR, BbF, BkF, BaP, DBA, BPE, and IPY. The latter compounds were mainly from pyrolytic sources. PC3 and PC4 were positively dominated by low-molecular-weight PAHs of petrogenic origin dominated by petroleum spills.
The three methods led to the conclusion that petroleum spills were the main petrogenic sources in surficial sediments of mangrove areas in China. Some areas also had pyrolytic sources. PAHs in surficial sediments of mangrove areas may come from exhaust from diesel engines of small ships of the growing tourism industries along the mangrove swamps. Ships, industrial emissions, heavy traffic, and coal heating near the mangrove swamps could also result in the increase in PAH loadings via atmospheric deposition and freshwater runoff. Heavy traffic and transportation may introduce large amounts of PAHs into nearby mangrove forests and may be responsible for major PAH contamination of surficial sediments in mangrove areas.
Risk assessments of PAHs in the mangrove surficial sediments
To evaluate the biological risk posed by PAHs in sediments, Long et al. (1995) proposed reliable guideline values based on the percentage of biotas adversely affected by exposure. A concentration of total PAHs lower than the effects range low (ERL) indicates that the PAHs in the sediments do not have any adverse effect on organisms; concentrations between the ERL and effects range median (ERM) imply that the PAHs may cause occasional damage to the biota in the region; if the concentration exceeds the ERM, then the PAHs cause frequent damage.
Table 3 presents toxicity guidelines for 12 PAHs and lists sites in three different ranges defined by the ERL and ERM. Concentrations of individual PAHs except those in sites DZ18 and SZ1 were all below the ERL, indicating that the PAHs in the sediments do not have any adverse effect on organisms. In sites DZ18 and SZ1, however, fluorene concentrations were between the ERL and ERM, indicating that biological effects related to fluorene occur occasionally at the two sites. All 16 PAHs were detected in the surficial sediments samples; thus, more attention should be paid, although the probability of the negative effects of the PAHs compounds is low.
Conclusions
Although the total PAH concentrations ranged from 3.16 to 464.05 ng g−1 dw (mean value of 72.80 ng g−1 dw), most sediments of mangrove swamps in China have relative low PAH contamination compared to other coastal ecosystems. The compositional patterns were dominated by four-ring PAHs, including fluoranthene, pyrene, benzo[a]anthracene and chrysene. The PAHs in the mangrove swamps under study mainly originated from petrogenic sources, in particular, petroleum spills. PAHs in the surficial sediments might have come from the exhaust from diesel engines of small ships of developing tourism industries along the mangrove swamps. Ships, industrial emissions, heavy traffic, and coal heating near the mangrove swamps could also have resulted in the increase in PAH loadings via atmospheric deposition and freshwater run off. The ecological risk assessment revealed that most of the sediments have low probability of negative effects caused by the 16 PAHs in mangrove wetlands of China. Due to the recent rapid urbanization and industrial development, and so pollution drainage induced from human activities inputs around the mangrove swamps should be properly managed to avoid further contamination by PAHs.
References
Bernard D, Pascaline H, Jeremie JJ (1996) Distribution and origin of hydrocarbons in sediments from lagoons with fringing mangrove communities. Mar Pollut Bull 32:734–739
Boonyatumanond R, Murakami M, Wattayakorn G, Togo A, Takada H (2007) Sources of polycyclic aromatic hydrocarbons (PAHs) in street dust in a tropical Asian mega-city, Bangkok, Thailand. Sci Total Environ 384(1–3):420–432
Bouloubassi I, Roussiez V, Azzoug M, Lorre A (2012) Sources, dispersal pathways and mass budget of sedimentary polycyclic aromatic hydrocarbons (PAH) in the NW Mediterranean margin gulf of lions. Mar Chem 142–144:18–28
Bragato M, Joshi K, Carlson JB, Tenório JAS, Levendis YA (2012) Combustion of coal, bagasse and blends thereof: Part II: speciation of PAH emissions. Fuel 96:51–58
Budzinski H, Jones I, Bellocq J, Pierrad C, Garrigues P (1997) Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Mar Chem 58:85–97
Callén MS, López JM, Iturmendi A, Mastral MA (2013) Nature and sources of particle associated polycyclic aromatic hydrocarbons (PAH) in the atmospheric environment of an urban area. Environ Pollut 183:166–174
Carver JH, Machado ML, MacGregor JA (1986) Application of modified salmonella/microsome prescreen to petroleum-derived complex mixtures and polynuclear aromatic hydrocarbons (PAH). Mutat Res 174(4):247–253
Cavalcante RM, Sousa FW, Nasciment RF, Silveira ER, Freire GS (2009) The impact of urbanization on tropical mangroves (Fortaleza, Brazil): evidence from PAH distribution in sediments. J Environ Manag 91:328–335
Chen CW, Chen CF (2011) Distribution, origin, and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in sediments of Kaohsiung Harbor, Taiwan. Mar Pollut Bull 63:417–423
Chen ZM, Gao XJ, Song ZG, Mai BX (2006) Distribution and source identification of polycyclic aromatic hydrocarbons in the tide-beach surface sediments of Hangzhou Bay. China Environ Sci 26(2):233–237
Deng W (2013) A preliminary study on the composition, distribution and source apportionment of aliphatic and polycyclic aromatic hydrocarbons in surface sediments from the South Yellow Sea and East China Sea. Colleage of Marine Geosciences, Qingdao
Dobbins RA, Fletcher RA, Benner JBA, Hoeft S (2006) Polycyclic aromatic hydrocarbons in flames, in diesel fuels, and in diesel emissions. Combust Flame 144(4):773–781
Fang MD, Hsieh PC, Ko FC, Baker JE, Lee CL (2007) Sources and distribution of polycyclic aromatic hydrocarbons in the sediments of Kaoping River and submarine canyon system, Taiwan. Mar Pollut Bull 54:1179–1189
Guo ZG, Lin T, Zhang G, Yang ZS, Fang M (2006) High-resolution depositional records of polycyclic aromatic hydrocarbons in the central continental shelf mud of the East China Sea. Environ Sci Technol 40:5304–5311
Guo GH, Wu FC, He HP, Zhang RQ, Li HX, Feng CL (2012) Distribution characteristics and ecological risk assessment of PAHs in surface waters of China. Sci China Earth Sci 55(6):914–925
Harrison RM, Smith DJT, Luhana L (1996) Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ Sci Technol 30(3):825–832
Hedges JI, Stren JH (1984) Carbon and nitrogen determinations of carbonate-containing solids. Limnol Oceanogr 29:657–663
Huang GP, Chen YJ, Lin T, Tang JH, Liu DY, Li J, Zhang G (2011) The distribution and ecological risk of polycyclic aromatic hydrocarbons of surface sediments in the intertidal zone of Bohai Bay, China. China Environ Sci 31(11):1856–1863
Kaivosoja T, Virén A, Tissari J, Ruuskanen J, Tarhanen J, Sippula O, Jokiniemi J (2012) Effects of a catalytic converter on PCDD/F, chlorophenol and PAH emissions in residential wood combustion. Chemosphere 88(3):278–285
Ke L, Wong TWY, Wong YS, Tam NFY (2002) Fate of polycyclic aromatic hydrocarbon (PAH) contamination in a mangrove swamp in Hong Kong following an oil spill. Mar Pollut Bull 45:339–347
Kim GB, Maruya KA, Lee RF, Lee JH, Koh CH, Tanabe S (1999) Distribution and sources of polycyclic aromatic hydrocarbons in sediments from Kyeonggi Bay, Korea. Mar Pollut Bull 38(1):7–15
Kimbrough KL, Dickhut RM (2006) Assessment of polycyclic aromatic hydrocarbon input to urban wetlands in relation to adjacent land use. Mar Pollut Bull 52:1355–1363
Lang YH, Li GL, Wang XM, Peng P (2015) Combination of Unmix and PMF receptor model to apportion the potential sources and contributions of PAHs in wetland soils from Jiaozhou Bay, China. Mar Pollut Bull 90:129–134
Larsen RK, Baker JE (2003) Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods. Environ Sci Technol 37(9):1873–1881
Lea-Langton AR, Ross AB, Bartle KD, Andrews GE, Jones JM, Li H, Pourkashanian M, Williams A (2013) Low temperature PAH formation in diesel combustion. J Anal Appl Pyrolysis 103:119–125
Li FL, Zeng XK, Yang JD, Zhou K, Zan QJ, Lei AN, Tam NFY (2014a) Contamination of polycyclic aromatic hydrocarbons (PAHs) in surface sediments and plants of mangrove swamps in Shenzhen, China. Mar Pollut Bull 85:590–596
Li GL, Lang YH, Yang W, Peng P, Wang XM (2014b) Source contributions of PAHs and toxicity in reed wetland soils of Liaohe estuary using a CMB-TEQ method. Sci Total Environ 490:199–204
Li PY, Xue R, Wang YH, Zhang RJ, Zhang G (2015) Influence of anthropogenic activities on PAHs in sediments in a significant gulf of low-latitude developing regions, the Beibu Gulf, South China Sea: distribution, sources, inventory and probability risk. Mar Pollut Bull 90:218–226
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Magi E, Bianco R, Ianni C, Carro M (2002) Distribution of polycyclic aromatic hydrocarbons in the sediments of the Adriatic Sea. Environ Pollut 119:91–98
Maliszewska-Kordybach B, Smreczak B, Klimkowicz-Pawlas A (2009) Concentrations, sources, and spatial distribution of individual polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in the Eastern part of the EU: Poland as a case study. Sci Total Environ 407:3746–3753
Marquez-Rocha FJ, Olmos-Soto J, Rosano-Hernandez MC, Muriel-Garca M (2005) Determination of the hydrocarbon-degrading metabolic capabilities of tropical bacterial isolates. Int Biodeterior Biodegrad 55:17–23
Marr LC, Kirchstetter TW, Harley RA, Miguel AH, Hering SV, Hammond SK (1999) Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environ Sci Technol 33(18):3091–3099
Nagelkerken I, Blaber SJM, Bouillon S, Green P, Haywood M, Kirton LG, Meynecke JO, Pawlik J, Penrose HM, Sasekumar A, Somerfield PJ (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185
Nicola FD, Maisto G, Prati MV, Alfani A (2005) Temporal variations in PAH concentrations in Quercus ilex L (holm oak) leaves in an urban area. Chemosphere 61:432–440
Ong JE, Gong WK, Wong CH (2004) Allometry and partitioning of the mangrove, Rhizophora apiculata. For Ecol Manag 188:395–408
Payne JR, Clayton JR, Kirstein BE (2003) Oil/suspended particulate material interactions and sedimentation. Spill Sci Technol Bull 8(2):201–221
Ramdine G, Fichet D, Louis M, Lemoine S (2012) Polycyclic aromatic hydrocarbons (PAHs) in surface sediment and oysters (Crassostrearhizophorae) from mangrove of guadeloupe: levels, bioavailability, and effects. Ecotoxicol Environ Saf 79:80–89
Raza M, Zakaria MP, Hashim NR, Yim UH, Kannan N, Ha SY (2013) Composition and source identification of polycyclic aromatic hydrocarbons in mangrove sediments of Peninsular Malaysia: indication of anthropogenic input. Environ Earth Sci 70:2425–2436
Readman JW, Fillmann G, Tolosa I, Bartocci J, Villeneuve JP, Catinni C, Mee LD (2002) Petroleum and PAH contamination of the Black Sea. Mar Pollut Bull 44(1):48–62
Ruiz-Fernandez AC, Portela JMB, Sericano JL, Sanchez-Cabeza JA, Espinosa LF, Cardoso-Mohedano JG, Perez-Bernal LH, Tinoco JAG (2016) Coexisting sea-based and land-based sources of contamination by PAHs in the continental shelf sediments of Coatzacoalcos River discharge area (Gulf of Mexico). Chemosphere 144:591–598
Sicre MA, Marty JC, Saliot A, Aparicio X, Grimalt J, Albaiges J (1987) Aliphatic and aromatic hydrocarbons in different sized aerosols over the Mediterranean Sea: occurrence and origin. Atmos Environ 21:2247–2259
Soclo HH, Garrigues PH, Ewald M (2000) Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Mar Pollut Bull 40:387–396
Soliman YS, Al Ansari EMS, Wade TL (2014) Concentration, composition and sources of PAHs in the coastal sediments of the exclusive economic zone (EEZ) of Qatar, Arabian Gulf. Mar Pollut Bull 85:542–548
Tam NFY, Ke L, Wang XH, Wong YS (2001) Contamination of polycyclic aromatic hydrocarbons in surface sediments of mangrove swamps. Environ Pollut 114:255–263
Venturini N, Tommasi LR (2004) Polycyclic aromatic hydrocarbons and changes in the trophic structure of polychaete assemblages in sediments of Todos os Santos Bay, Northeastern, Brazil. Mar Pollut Bull 48:97–107
Wang ZC, Liu ZF, Yang Y, Li T, Liu M (2012) Distribution of PAHs in tissues of wetland plants and the surrounding sediments in the Chongming wetland, Shanghai, China. Chemosphere 89:221–227
Weinsteina JE, Crawford KD, Garner TR, Flemming AJ (2010) Screening-level ecological and human health risk assessment of polycyclic aromatic hydrocarbons in stormwater detention pond sediments of Coastal South Carolina, USA. J Hazard Mater 178:906–916
Wu Y, Zhang J, Zhu ZJ (2003) Polycyclic aromatic hydrocarbons in the sediments of the Yalujiang Estuary, North China. Mar Pollut Bull 46:619–625
Xiao R, Bai JH, Wang JJ, Lu QQ, Zhao QQ, Cui BS, Liu XH (2014) Polycyclic aromatic hydrocarbons (PAHs) in wetland soils under different land uses in a coastal estuary: toxic levels, sources and relationships with soil organic matter and water-stable aggregates. Chemosphere 110:8–16
Yuan DX, Yang DN, Chen M, Xu PX, Qian YR, Wang JL (2001) Concentrations and distribution of polycyclic aromatic hydrocarbons and organo-chlorides in surface sediment of Xiamen Western Harbour and Minjiang Estuary. Acta Sci Circumstantiae 21:107–112
Yuan ZJ, Liu GJ, Wang RW, Da CN (2014) Polycyclic aromatic hydrocarbons in sediments from the Old Yellow River Estuary, China: occurrence, sources, characterization and correlation with the relocation history of the Yellow River. Ecotoxicol Environ Saf 109:169–176
Yuan K, Wang XW, Lin L, Zou SC, Li Y, Yang QS, Luan TG (2015) Characterizing the parent and alkyl polycyclic aromatic hydrocarbons in the Pearl River Estuary, Daya Bay and northern South China Sea: influence of riverine input. Environ Pollut 199:66–72
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zhang J (2006) Study on concentration, source and transportation of PAHs in typical coastal mangrove swamps. Xiamen University, China
Zhang J, Cai LZ, Yuan DX, Chen M (2004) Distribution and sources of polynuclear aromatic hydrocarbons in Mangrove surficial sediments of Deep Bay, China. Mar Pollut Bull 49:479–486
Zhang Y, Guo CS, Xu J, Tian YZ, Shi GL, Feng YC (2012) Potential source contributions and risk assessment of PAHs in sediments from Taihu Lake, China: comparison of three receptor models. Water Res 6:3065–3073
Zhang ZW, Xu XR, Sun YX, Yu S, Chen YS, Peng JX (2014) Heavy metal and organic contaminants in mangrove ecosystems of China: a review. Environ Sci Pollut Res 21:11938–11950
Zhang DL, Liu JQ, Jiang XJ, Cao K, Yin P, Zhang XH (2016) Distribution, sources and ecological risk assessment of PAHs in surface sediments from the Luan River Estuary, China. Mar Pollut Bull 102(1):223–229
Zhao ZH, Zhang L, Cai YJ, Chen YW (2014) Distribution of polycyclic aromatic hydrocarbon (PAH) residues in several tissues of edible fishes from the largest fresh water lake in China, Poyang Lake, and associated human health risk assessment. Ecotoxicol Environ Saf 104:323–331
Zheng GJ, Man BKW, Lam JCW, Lam MHW, Lam PKS (2002) Distribution and sources of polycyclic aromatic hydrocarbons in the sediment of a sub-tropical coastal wetland. Water Res 36:1457–1468
Acknowledgments
This study was supported by the Basic Fund of Ministry of Science and Technology (Grant No. 2013FY112200), the Marine Geology Survey Project (Grant No. GZH201100203), and the National Natural Science Foundation of China (Grant No. 41306064).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Dl., Liu, N., Yin, P. et al. Characterization, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in surface sediments from the mangroves of China. Wetlands Ecol Manage 25, 105–117 (2017). https://doi.org/10.1007/s11273-016-9505-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-016-9505-z