Abstract
Key message
A novel locus, qCSS3, involved in the non-seed-shattering behaviour of Japonica rice cultivar, ‘Nipponbare’, was detected by QTL-seq analysis using the segregating population with the fixed known seed-shattering loci.
Abstract
Asian cultivated rice, Oryzasativa, was domesticated from its wild ancestor, O.rufipogon. Loss of seed shattering is one of the most recognisable traits selected during rice domestication. Three quantitative trait loci (QTLs), qSH1, qSH3, and sh4, were previously reported to be involved in the loss of seed shattering of Japonica cultivated rice, O.sativa ‘Nipponbare’. However, the introgression line (IL) carrying ‘Nipponbare’ alleles at these three loci in the genetic background of wild rice, O.rufipogon W630, showed a lower value for detaching a grain from the pedicel than ‘Nipponbare’. Here, we investigated abscission layer formation in the IL and found a partially formed abscission layer in the central region between the epidermis and vascular bundles. Based on QTL-seq analysis using the F2 population obtained from a cross between ‘Nipponbare’ and the IL, we detected two novel loci qCSS3 and qCSS9 (QTL for the Control of Seed Shattering in rice on chromosomes 3 and 9), which were found to be involved in the difference in seed-shattering degree between ‘Nipponbare’ and W630. Then, we further focused on qCSS3 in order to understand its potential role on the loss of seed shattering. The candidate region of qCSS3 was found to be located within a 526-kb region using substitution mapping analysis. Interestingly, the qCSS3 candidate region partially overlaps the selective sweep detected for Japonica but not for Indica rice cultivars, suggesting that this region harbours the mutation at a novel seed-shattering locus specifically selected for non-seed-shattering behaviour in Japonica cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, the process of crop domestication involved the selection of several naturally occurring variations in wild plants that provided useful agronomic traits. These traits were related to seed size, plant architecture, seed shattering, seed dormancy, and photoperiod sensitivity (Doebley et al. 2006). Seed shattering is one of the most important characteristics for the propagation of wild plants (Dong and Wang 2015) and is caused by the degradation of the abscission layer formed between the grain and pedicel. Because seed shattering affects yield, hunter-gatherers and early farmers must have selected plants with non-seed-shattering behaviour to increase their yield (Fuller and Allaby 2009). Thus, loss of seed shattering is regarded as one of the most important domestication traits (Harlan 1975; Fuller 2007).
Oryzasativa L., Asian cultivated rice, was domesticated from its wild ancestor, O.rufipogon Griff. (Oka 1988; Fuller 2007). During the process of domestication, the seed-shattering ability of rice plants weakened or was lost as a result of the inhibition of abscission layer formation. Asian cultivated rice is generally classified into two groups: Indica and Japonica. The former normally exhibits a weak shattering habit with partial abscission layer formation, whereas the latter has a non-shattering habit with complete inhibition of abscission layer formation. Previous studies have shown that three quantitative trait loci (QTLs): sh4, qSH1, and qSH3, are involved in the loss of seed shattering (Li et al. 2006; Konishi et al. 2006; Htun et al. 2014). The major QTL is sh4, which was identified from a cross between O.nivara and O.sativa Indica explaining 69% of the phenotypic variation (Li et al. 2006). The cultivated allele of sh4 is recessive and inhibits the formation of the abscission layer, resulting in the reduction of the degree of seed shattering (Li et al. 2006; Lin et al. 2007). qSH1 was identified from a cross between O.sativa Indica ‘Kasalath’ and O.sativa Japonica ‘Nipponbare’, which explained 68.6% of the phenotypic variation (Konishi et al. 2006). A single nucleotide polymorphism (SNP) in the regulatory region of a downstream gene was shown to result in the absence of an abscission layer in ‘Nipponbare’ (Konishi et al. 2006). These two QTLs were found to be major loci involved in non-seed-shattering behaviour of most Japonica rice cultivars. However, we previously evaluated seed-shattering degree of introgression lines of wild rice carrying ‘Nipponbare’ alleles at sh4 and qSH1 (Ishikawa et al. 2010). Results show that a single introgression of ‘Nipponbare’ alleles at sh4 or qSH1 display complete shattering behaviour as wild rice, and even a double introgression at the two loci display weak seed-shattering inhibition. These findings suggest that additional loci may still be involved in a loss of seed shattering in Japonica rice cultivars. The third QTL is qSH3, which was originally detected from a cross between O.rufipogon and O.sativa Japonica (Onishi et al. 2007). We also detected qSH3 by QTL analysis in a previous study, using an F2 population generated by a cross between ‘Nipponbare’ and an introgression line (IL) carrying the ‘Nipponbare’ alleles at both qSH1 and sh4 in the genetic background of O.rufipogon W630 (Htun et al. 2014). Gene interaction of qSH3 and sh4 was also investigated in the genetic background of wild rice to understand the seed-shattering behaviour in early rice domestication (Inoue et al. 2015; Ishikawa et al. 2017), because the selection of qSH1 is specific to Japonica rice cultivars. We also evaluated the seed-shattering degree of an F2 segregating population between Indica rice cultivar O.sativa ‘IR36′ and an introgression line carrying ‘Nipponbare’ allele at qSH3 and sh4 in the genetic background of wild rice O.rufipogon W630 (Tsujimura et al. 2017). We observed the segregation of seed-shattering behaviour in the F2 population, confirming that unknown mutation(s) other than qSH3 and sh4 may underlie the non-shattering behaviour of ‘IR36′. These studies suggest that several unidentified loci may still be involved in the non-shattering of seeds during rice domestication or breeding.
Here, we first investigated the abscission layer formation in an IL having the cultivated alleles of ‘Nipponbare’ at qSH1, qSH3, and sh4 in the genetic background of wild rice, O.rufipogon W630. The IL was crossed with ‘Nipponbare’ and the seed-shattering degrees of their F2 plants were measured to detect QTL(s) other than the three known seed-shattering loci. We performed QTL-seq analysis, and two novel loci on chromosomes 3 and 9 were detected. We further analysed the effect of the putative locus on chromosome 3, and the effect of the wild allele on seed-shattering degree was confirmed with two backcross recombinant inbred lines (BRILs) in the genetic background of ‘Nipponbare’ and by a progeny test of the F2 plants. The candidate region of the locus on chromosome 3 was restricted to a 526-kb region by substitution mapping analysis.
Material and methods
Plant materials
A Japonica rice cultivar, O.sativa ‘Nipponbare’, and a wild accession of O.rufipogon W630 originated from Myanmar were used in this study. By backcrossing with W630, an IL carrying the ‘Nipponbare’ alleles at the sh4, qSH1, and qSH3 loci in the genetic background of wild rice was produced. The IL was crossed with ‘Nipponbare’, and the resulting F1 plant was self-pollinated to obtain the segregating F2 population. A total of 174 individuals were grown in pots at Kobe University, Japan, and their seed-shattering behaviour was evaluated. To minimise the effect of the difference in heading time, short-day treatment was used for all the plants. The validation of the locus detected in this study was conducted using backcross recombinant inbred lines as previously reported by Thanh et al. (2011). A progeny test was also conducted using three lines having a heterozygous chromosomal constitution at the locus detected in this study. Further substitution mapping analysis was conducted by the progeny tests using one of the three lines.
Evaluation of seed-shattering degree
The seed-shattering degree was evaluated by measuring the breaking tensile strength (BTS, gf: gramme-force), which is the value required to detach a grain from the pedicel, measured with a digital force gauge (FGP 0.5, Nidec-Shimpo Co., Japan). The BTS values of 75 seeds (25 randomly selected seeds from three panicles) were measured, approximately a month after heading, and their average BTS values were calculated.
DNA extraction, bulking, and library construction for next-generation sequencing analysis
For QTL-seq analysis, bulked DNA samples were prepared as described in previous studies (Abe et al. 2012; Takagi et al. 2013). DNA was extracted from 100 mg of fresh rice leaves using DNeasy Plant Mini Kit (QIAGEN Sciences, Germany) and was quantified using QubitⓇ 3.0 Fluorometer and QubitⓇ dsDNA BR Assay Kit (Life Technologies, Japan). The extracted DNA samples were mixed in an equal ratio and were regarded as the bulked DNA. To survey the genotypes at the detected loci, simple sequence repeat (SSR) or InDel markers were used (Supplemental Table 1).
Detection of novel seed-shattering loci by QTL-seq analysis
All analyses were carried out using the previously developed QTL-seq Pipeline (Department of Genomics and Breeding, Iwate Biotechnology Research Center; https://genome-e.ibrc.or.jp). The short reads obtained from the IL were aligned to the ‘Nipponbare’ reference genome sequence obtained from the Rice Annotation Database Project (https://rapdb.dna.affrc.go.jp). Thereafter, the genome sequence of the IL was developed as a ‘reference sequence’. The short reads obtained from high (H-) and low (L-) bulks were aligned to the reference sequence using the Burrows–Wheeler Aligner software (Li and Durbin 2009). The aligned sequence files were converted to SAM/BAM files using SAM tools (Li et al. 2009) and were applied to Coval (Kosugi et al. 2013) to increase the SNP-calling accuracy. SNP-index was calculated for all the SNP positions. Then Δ(SNP-index) was calculated by subtracting the SNP-index values of L-bulk from H-bulk. A sliding window analysis was applied by averaging the Δ(SNP-index) values within a 4-Mb window size and a 50-kb increment.
Morphological and histological analysis of abscission layer formation
The abscission layer (axial images of detached spikelet) was examined using a LEICA S6D microscope and photographs were taken with the MC170HD and Leica Application Suite (Leica Biosystems, Germany). The samples for histological analysis were collected from the pedicel tissue of grains before heading, as previously reported (Htun et al. 2014; Inoue et al. 2015). The samples were fixed in FAA solution (formaldehyde: acetic acid: 70% ethanol = 1:1:18 (volume ratio)) with vacuum infiltration and were preserved at 4 °C. They were dehydrated in an ethanol series (70%, 80%, and 90% ethanol) for 2 days at each stage and then embedded in Technovit 7100 resin (Heraeus Kulzer, Germany), according to the manufacturer’s instructions. The samples were cut into 3-μm sections with a rotary microtome, RM1215RT (Leica Biosystems, Germany), and stained with toluidine blue O solution. These sections were observed under a microscope and photographed with a digital camera using the imaging software, ToupView ( × 86) (Amscope.com, USA).
Results
Seed-shattering behaviour of the IL (qSH1, qSH3, and sh4)
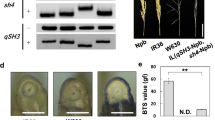
We first surveyed backcross plants carrying O.sativa ‘Nipponbare’ alleles at the qSH1, qSH3, and sh4 loci in the genetic background of wild rice, O.rufipogon W630. From these, the IL with the least other ‘Nipponbare’ chromosomal segments was selected. The graphical genotype of the IL with the ‘Nipponbare’ chromosomal segments covering the three seed-shattering loci and on chrs. 5, 7, 10, and 11 is shown in Fig. 1a. The appearance of the seed of the IL was similar to that of wild rice, O.rufipogon W630 (Fig. 1b). The IL showed an inhibition of seed-shattering behaviour, but it had a lower BTS value than ‘Nipponbare’ (Fig. 1c). We investigated the abscission layer formation in the IL after the detachment of seeds. In the basal part of the grain, no abscission layer was produced in ‘Nipponbare’ and the pedicel tissues were broken when a seed was strongly detached from the pedicel (Fig. 1d). In contrast, W630 formed a complete abscission layer from the epidermis to the region surrounding the vascular bundle (Fig. 1d). A partial abscission layer formation was observed in the inner region of the IL corresponding to the area where the complete abscission layer is formed in W630. The abscission layer formation in the IL was different from those of the parents. Furthermore, we also compared abscission layer formation using longitudinal sections. The abscission layer was inhibited outside the region of the pedicel and around the vascular bundle and partially formed only in the central region of the IL, whereas a complete abscission layer is formed in W630 (Fig. 1e). Because the IL had significantly lower BTS values than ‘Nipponbare’, the partially formed abscission layer probably contributes to lowering the BTS value. These results indicated that mutations at qSH1, qSH3, and sh4 are insufficient to explain the non-seed-shattering phenotype of ‘Nipponbare’, and other unknown loci are probably involved.
Characterisation of the introgression line (IL) carrying Oryzasativa ‘Nipponbare’ (Npb) alleles at qSH1, qSH3, and sh4 in the genetic background of wild rice, O.rufipogon W630. a Graphical genotype of the IL. The positions of the three seed-shattering loci qSH1, qSH3, and sh4 are shown. b Seeds of ‘Nipponbare’ (Npb), W630, and the IL. Scale bar = 5 mm. c The breaking tensile strength (BTS) values for ‘Nipponbare’ (Npb), W630, and the IL. Data are mean ± SD (n = 4). ** indicates P < 0.01 by unpaired Student’s t test. N.D. indicates ‘Not Determined’ owing to complete seed shattering. d An overview of spikelet base view (upper panels) and a close view of the spikelet base (lower panels) in ‘Nipponbare’ (Npb), W630, and the IL. The abscission scars were observed from the side of each dotted-line square (bottom). Scale bars = 1 mm. e Longitudinal sections of the spikelet base after seed detachment in ‘Nipponbare’ (Npb), W630, and the IL. vb = vascular bundle. ab = abscission layer. Black triangles indicate both edges of the abscission layer. Scale bars = 50 μm
Seed-shattering degree in an F2 population obtained by a cross between the IL and ‘Nipponbare’
We obtained a total of 174 F2 plants from a cross between the IL and ‘Nipponbare’ and their BTS values were measured. In this segregating population, the three seed-shattering loci were all fixed with the ‘Nipponbare’ alleles. Differences in the BTS were approximately 70–210 gf (Fig. 2, Supplemental Table 2). The transgressive segregation of the BTS values in the population indicates that several genes might be involved in the difference in seed-shattering degree between the parent lines.
Frequency distribution of breaking tensile strength (BTS) values for 174 F2 individuals between Oryzasativa ‘Nipponbare’ and the introgression line (IL) carrying the ‘Nipponbare’ alleles at the qSH1, qSH3, and sh4 loci. The BTS values for the parent lines and their F1 plants are shown with black dots with SD. ‘Nipponbare’: 157.7 ± 5.1 gf (n = 4), the IL: 111.4 ± 3.3 (n = 4), and F1: 129.6 ± 2.1 (n = 3) (mean ± SD). The DNA samples of F2 plants with BTS values between 70 and 100 gf were selected as low (L) bulk and those with BTS values between 150 and 210 gf were selected as high (H) bulk
Detection of novel loci controlling the seed-shattering behaviour in rice
To detect the loci involved in the difference in seed-shattering degree between the IL and ‘Nipponbare’, we employed QTL-seq analysis (Takagi et al. 2013). The analysis requires bulked DNA of progeny showing extreme phenotypes (i.e. those exhibiting high and low BTS values in the population). We selected 12 and 14 F2 plants with low (70–100 gf) and high (150–210 gf) BTS values, respectively (Fig. 2 and Supplemental Table 2). The DNA of each bulk as well as that of the IL was subjected to whole-genome resequencing analysis. A total of 104.3, 107.1, and 48.2 million sequence reads (each 100 bp) were obtained from the DNA of H-bulk, L-bulk, and IL, respectively. By examining the Δ(SNP-index) plot, we identified the two genomic regions exhibiting the Δ(SNP-index) values exceeding the 95% confidence interval by a sliding window analysis (statistical significance under the null hypothesis: P < 0.05): the regions on chr. 3 from 8.30 to 13.65 Mb with maximum Δ(SNP-index) = 0.49 (statistical significance under the null hypothesis: P < 0.05) and on chr. 9 from 21.1 Mb to the distal end with maximum Δ(SNP-index) = −0.46 (Fig. 3, Supplemental Fig. 1). We named them as QTL for theControl ofSeedShattering in rice on chrs. 3 and 9 (qCSS3 and qCSS9). No other region over the confidence interval was detected in this analysis (Supplemental Fig. 1). According to the Δ(SNP-index), ‘Nipponbare’ allele at qCSS3 and W630 allele at qCSS9 were found to inhibit seed shattering. To confirm the results of the QTL-seq analysis, each F2 plant was genotyped using the five and four DNA markers covering the entire significant regions on chrs. 3 and 9, respectively (Supplemental Table 1). For qCSS3 region, 32 (18%) and 20 (11%) plants were found to carry the ‘Nipponbare’ and W630 homozygous chromosomal segments, respectively. Moreover, 33 (19%) plants had heterozygous chromosomal segments and the rest (52%) showed recombination between any of the five SSR markers (Supplemental Table 3). We compared the BTS values of two homozygous groups based on the chromosomal constitutions (Supplemental Fig. 2) and found that the ‘Nipponbare’ and W630 chromosomal segments at the region were responsible for increasing and decreasing the BTS values, respectively. Similarly, the result of QTL-seq analysis at qCSS9 region was also confirmed (Supplemental Table 2, Supplemental Fig. 2). These results confirm that both qCSS3 and qCSS9 detected in QTL-seq analysis are involved in the control of seed shattering in rice.
Detection of novel loci for seed shattering on chromosomes 3 (qCSS3) and 9 (qCSS9) by QTL-seq analysis. qCSS3 and qCSS9 were detected in approximately the 5.4-Mb region on chromosome 3 and the 1.9-Mb region on chromosome 9. The Δ(SNP-index) plots with statistical intervals under the null hypothesis of no QTL (orange, P < 0.01; green; P < 0.05). The red line indicates the average ΔSNP-index calculated by a sliding window analysis
Validation of the effect of qCSS3 on the seed-shattering degree
As we found that the ‘Nipponbare’ allele at qCSS3 contributed to inhibition of seed shattering and possibly involved in non-shattering behaviour of O.sativa ‘Nipponbare’, we further studied qCSS3. To validate the effect of qCSS3 on the seed-shattering degree, we first screened the individual lines of the previously produced backcross recombinant inbred lines (BRILs) (Thanh et al. 2011). These BRILs were generated by crossing W630, as the donor parent, and ‘Nipponbare’, as the recurrent parent. Using two SSR markers, flanking the qCSS3 region (RM1002 and RM6080; Supplemental Table 1), we screened 159 BRILs. We found that lines 42 and 86 had homozygous chromosomal segments of W630, covering the candidate region of qCSS3 (Supplemental Fig. 3a). Both lines showed significantly lower BTS values than ‘Nipponbare’, although the differences were very small (Supplemental Fig. 3b). Next, we carried out the progeny test of F2 lines obtained by crossing the IL and ‘Nipponbare’. One of the F2 plants (No. 57), with heterozygous chromosomal constitution between RM1002 and RM6080 covering the whole candidate region of qCSS3 was selected (Fig. 4a). The self-pollinating seeds (F3 generation) were germinated, and their chromosomal constitutions were surveyed using the five SSR markers (Supplemental Table 1). The F3 progeny of this plant showed similar days to heading, minimising the effect of differences in heading date on the seed-shattering degree (Supplemental Table 3). A significant difference in the BTS values was observed between the F3 lines with the ‘Nipponbare’ and W630 homozygous chromosomal segments (Fig. 4b, P < 0.01). In addition, partial abscission layer formation was found to be associated with the seed-shattering degree, depending on the genotypes at the region (Fig. 4b, c). To restrict the border of qCSS3, we selected two other F2 plants having recombination within the qCSS3 candidate region. Numbers 54 and 129 F2 plants were found to have recombination between RM5639 and RM232 and between RM5551 and RM3297, respectively (Fig. 4a). They produced F3 progeny with similar heading dates (Supplemental Table 3). Their progeny tests showed a significant difference in the BTS values between F3 lines having recombinant and W630 homozygous chromosomal segments (Fig. 4a, P < 0.01). These results indicated that the candidate region of qCSS3 was within a 5.1-Mb region between RM5639 and RM3297.
Genetic dissection of qCSS3. a, d Graphical genotypes of three F2 plants (Nos. 54, 57, and 129) and six critical recombinant F3 plants (Nos. 129–43, 55, 88, 185, 195, and 282) in the candidate region of qCSS3. The BTS values of the F3 lines from the three F2 plants are shown on right. Data are mean ± SD (n = 6). Significant differences in the BTS values were observed for all pairs between the F3 and F4 lines having ‘Nipponbare’ and W630 homozygous chromosomal segments at the qCSS3 candidate region. **, *, and n.s. indicates P < 0.01, P < 0.05, and not significant (P ≥ 0.05) by unpaired Student’s t test. b, c Abscission layer formation for the F3 progeny of No. 57 having ‘Nipponbare’ and W630 heterozygous chromosomal segments. vb = vascular bundle. ab = abscission layer. Black triangles indicate both edges of the partially formed abscission layer. Scale bars = 50 μm
We further carried out genetic dissection at qCSS3 candidate region. A total of 78 recombinants out of 200 segregating plants were selected between RM5639 and RM3297 using F3 generation of F2-129. We surveyed these recombinant plants with additional DNA markers and 25 of recombinant plants were self-pollinated to conduct substitution mapping analysis. The mean BTS values of recombinant lines were compared at P < 0.05 level. Comparison of the BTS values in the progeny test showed that the qCSS3 candidate region was narrowed down to 1.3 Mb region between RM5639 and RM1284 by six critical recombinants (Nos. 129-43, -55, -88, -185, -195, and -282, Fig. 4d). We further investigated their genotypes at qCSS3 using ten more additional DNA markers (Supplemental Table 1). No significant differences were observed for both progeny tests of 129–55 (recombination between RM14731 and OTS1) and 129–185 (recombination between RM232 and RM14764), respectively (Fig. 4d). These results indicated that the putative candidate poison of qCSS3 is mapped to a 526 kb interval defined by two SSR markers RM14731 and RM14764. In this 526 kb region, we found 96 genes based on the published sequence annotation for O. sativa Japonica ‘Nipponbare’ (Supplemental Table 4), although there might be additional genes in the corresponding region of O.rufipogon W630.
Discussion
Abscission layer formation in ILs with non-functional alleles at seed-shattering loci in the genetic background of wild rice
In the previous study, we evaluated the ‘Nipponbare’ allele at any of the qSH1, qSH3, or sh4 loci in the W630 genetic background (Htun et al. 2014). All of them showed complete abscission layer formation as in W630, indicating that single mutation at any of the three seed-shattering loci was not sufficient to disrupt the abscission layer formation. In this study, we characterised the IL having the ‘Nipponbare’ alleles at the qSH1, qSH3, and sh4 loci in the genetic background of wild rice, O.rufipogon W630. Although these three seed-shattering loci were fixed with the ‘Nipponbare’ alleles, the average BTS value was significantly lower than that of ‘Nipponbare’ (Fig. 1a, c). Microscopic and longitudinal section analyses showed the presence of a ring-shaped abscission layer in the IL (Fig. 1d, e). Although the mechanism underlying the partial formation of a ring-shaped abscission layer is not understood, the expression gradient of the genes involved in abscission layer formation might be responsible.
As the SNP at qSH1 was specific to most Japonica rice cultivars and probably occurred late in the domestication process of rice (Konishi et al. 2006), we previously focused on the gene interaction at the other two loci (sh4 and qSH3) in the genetic background of W630 to understand the process of loss of seed shattering in rice domestication. A couple of abscission cells around the vascular bundles were found to be disrupted in the IL having the ‘Nipponbare’ alleles at qSH3 and sh4, suggesting that the interaction of the mutations at the two loci might have played an important role in the selection of the initial non-seed-shattering rice.
The ILs with the ‘Nipponbare’ alleles at the three (qSH1, sh4, and qSH3) and two (sh4 and qSH3; Inoue et al. 2015) loci showed different abscission layer formation at the epidermis region (Fig. 1d, e). The disruption of abscission layer at the epidermis region increases the BTS values tremendously (Fig. 1c). The progeny test of No. 57 F2 plant gave further interesting results. The F3 lines with ‘Nipponbare’ homozygous allele at the qCSS3 showed no abscission layer formation, similar to ‘Nipponbare’, whereas those with W630 homozygous allele had a partial abscission layer formation as the parental IL with the ‘Nipponbare’ alleles at the three seed-shattering loci (Fig. 4b, c). A similar allele effect was also observed in the two BRILs with the W630 allele at qCSS3 in the genetic background of ‘Nipponbare’. Both lines showed significantly lower BTS values than ‘Nipponbare’ (Supplemental Fig. 3). These results strongly indicate that the wild allele at qCSS3 may act to promote the abscission layer formation. It would be of interest to investigate the tissue specific expression of the seed-shattering gene(s) in the developing stage of spikelets with or without the functional allele at qCSS3.
Allele effects of qCSS3 and qCSS9 on the seed-shattering degree
The QTL-seq analysis successfully detected qCSS3 in a 5.1-Mb region on chr. 3, which is different form qSH3, the seed-shattering locus that was previously detected (Htun et al. 2014). We also detected qCSS9 in a 1.9-Mb region on chr. 9. As ‘Nipponbare’ allele at qCSS9 was found to promote seed shattering, we speculated that the allele effect of qCSS9 is not involved in a loss of seed shattering in ‘Nipponbare’. The allele effect at qCSS3 on the seed-shattering degree was examined using two BRILs in the ‘Nipponbare’ genetic background (Supplemental Fig. 3) and in the progeny test of F3 lines between the IL and ‘Nipponbare’ (Fig. 4). Although the BTS value of ‘Nipponbare’ was significantly higher than those of the two BRILs, their difference was about 15 gf. In the progeny test, large differences in the BTS values (30 gf) were observed between the F3 lines from three independent F2 plants. This may be owing to the different genetic background of the lines. Probably, ‘Nipponbare’ possesses cultivated alleles at some other minor QTLs for seed shattering.
Mapping of qCSS3 and the selection signature at the candidate region
On the basis of progeny tests, the candidate region of qCSS3 was estimated to be in a 526-kb region between the two SSR markers: RM14731 and RM14764. In the region, we found a total of 96 annotated genes based on Rice Annotation Database for O.sativa ‘Nipponbare’ (Supplemental Table 4). Once the causal gene is identified by further genetic mapping experiments, expression analysis of the candidate genes should be conducted in wild genetic background that has a functional pathway for promoting seed shattering.
In many domestication-related genes, reduction in nucleotide diversity is often observed (Doebley et al. 2006). Interestingly, a 526-kb candidate region partially overlaps with the selective sweep reported previously (Xu et al. 2012; Huang et al. 2012). Approximately, a 1.0-Mb region (9.0–10.0 Mb) on chr. 3 shows a reduction in diversity in Japonica rice cultivars (Xu et al. 2012), and the region carries the putative candidate region of qCSS3. This signature is specific to Japonica but not to Indica rice cultivars. Furthermore, a large scale genomic study using 446 wild and 1083 cultivated rice shows that the putative region from 9.7 to 10.1 Mb on chr. 3 exhibits quite low diversity in Japonica rice population (Huang et al. 2012). An approximately 130-kb region on RM14764 side of qCSS3 was found to overlap partially the sweep region. Interestingly, the selective sweep at the region was not detected in Indica nor full populations. These findings suggest that Japonica-specific domestication-related region may be located within a putative candidate region of the qCSS3. If a loss-of-function mutation at qCSS3 is common only to Japonica cultivars, this mutation may be selected after the differentiation of Japonica rice cultivars diverged from the initial cultivated rice. In contrast, the qSH3 region was found to be overlapping with the selective sweep region, both for the Japonica and Indica rice cultivars (Xu et al. 2012). These results suggest different evolutionary trajectories for the seed-shattering loci, which might be useful in understanding the process of rice domestication and distribution.
Loci involved in non-seed-shattering behaviour of cultivated rice
In previous studies, sh4 was identified as a major locus selected for during rice domestication (Li et al. 2006; Lin et al. 2007). The mutation at sh4 is conserved in all cultivated rice examined, indicating that sh4 is a key locus playing an important role in the loss of seed shattering (Zhang et al. 2009). On the other hand, mutation at qSH1 was found only in Japonica rice cultivars, suggesting that the mutation might have been selected after Japonica rice differentiated (Konishi et al. 2006; Zhang et al. 2009). In our recent study, we evaluated the non-seed-shattering behaviour of the Indica rice cultivar, ‘IR36′ (Ishikawa et al. 2017). The evaluation of the BRILs having ‘IR36′-derived chromosomal segments in the W630 genetic background identified a strong QTL on chr. 4, which overlaps with sh4. Additional genetic analysis suggested the involvement of qSH3 and other minor loci in the non-seed-shattering behaviour of ‘IR36′. At present, it remains unclear whether qCSS3 is involved in the loss of seed shattering in Indica rice. However, once the causal mutation at qCSS3 is identified, genotyping survey at qCSS3 in the Indica rice cultivars will clarify involvement of qCSS3 in the non-shattering behaviour. The gene interaction among the four seed-shattering loci provides scope for future studies that will help in understanding the process of loss of seed shattering during rice domestication.
Conclusions
We successfully identified novel loci involved in the difference in seed-shattering behaviour between ‘Nipponbare’, a typical Japonica rice cultivar, and wild rice O.rufipogon. Among two loci, ‘Nipponbare’ allele at qCSS3 may have contributed to a loss of seed shattering in rice domestication. To our knowledge, qCSS3 is the fourth detected locus involved in a loss of seed shattering in cultivated rice after sh4, qSH1, and qSH3. The genetic dissection of the non-seed-shattering behaviour of ‘Nipponbare’ reveals that at least four mutations (i.e. qSH1, qSH3, sh4, and qCSS3) are required to fully lose the abscission layer formation. Identification of the causal mutation at qCSS3 will provide important information for rice breeding that will help in manipulating the degree of seed shattering. Moreover, combination of the alleles at the four seed-shattering loci will be useful in understanding the process and history of rice domestication.
References
Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, Matsumura H, Yoshida K, Mitsuoka C, Tamiru M, Innan H, Cano L, Kamoun S, Terauchi R (2012) Genome sequencing reveals agronomically important loci in rice using MutMap. Nat Biotechnol 30:174–178. https://doi.org/10.1038/nbt.2095
Doebley JF, Gaut BS, Smith BD (2006) The molecular genetics of crop domestication. Cell 127:1309–1321. https://doi.org/10.1016/j.cell.2006.12.006
Dong Y, Wang YZ (2015) Seed shattering: from models to crops. Front Plant Sci 6:476. https://doi.org/10.3389/fpls.2015.00476
Fuller DQ (2007) Contrasting patterns in crop domestication and domestication rates: recent archaeobotanical insights from the Old World. Ann Bot 100:903–924. https://doi.org/10.1093/aob/mcm048
Fuller DQ, Allaby R (2009) Seed dispersal and crop domestication: shattering, germination and seasonality in evolution under cultivation. In: Østergaard L (ed) Annual plant reviews, vol 38: fruit development and seed dispersal. Wiley, Oxford, pp 238–295
Harlan JR (1975) Crops and man. American Society of Agronomy, Madison
Htun TM, Inoue C, Chhourn O, Ishii T, Ishikawa R (2014) Effect of quantitative trait loci for seed shattering on abscission layer formation in Asian wild rice Oryza rufipogon. Breed Sci 64:199–205. https://doi.org/10.1270/jsbbs.64.199
Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, Zhao Y, Liu K, Lu H, Li W, Guo Y, Lu Y, Zhou C, Fan D, Weng Q, Zhu C, Huang T, Zhang L, Wang Y, Feng L, Furuumi H, Kubo T, Miyabayashi T, Yuan X, Xu Q, Dong G, Zhan Q, Li C, Fujiyama A, Toyoda A, Lu T, Feng Q, Qian Q, Li J, Han B (2012) A map of rice genome variation reveals the origin of cultivated rice. Nature 490:497–501. https://doi.org/10.1038/nature11532
Inoue C, Htun TM, Inoue K, Ikeda K, Ishii T, Ishikawa R (2015) Inhibition of abscission layer formation by an interaction of two seed-shattering loci, sh4 and qSH3, in rice. Genes Genet Syst 90:1–9. https://doi.org/10.1266/ggs.90.1
Ishikawa R, Thanh PT, Nimura N, Htun TM, Yamasaki M, Ishii T (2010) Allelic interaction at seed-shattering loci in the genetic backgrounds of wild and cultivated rice species. Genes Genet Syst 85:265–271. https://doi.org/10.1266/ggs.85.265
Ishikawa R, Nishimura A, Htun TM, Nishioka R, Oka Y, Tsujimura Y, Inoue C, Ishii T (2017) Estimation of loci involved in non-shattering of seeds in early rice domestication. Genetica 145:201–207. https://doi.org/10.1007/s10709-017-9958-x
Konishi S, Izawa T, Lin SY, Ebana K, Fukuta Y, Sasaki T, Yano M (2006) An SNP caused loss of seed shattering during rice domestication. Science 312:1392–1396. https://doi.org/10.1126/science.1126410
Kosugi S, Natsume S, Yoshida K, MacLean D, Cano L, Kamoun S, Terauchi R (2013) Coval: improving alignment quality and variant calling accuracy for next-generation sequencing data. PLoS ONE 8:e75402. https://doi.org/10.1371/journal.pone.0075402
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25:1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Li C, Zhou A, Sang T (2006) Rice domestication by reducing shattering. Science 311:1936–1939. https://doi.org/10.1126/science.1123604
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Lin Z, Griffith ME, Li X, Zhu Z, Tan L, Fu Y, Zhang W, Wang X, Xie D, Sun C (2007) Origin of seed shattering in rice (Oryza sativa L.). Planta 226:11–20. https://doi.org/10.1007/s00425-006-0460-4
Oka HI (1988) Origin of cultivated rice. Elsevier, Amsterdam
Onishi K, Horiuchi Y, Ishigoh-Oka N, Takagi K, Ichikawa N, Maruoka M, Sano Y (2007) A QTL cluster for plant architecture and its ecological significance in Asian wild rice. Breed Sci 57:7–16. https://doi.org/10.1270/jsbbs.57.7
Takagi H, Abe A, Yoshida K, Kosugi S, Natsume S, Mitsuoka C, Uemura A, Utsushi H, Tamiru M, Takuno S, Innan H, Cano LM, Kamoun S, Terauchi R (2013) QTL-seq: rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J 74:174–183. https://doi.org/10.1111/tpj.12105
Thanh PT, Phan PDT, Mori N, Ishikawa R, Ishii T (2011) Development of backcross recombinant inbred lines between Oryza sativa Nipponbare and O. rufipogon and QTL detection on drought tolerance. Breed Sci 61:76–79. https://doi.org/10.1270/jsbbs.61.76
Tsujimura Y, Inoue C, Htun TM, Oka Y, Ishii T, Ishikawa R (2017) Investigation of genetic loci controlling non-shattering behaviour in an Indica rice cultivar ‘IR36’. J Crop Res 62:19–23. https://doi.org/10.18964/jcr.62.0_19
Xu X, Liu X, Ge S, Jensen JD, Hu F, Li X, Dong Y, Gutenkunst RN, Fang L, Huang L, Li J, He W, Zhang G, Zheng X, Zhang F, Li Y, Yu C, Kristiansen K, Zhang X, Wang J, Wright M, McCouch S, Nielsen R, Wang J, Wang W (2012) Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat Biotechnol 30:105–111. https://doi.org/10.1038/nbt.2050
Zhang LB, Zhu Q, Wu ZQ, Ross-Ibarra J, Gaut BS, Ge S, Sang T (2009) Selection on grain shattering genes and rates of rice domestication. New Phytol 184:708–720. https://doi.org/10.1111/j.1469-8137.2009.02984.x
Acknowledgments
The wild rice accession, O. rufipogon W630, was provided by the National Institute of Genetics supported by the National Bioresource Project, MEXT, Japan. We thank Satoshi Natsume (Iwate Biotechnology Research Center), Kentaro Yoshida (Kobe University), Soichiro Nishiyama and Takuya Morimoto (Kyoto University) for their useful advices on our QTL-seq analysis. This study was supported in part by Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS) to R.I. (Nos. 15KK0280 and 18K05594) and by the JSPS Bilateral Open Partnership Joint Research Project to R.I.
Author information
Authors and Affiliations
Contributions
RI conceived and designed the study. YT, SS, KO, TMT, CC, and RI performed the experiments. YT, SS, KO, TI, and RI analysed the data. YT, KN, and TA contributed to bioinformatic analysis. YT, TI, and RI prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Yunbi Xu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Results of the QTL-seq analysis for all chromosomes. The Δ(SNP-index) plots with statistical intervals under the null hypothesis of no QTL (orange, P < 0.01; green; P < 0.05). The red line indicates the average ΔSNP-index calculated by a sliding window analysis (PDF 822 kb)
Supplemental Fig. 2
Box plots of the BTS values of two F2 groups based on the genotypes at the candidate regions of qCSS3 and qCSS9. All F2 individuals were surveyed with five and four PCR-based molecular markers covering qCSS3 and qCSS9 regions, respectively (Supplemental Tables 1 and 2). Plants carrying the ‘Nipponbare’ (Npb) and W630 homozygous chromosomal segments for the entire candidate regions of qCSS3 and qCSS9 were selected (PDF 327 kb)
Supplemental Fig. 3
Evaluation of seed-shattering degree of the two backcross recombinant inbred lines (BRILs) carrying the W630 chromosomal segment covering the qCSS3 region in the ‘Nipponbare’ genetic background. (a) Graphical genotypes of two BRILs, BRIL42 and BRIL86. Red lines indicate the region covering qCSS3. (b) Seed-shattering degree of ‘Nipponbare’ (Npb) and the two BRILs. Data are mean ± S.D. (n = 6). ** indicates P < 0.01 by unpaired Student’s t-test (PDF 465 kb)
Supplemental Table 1
PCR-based molecular markers used in this study (PDF 79 kb)
Supplemental Table 2
Information of each 174 F2 plant used for QTL-seq analysis (PDF 98 kb)
Supplemental Table 3
Days to heading observed for F3 progeny from three F2 plants (Nos. 57, 54, and 129). Data are mean ± S.D. (n = 6) (PDF 29 kb)
Supplemental Table 4
List of the 96 genes annotated in a 526-kb candidate region of qCSS3 (PDF 36 kb)
Rights and permissions
About this article
Cite this article
Tsujimura, Y., Sugiyama, S., Otsuka, K. et al. Detection of a novel locus involved in non-seed-shattering behaviour of Japonica rice cultivar, Oryzasativa ‘Nipponbare’. Theor Appl Genet 132, 2615–2623 (2019). https://doi.org/10.1007/s00122-019-03376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-019-03376-3