Abstract
Key message
Selection for QLr.cau - 1AS (a major QTL detected in wheat for reducing leaf rust severity) based on the DNA marker gpw2246 was as effective as selection for Lr34 based on cssfr5.
Abstract
Leaf rust is an important disease of wheat worldwide. Utilization of slow-rusting resistance constitutes a strategy to sustainably control this disease. The American wheat cultivar Luke exhibits slow leaf-rusting resistance at the adult plant stage. The objectives of this study were to detect and validate QTL for the resistance in Luke. Three winter wheat populations were used, namely, 149 recombinant inbred lines (RILs) derived from the cross Luke × Aquileja, 307 RILs from Luke × AQ24788-83, and 80 F2:3 families selected from Lingxing66 × KA298. Aquileja and Lingxing66 are highly susceptible to leaf rust. AQ24788-83 shows high (susceptible) infection type but contains the slow-rusting gene Lr34 as diagnosed by the gene-specific marker cssfr5. KA298, an F9 RIL selected from Luke × AQ24788-83, contains Lr34 and QLr.cau-1AS (a major QTL originated from Luke, this study). These wheats were evaluated for leaf rust in 12 field and greenhouse environments involving four locations and five seasons. Genotyping was done using simple sequence repeat (SSR) and diversity arrays technology markers. Of the detected QTLs, QLr.cau-1AS was significant consistently across all the genetic backgrounds, test environments, and likely a wide range of pathogen races. QLr.cau-1AS explained 22.3–55.2 % of leaf rust phenotypic variation, being comparable to Lr34 in effect size. A co-dominant SSR marker (gpw2246, http://wheat.pw.usda.gov/GG2/index.shtml) was identified to be tightly linked to QLr.cau-1AS. Selection based on gpw2246 for QLr.cau-1AS was as effective as the selection based on cssfr5 for Lr34. QLr.cau-1AS will be helpful for increasing the genetic diversity of slow leaf-rusting resistance in wheat breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaf rust disease (caused by Puccinia triticina Eriks.) occurs on wheat (Triticum aestivum L.) in many countries (Roelfs et al. 1992; Kolmer 2005). Wheat cultivars that are susceptible to leaf rust regularly suffer yield reductions of 5–15 % (Kolmer 1996). There were destructive epidemics that caused yield reductions up to 40 % (Khan et al. 2013) and 70 % (Dubin and Torres 1981). In China, wheat is grown on about 29 million hectares in diverse environments (Singh et al. 1999). In terms of acreage affected by leaf rust, China is one of the largest epidemic regions in the world. In 2012, substantial yield reductions were caused by leaf rust in several provinces (including Gansu and Shandong) (Zhou et al. 2013). Utilization of genetic resistance in wheat can reduce the need for fungicides and thus is an economical and environmentally friendly strategy to control leaf rust. A great effort has been dedicated to searching for leaf rust-resistance worldwide, and more than 70 leaf rust-resistance genes have been formally cataloged in wheat (McIntosh et al. 2013; Park et al. 2014). However, many of these genes are no longer effective owing to their race specificity (Bolton et al. 2008). P. triticina interacts with wheat in a gene-for-gene hypothesis (Flor 1971) as observed in many plant–pathogen systems (Keen 1990; Heath 2000; Van der Hoorn and Kamoun 2008). Plant resistance (R) gene products act as receptors that directly interact with the elicitors or cognate avirulence (avr) gene products of pathogen (Keen 1990; Heath 2000). Alternatively, R gene products may indirectly recognize avr gene products (effectors) in a guard or decoy model (Van der Hoorn and Kamoun 2008). Either the direct or indirect perceptions underlying the gene-for-gene hypothesis are highly specific and often elicit rapid and localized plant cell death, known as a hypersensitive response, consequently, the growth of invading pathogen is strongly inhibited or completely stopped. Directional selection occurs when a race-specific resistance gene becomes widely distributed over a large geographical area of wheat. This leads to the establishment of some virulent mutants that have lost the function of elicitor/effector (McDonald and Linde 2002). Newly deployed resistance genes in commercial wheat fields can be rendered ineffective within a few years by the established virulent races (Kolmer 2005). Of these race-specific resistance genes, most begin to express at the seedling stage, while some (such as Lr12, Lr22b, LrSV1, and LrSV2) express at the adult plant stage only (Park and McIntosh 1994; Ingala et al. 2012). Either the former or the latter are phenotypically characterized by a low infection type (Park and McIntosh 1994; William et al. 2006).
On the other hand, there are wheat cultivars that exhibit slow leaf rust development, although they display a high (susceptible) infection type (Caldwell et al. 1957; Caldwell 1968; Ohm and Shaner 1976; Singh 1992; Kolmer 1996; Michelmore et al. 2013). Slow-rusting (or partial) resistance has been suggested to be generally more durable than race-specific resistance (e.g., Singh 1992; Kolmer 1996; Bolton et al. 2008; Krattinger et al. 2009). This is likely because slow-rusting resistance is not based on the recognitions of R-avr gene products and thus appears to work equally across all races of a pathogen with no directional selection (McDonald and Linde 2002). This type of resistance is often quantified in the field by lower disease severity or smaller area under the disease progress curve (AUDPC) compared with a susceptible genotype, or in the greenhouse by longer latent period, smaller pustule size, lower pustule density, etc. (Caldwell 1968; Ohm and Shaner 1976). Slow-rusting resistance can be genetically mapped in chromosome regions delimited by DNA markers through QTL analysis, and it can be selected on the basis of tightly linked DNA markers. Li et al. (2014) provided a review about QTL for leaf rust-resistance in wheat published before 2013, summarizing about 80 QTLs assigned to 16 chromosomes, and additional QTLs were reported recently (e.g., Buerstmayr et al. 2014; Singh et al. 2014; Tsilo et al. 2014; Zhou et al. 2014). These QTLs illustrate a genetic diversity for this trait, although some of them may be redundant. The diverse resources constitute a great help for combining different QTLs to achieve high level resistance. These QTLs show different sizes of effect, explaining 4.37–76.9 % of phenotypical variance individually (Li et al. 2014). The term “major QTL” is often used to indicate the QTL that has large effect. Lr34 is the best characterized slow-rusting gene of major effect. It has remained durable, and no P. triticina race of increased virulence toward it has been observed for more than 50 years (Krattinger et al. 2009). The gene-specific DNA markers developed from the cloned Lr34 sequences (Krattinger et al. 2009; Lagudah et al. 2009) can be readily applied to diagnosing this gene. Lr34 pleiotropically confers leaf tip necrosis and partial resistance to multiple diseases such as yellow (stripe) rust and powdery mildew (Singh 1992; Spielmeyer et al. 2005; Krattinger et al. 2009). Similarly, Lr46 and Lr67 are well characterized for their durable slow leaf-rusting resistance of major effect (Singh et al. 1998; Hiebert et al. 2010; Herrera-Foessel et al. 2011). Of the currently reported QTLs, six can be comparable to Lr34, Lr46 and Lr67 with respect to effect size, namely, QLr.sfr-1BS (Schnurbusch et al. 2004), QLr.hebau-2BS (Zhou et al. 2014), QLr.ubo-3A (Maccaferri et al. 2008), QLr.osu-7BL (Xu et al. 2005), QLr.sfrs-7B.2 (Messmer et al. 2000), and QLr.ubo-7B.2 (Maccaferri et al. 2008). These QTLs individually explain >20 % of leaf rust phenotypic variation with a good or fair consistency across different environments, and can be more easily deployable for practical breeding in comparison with minor QTLs.
It has been suggested that over-reliance on limited number of resistance genes/QTLs would be imprudent for deployment over vast wheat-growing areas (McDonald and Linde 2002; Xu et al. 2005; William et al. 2006; Singh et al. 2009). Therefore, it is essential to search for additional leaf rust-resistance QTLs to increase the diversity of resistance for sustainable control of leaf rust. Milus and Line (1980b) reported that the American wheat cultivar Luke could limit leaf rust development at the adult plant stage. We have observed Luke in our wheat nurseries located in Gansu and Shandong for a long period, and found that Luke was stably resistant to leaf rust in a slow-rusting nature at the adult plant stage. The objectives of this study were to (1) detect QTL for reducing leaf rust severity in Luke across diverse environments and identify linked DNA marker, (2) validate QLr.cau-1AS (a major QTL subsequently detected in Luke) in different genetic backgrounds and evaluate its effect size with Lr34 as a reference, and (3) test the applicability of a DNA marker (gpw2246) to marker-based selection for QLr.cau-1AS.
Materials and methods
Wheat materials and population development
Two winter wheat populations were used to detect and validate slow leaf-rusting QTL, i.e., 149 RILs derived from the cross Luke × Aquileja (hereafter referred to as Luke × AL) and 307 RILs from Luke × AQ24788-83 (Luke × AQ). AL (pedigree: Tevere/Giuliani//Gallini) and Luke (pedigree: PI 178383/2*Burt//CItr 13438) have the accession numbers PI 393993 and CItr 14586, respectively, at the National Small Grain Collection, Aberdeen, Idaho 83210, USA. AL was introduced from Italy to China and was commercially planted in several counties of Gansu province for more than 20 years before 1990. Luke was commercially planted in the Pacific Northwest of North America from 1970 to 1986 (Milus and Line 1986; Line 2002) where leaf rust was considered to be one of the important diseases (Milus and Line 1980a, b; Bjarko and Line 1988). AQ was selected for accumulating disease resistance from the progeny of a double cross among four Chinese wheat landraces: Ma Zhamai/Bai Qimai//Hong Qimai/Qing Shoumai. AQ was advanced to F14 generation by 2002 when it was crossed with Luke. AL, AQ, and Luke were valued originally for their durable resistance to yellow (stripe) rust. AL and the four parents of AQ exhibited durable slow-rusting resistance to yellow (stripe) rust (Zhang 1995; Guo et al. 2008; Quan et al. 2013). Luke was reported to possess durable high-temperature, adult plant resistance to yellow (stripe) rust (Qayoum and Line 1985; Line 2002). AQ and Luke were also observed to harbor QTLs for limiting sharp eyespot disease (Chen et al. 2013) and to display obviously reduced leaf rust severity at the adult plant stage. AL was highly susceptible to leaf rust on the basis of both infection type and disease severity (Zhang and Du, unpublished data).
The construction of Luke × AL population began in 2002 when F1 seeds from the cross between a single female (Luke) and male (AL) plant were produced in isolation in a growth chamber. The 149 RILs derived from one F1 seed were advanced to F6 plants by single-seed descent. From the F6 plants of each RIL, one spike was sampled to grow F7 spike-row plants. One seed was then sampled from the spike-row plants to serve as the representative of F8 generation, and the remaining seeds of the spike-row were bulked to grow F6:8 plants. Each RIL traces back to a single F6 plant. Likewise, the representative seed and bulked seeds of the subsequent generations were developed. During this process, selfing was controlled by bagging the sampled spikes. F6:8 and subsequent generations were used for phenotyping. The seed representative of F8 of each RIL was used to grow the plant from which DNA was extracted for genotyping. In the same way, 307 Luke × AQ RILs were developed from two F1 seeds. The seed representative of F9 generation from each Luke × AQ RIL was used to grow plant from which DNA was extracted for genotyping, and F10 was used for replacing missing genotype data. F6:8 and subsequent generations were used for phenotyping.
A third winter wheat population, Lingxing 66 × KA298 (referred to as LX × KA298), was constructed for examining the applicability of the DNA marker gpw2246 to marker-based selection for QLr.cau-1AS. The cross was done in 2011 and 80 F2:3 families were selected by 2014. LX is an elite wheat cultivar (Huang et al. 2012), but it is highly susceptible to leaf rust in both terms of infection type and disease severity. The parents of LX are Ji 91102 and Ji 935031 (Huang et al. 2012), and the grand parents of it are Little Poppy from Japan, Oro from Chile, Lovrin 13 from Romania, and Bai Youbao from China (http://www.chinaseed114.com/seed/8/seed_36812.html). KA298 was selected from the 307 Luke × AQ F9 RILs based on the six DNA markers as detailed in “Results” section, carrying Lr34 and QLr.cau-1AS. The Chinese wheat land race Mingxian 169 (referred to as MX) was used as the susceptible control throughout this study. MX is highly susceptible to all of the 24 P. triticina races that are representative of the diverse virulences of P. triticina populations in China (Li et al. 2010).
The field trials
The field trials were conducted in 11 environments involving three locations (Beijing, Gansu, and Shandong) and five winter wheat seasons (2009–10 to 2013–14), with each year × location combination being considered as an individual environment. The 149 Luke × AL RILs were tested in a commercial field in Gansu (Wushan county; 34°42′15′′N, 104°40′08″E, elevation 1680 m) in two seasons (2009–10 and 2010–11). The 307 Luke × AQ RILs were tested in all three locations, i.e., the CAU Experimental Station in Beijing (39°54′20′′N, 116°25′29′′E, elevation 50 m) in three seasons (2009–10 to 2011–12), the field of Gansu in three seasons (2011–12 to 2013–14), and a commercial wheat field in Shandong (Taian; 36°18′09′′N, 117°13′05′′E, elevation 90 m) in three seasons (2011–12 to 2013–14). The 80 LX × KA298 F2:3 families were tested in the field of Shandong in one season (2013–14). An individual trial was defined as a trial that was conducted in one location during one season for a single wheat population, and the study included 12 field trials in total. Each trial was in a randomized complete block design with three replicates in Gansu and Shandong, but with a single replicate in Beijing. An individual plot consisted of a single 1-m-long row with 25 cm between adjacent rows. Each plot was sown with approximately 40 seeds of an RIL for the Luke × AL and Luke × AQ populations, while with 15 seeds of an F2:3 family for the LX × KA298 population. A set of parents and the susceptible control MX were included after every 80 rows. A row of MX perpendicular and adjacent to the plot rows was sown along each field block to serve as spreader plants. In wheat-growing months, the highest and lowest temperatures in the field plots were recorded daily.
In Gansu and Shandong where the environments are highly conducive to leaf rust and epidemics of this disease occurred naturally every year during the course of this study, the test wheats were infected with the naturally occurring P. triticina populations. Winter wheat was harvested in late July (Gansu) or mid-June (Shandong) and sown in mid-September (Gansu) or early October (Shandong). Volunteer wheat seedlings constituted a “green bridge” for P. triticina urediniospores to move from the harvested winter wheat plants to the autumn-sown seedlings. To reinforce the green bridge, the susceptible wheat MX was sown in the rows bordering the trial areas in mid-May (Shandong) or early July (Gansu). Autumn-sown test wheat seedlings were infected by the urediniospores released from the green bridge plants. The infected autumn-sown test seedlings were covered with plastic film during winter months to facilitate P. triticina to overwinter and thus to increase infection in the coming spring. In the cases of drought in spring and/or summer, the test plants were frequently misted to facilitate re-infection. The trial plots in Shandong were surrounded by commercial wheat fields where fungicides were applied by an airplane (authorized by local government) in 2012–13 season, and consequently, leaf rust in the plots was constrained in this season. Under such a low disease pressure, resistance or susceptibility of the wheat plants could not be well revealed/evaluated; therefore, the Shandong trial of 2012–13 will be excluded from the subsequent analyses.
At the CAU Experimental Station in Beijing, leaf rust is unlikely to occur naturally due to lack of commercial wheat fields in the surrounding areas, thus artificial inoculation is needed. A P. triticina race designated as THTT was used as inoculums. The designation follows the three letter code system of Long and Kolmer (1989), with an additional fourth letter that described the high or low infection type to Thatcher wheat lines with genes LrB, Lr10, Lr14a, and Lr18. Li et al. (2010) and Zhou et al. (2013) reported that THTT has the avirulent/virulent formula Lr9, Lr19, Lr24, Lr28, Lr38, Lr39, Lr42, Lr45/Lr1, Lr2a, Lr2b, Lr2c, Lr3, Lr3bg, Lr3 ka, Lr10, Lr11, Lr14a, Lr14b, Lr15, Lr16, Lr17a, Lr18, Lr20, Lr21, Lr23, Lr25, Lr26, Lr29, Lr30, Lr32, Lr33, Lr36, Lr50, LrB. THTT has been one of the prevalent races in China over the last years (Li et al. 2010; Zhou et al. 2013). The stock of THTT is deposited at the Biological Control Center for Plant Diseases and Plant Pests of Hebei, Heibei Agricultural University, 289 Lingyusi Street, Baoding 071001, Hebei, P. R. China. In mid-April when the test plants were at the stem elongation stage, inoculation was done on clear afternoons when there was a high likelihood of overnight dew. The spreader plants were sprayed with fresh THTT urediniospores suspended in water containing 0.04 % Tween 20. The inoculated plants were then covered with plastic films to ensure long-lasting dew, and 18 h later the films were removed. About 2 weeks after inoculation when some urediniospores were obviously released from the spreader plants, the test plants were frequently misted to facilitate re-infection.
Leaf rust severity in the form of percentage of infected leaf area was scored on flag leaves using the modified Cobb scale (Peterson et al. 1948) in all locations. The estimation was on plot basis, i.e., by visually averaging over all the flag leaves of each plot. For the Luke × AL and Luke × AQ populations, recording was done three times when the leaf rust severities on MX flag leaves reached approximately 10–30, 50–60, and 80–90 %, respectively. For the LX × KA298 population, recording was done five times at 5-day intervals beginning when MX was showing 10–30 % severities. The area under the disease progress curve (AUDPC) was calculated on the basis of the multiple recordings for each plot entry with the formula: \( {\text{AUDPC}} = \sum {\left[ {{{\left( {x_{i} + x_{(i\; + \; 1)} } \right)} \mathord{\left/ {\vphantom {{\left( {x_{i} + x_{(i\; + \; 1)} } \right)} 2}} \right. \kern-0pt} 2}} \right] \times (t_{(i\; + \; 1)} - t_{i} )} \), where x i and x (i + 1) are scores for a plot entry on date t i and date t (i + 1), respectively, and t (i + 1) − t i is the number of days between date t (i + 1) and date t i . QTL mapping was done on mean AUDPCs calculated for each RIL by averaging over all replicates within individual trial.
Host infection response was scored in Beijing using the scale described by Roelfs et al. (1992) where R is the resistant or miniature uredinia surrounded by necrosis and chlorosis, MR the moderately resistant or small uredinia surrounded with chlorosis or necrosis, MS the moderately susceptible or moderate-sized uredinia without chlorosis or necrosis, and S the susceptible or large uredinia without chlorosis and necrosis. The scoring was done when the susceptible control MX expressed high host infection response.
The greenhouse trials
The greenhouses were located in Inner Mongolia (Chifeng county; 42°16′36′′N, 119°01′37″E, elevation 560 m) where the weather was not very hot even in summer and the greenhouse temperature could be easily controlled to a degree lower than 28 °C. The 149 Luke × AL RILs, 307 Luke × AQ RILs, and 80 LX × KA298 F2:3 families were sown in early October 2013 in 1.2-m-long rows with 40 cm between rows. Each row consisted of four hills 30 cm apart. Each population was in a randomized complete block design with three replicates. An individual plot consisted of a single hill sown with eight seeds of an RIL or an F2:3 family. A set of parents and MX were included after every forty hills. When the primary leaves of the test seedlings unfolded completely, the seedlings were sprayed with fresh urediniospores of THTT suspended in water containing 0.03 % Tween 20. The seedlings were then covered with plastic films and incubated overnight (18 h) at 18 ± 1 °C. Then, the films were removed and the greenhouse was operated at the temperatures between 18 and 22 °C daily under natural daylight supplemented with high-pressure sodium lamps (8000–10,000 lux at top leaves) in a photoperiod of 16 h. Infection types were recorded at 12–15 days after inoculation on a 0–4 scale as described by Roelfs et al. (1992) in which 0 = neither uredinia nor hypersensitive flecks; semicolon (;) = no uredinia but hypersensitive necrotic or chlorotic flecks; 1 = small uredinia surrounded by distinct necrosis; 2 = small to medium uredinia surrounded by necrosis or chlorosis; 3 = moderate size uredinia without chlorosis; and 4 = large size uredinia without chlorosis. From late November 2013 to mid-February 2014, heaters of the greenhouse were closed, and on sunny days the wheat seedlings were sheltered from sunlight using the greenhouse facilities. This resulted in the low temperatures of −4 °C (the lowest, night) to 8 °C (the highest, daytime) for vernalizing the seedlings. Afterwards, the greenhouse was operated at 18–25 °C daily under natural daylight supplemented to 16 h. In early March, thinning was done to retain up to five plants in each hill, and the plants were inoculated with THTT in the same way as detailed above. The plants were frequently misted to facilitate re-infection. Leaf rust severity was scored on flag leaves for three times for the Luke × AL and Luke × AQ populations and five times for LX × KA298, and AUDPC was calculated as detailed above. Infection type was scored when MX expressed high infection type.
Construction of genetic linkage map and QTL analysis
Map construction was performed using 149 Luke × AL F8 RILs and 307 Luke × AQ F9 RILs by the procedures described in Chen et al. (2013). Briefly, DNA was extracted using CTAB method (Rogers and Bendich 1985). SSR and EST primer sequences were acquired from the public domain (e.g., http://wheat.pw.usda.gov). PCR products were separated in 6 % denaturing polyacrylamide gels and visualized with the silver staining method (Bassam et al. 1991). Linkage analysis was conducted using the software MAPMAKER/EXP 3.0 (Lander et al. 1987) set to Kosambi mapping function in centiMorgan (cM). QTL mapping was done using the Windows QTL Cartographer 2.5 (Wang et al. 2010). Determination coefficient (R 2), a measurement of proportion of phenotypic variation explained by a QTL, was calculated using Windows QTL Cartographer for Luke × AL and Luke × AQ and using the SAS statistics package (SAS Institute Inc., Cary, NC, USA) for LX × KA298. Chi-square test on the segregation of marker loci and calculation of correlation coefficient of leaf rust intensity were performed using the SAS statistics package. Several works were included. First, 2018 SSR and EST primer pairs were screened against the parental wheat lines Luke and AQ for clearly distinct and readily repeatable polymorphic bands that were then used to genotype the 307 Luke × AQ F9 RILs. Second, to bridge chromosome “gaps” (>30 cM) on Luke × AQ map where no polymorphic SSR and EST markers were found, Diversity arrays technology (DArT) marker assays were conducted by Triticarte Pty. Ltd (Canberra, Australia; http://www.triticarte.com.au) as previously described by Wenzl et al. (2004) and Akbari et al. (2006). Third, to determine the presence/absence of the genes Lr10 and Lr34 in parental wheat lines, and to determine that QLr.cau-1AS was different from Lr10, the gene-specific DNA markers STSLrk10-6 (for Lr10; Schachermayr et al. 1997), cssfr5 (for Lr34; Lagudah et al. 2009), and (Glu-3)-1A (for chromosome arm 1AS; Devos et al. 1995) were used to genotype the Luke × AL and Luke × AQ populations. PCR amplifications and separations of the products were done by the procedures described in Lagudah et al. (2009) for Lr34, in Schachermayr et al. (1997) for Lr10, and in Devos et al. (1995) for (Glu-3)-1A. But, the PCR products amplified with (Glu-3)-1A primer set were separated in 6 % denaturing polyacrylamide gels instead of agarose gels. Fourth, the threshold LOD score to declare significant QTL was determined by running the permutation program, set with 3000 replications and P = 0.01, in the Windows QTL Cartographer 2.5 (Wang et al. 2010). Permutation analyses were conducted for each phenotypic dataset and, for simplicity, the highest threshold LOD value (4.2) was chosen as a uniform threshold for all datasets. Last, a bulked segregant analysis was applied to the Luke × AL population. The resistant bulk (R-bulk) contained equal amount of DNA from each of the ten RILs that showed the lowest leaf rust severities, and the susceptible bulk (S-bulk) contained equal amount of DNA from each of the ten RILs that showed the highest leaf rust severities. In total, 1586 SSR and EST primer pairs were screened against the two bulks.
Results
Identification of P. triticina races and infection types
Leaves bearing P. triticina uredinia were collected from the MX plants in the field plots of Gansu and Shandong during 2012–2014. Sixty single uredinial isolates (30 each location) derived from the collections were analyzed using the methods in Online Resource 1. From these isolates, nine P. triticina races were identified (Table 1) which should represent the prevalent ones in the field plots. Of these nine races, four (THTT, PHTT, THSS, and THST) were detected in both locations, three (PHRT, PHKT, and PHSS) in Gansu only, and two (THTS and PHTS) in Shandong only. These races were collectively virulent to the 14 genes Lr1, Lr2a, Lr2c, Lr3, Lr3 ka, Lr10, Lr11, Lr14a, Lr16, Lr17, Lr18, Lr26, Lr30, and LrB. The infection types of the parental wheat seedlings against each of the nine races, tested with the methods in Online Resource 2, were listed in Table 2. These wheats showed the susceptible infection types of 4 or 3. THTT accumulated the virulences against all of the 14 Lr genes listed in Table 1. With THTT as inoculums in the greenhouse, infection types were recorded on the 149 Luke × AL RILs, 307 Luke × AQ RILs, 80 F2:3 families of LX × KA298, and their parents. These wheats showed the infection types of 4 or 3 at both the seedling and the adult plant stages (Table 2). Also in Beijing field plots, the adult plants of the 307 Luke × AQ RILs were inoculated with THTT, and they showed the host infection responses of MS or S. It is noticed that the Lr34-carrying line KA298 displayed the highest infection type of 4 to THTT at both the seedling and adult plant stages. This agrees with the study of Li et al. (2010) where Saar (an Lr34-carrying line) exhibited the infection type 4 to THTT. These repeated observations indicate that no hypersensitive response gene was segregating in these wheat populations with respect to the involved P. triticina races. Therefore, infection type and host infection response data will be omitted from the subsequent analyses.
Leaf rust severity at the adult plant stage
Severe leaf rust was induced in both the fields and the greenhouse. The final mean severities of the susceptible control wheat MX were between 82 % (Beijing field plots in 2010–11) and 96 % (greenhouse). The severities (79–92 %) of the susceptible parents AL and LX were approximately equal to those of MX. In comparison with MX, the slow-rusting resistance parents AQ and Luke reduced severity by 48 and 44 % on average, respectively. To improve QTL mapping, AUDPC was calculated for each RIL. The ranges of AUDPC were shown for the 149 Luke × AL RILs in Fig. 1a and for the 307 Luke × AQ RILs in Fig. 1b, c. For the sake of simplifying the figures, trials within the same location were jointly depicted. The Luke × AL RILs were tested in three trials, i.e., two in Gansu fields and one in the greenhouse. The two trials of Gansu were highly correlated (r = 0.89, P < 0.0001, df = 147) for AUDPC and thus, mean AUDPC was calculated for each RIL by averaging over the two trials. AUDPC of an individual RIL of Luke × AL varied from 266 to 1294 (Fig. 1a). The Luke × AQ RILs were tested in nine trials, i.e., three in Beijing field, three in Gansu field, two in Shandong field, and one in the greenhouse. Highly significant correlations for AUDPC were detected among the different trials within each of the first three locations (r = 0.81–0.88 in Beijing, 0.79–0.85 in Gansu, and 0.89 in Shandong; P < 0.0001, df = 305). Therefore, mean AUDPC was calculated for each RIL by averaging over the two trials (Shandong) or the three trials (Beijing, and Gansu). AUDPC of an individual RIL of Luke × AQ varied from 83 to 1258 (Fig. 1b, c). The distributions were continuous and mono-modal in both populations, suggesting a quantitative inheritance. The distribution peaks of Luke × AQ RILs occurred in a region of lower AUDPC values (201–400, Fig. 1b) in Beijing and Gansu, whereas in a region of higher AUDPC (401–600, Fig. 1c) in Shandong and the greenhouse. This difference might be attributable to the higher leaf rust infection pressures in Shandong and the greenhouse where the environments were more conducive to leaf rust than those in Beijing and Gansu. The comparison between Luke × AL and Luke × AQ showed the occurrence of transgression in the latter (Fig. 1b, c) but not in the former (Fig. 1a), suggesting that both Luke and AQ should carry slow leaf-rusting QTL. However, AL appeared unlikely to harbor such QTL.
Distributions of recombinant inbred lines (RILs) over the ranges of leaf rust intensity as measured in the area under the disease progress curve (AUDPC) in the wheat populations Luke × Aquileja (a), and luke × AQ24788-83 (b, c). The test plants were artificially inoculated with the Puccinia triticina race THTT in the greenhouse of inner Mongolia and the fields of Beijing, while infected by the natural Puccinia triticina populations in Gansu and Shandong. The approximate AUDPC means and standard deviations were indicated, respectively, with arrows and bars for the parental lines
Linkage map and QTL analysis
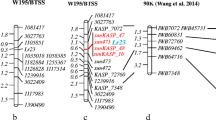
The Luke × AL cross was used as the original population for detecting slow leaf-rusting QTL since the two parents were quite contrasting in leaf rust phenotype (Fig. 1a). The initial screening against SSR and EST primer pairs for polymorphism between the R- and S-bulks showed that the SSR markers gpw2277, wmc24, and wmc95 were polymorphic. According to the wheat SSR maps of Somers et al. (2004) and Sourdille et al. (2004), these three SSRs are all located on 1AS. Then, 1A was focused on for mapping, and a major QTL (designated as QLr.cau-1AS) was detected on 1AS with DNA marker locus gpw2246 located rightly at the peaks of the LOD curves (Fig. 2a). In all the field and greenhouse trials, QLr.cau-1AS was significant at the threshold LOD of 4.2 with its peak LOD values being 14.1–17.8 (Fig. 2a; Table 3), and explained 44.3–52.6 % of leaf rust phenotypic variation (Table 3). The resistance allele at QLr.cau-1AS was contributed by Luke.
Linkage groups assigned to chromosome 1A of the Luke × Aquileja population (a), and 1A (b) and 7D (c) of the Luke × AQ24788-83 population, showing the logarithm of the odds (LOD) curves for the slow leaf-rusting quantitative trait loci (QTLs) of QLr.cau-1AS and Lr34. Distances are in centiMorgans (cM) of Kosambi function. Each of the chromosomes is oriented with the telomere of the short arm to the left positioned at 0 cM. Arrow the approximate position of centromere estimated by referring to the previously published wheat SSR maps (Röder et al. 1998; Paillard et al. 2003; Somers et al. 2004; Sourdille et al. 2004). The positions of the DNA markers are shown along the chromosomes. Horizontal line the threshold LOD of 4.2
A second population (Luke × AQ) was used as a different genetic background for validating QLr.cau-1AS. AQ contains the resistant allele as diagnosed by the 751 bp fragment amplified from Lr34 locus with the cssfr5 primer set (Fig. 3). Thus, the Luke × AQ population could provide an opportunity for comparing QLr.cau-1AS with Lr34 (see “Discussion” section). The Luke × AQ map consisted of 605 SSR and EST loci and 207 DArT loci after excluding the unsatisfactory markers such as those that distorted from the 1:1 segregating ratio at P = 0.01 and those that could not be assigned to any chromosome. The retained 812 markers were grouped into 21 linkages that were then assigned to the 21 hexaploid wheat chromosomes by referring to the previously published wheat SSR maps (Röder et al. 1998; Paillard et al. 2003; Somers et al. 2004; Sourdille et al. 2004). The Luke × AQ map covered 3329 cM with a marker density of 4.1 cM per marker. No gap longer than 30 cM existed in the Luke × AQ map. For the sake of simplicity, only the two chromosomes that were associated with major QTLs were presented here (Fig. 2b, c). On the basis of this map and AUDPC data, QTL mapping was done separately for each of the nine field and greenhouse trials. The mapping results showed two major QTLs on 1AS and 7DS, respectively (Fig. 2b, c). The DNA marker locus gpw2246 was located rightly at the peaks of the LOD curves for 1AS QTL (Fig. 2b), and the resistant allele at the 1AS QTL was contributed by Luke (Table 3). These results indicate that the 1AS QTL in Luke × AQ corresponds to QLr.cau-1AS that was originally detected in Luke × AL. The 7DS QTL was located at the same location of the DNA marker locus cssfr5 that diagnoses Lr34 (Fig. 2c) and hence, the 7DS QTL corresponds to Lr34. In each of the field and greenhouse trials, both QLr.cau-1AS and Lr34 were highly significant. QLr.cau-1AS and Lr34 showed their peak LOD scores of 10.6–15.9 and 9.9–15.5, respectively, and explained 22.3–32.8 and 20.6–32.5 % of leaf rust phenotypic variation (Table 3). In addition, four minor QTLs were detected on 3DL, 4AL, 5AS, and 6DS, respectively, with their closest DNA markers being gdm72, wmc468, BE40413, and barc196. Each of these markers was less than 3 cM away from the LOD peak of its represented QTL (LOD curves not shown). The resistant alleles at these four QTLs were contributed by AQ. These QTLs, however, showed a limited size of effect (explaining <13 % of leaf rust phenotypic variation) and an inadequate consistency across different environments (being significant only in one to four of the seven data sets used for mapping QTL). Further works are underway to clarify their involvement in the interactions of QTL × QTL and QTL × environment. These four minor QTLs will not be addressed below, and will be reported in a separate paper.
Detection of the resistance gene Lr34 in the parental wheat line AQ24788-83, with Thatcher as negative control and ThLr34 as positive control. ThLr34 denotes the near-isogenic line for Lr34 in the genetic background of Thatcher. Arrow the 751 bp fragment which is specifically amplified from the wheat lines containing resistant allele at Lr34
It is noticed that Luke contributed resistance allele at only one (i.e., QLr.cau-1AS) of the six QTLs. This result suggests that the slow leaf-rusting resistance in Luke was conferred mainly or likely solely by QLr.cau-1AS.
Selection for QLr.cau-1AS on the basis of the DNA marker gpw2246
To examine the applicability of gpw2246 to selection for QLr.cau-1AS, and to compare QLr.cau-1AS with Lr34, marker-based selection was investigated using the LX × KA298 population. The selection was done in two steps. First, KA298 was selected from the 307 Luke × AQ F9 RILs on the basis of the DNA markers gpw2246, cssfr5, gdm72, wmc468, BE40413, and barc196. KA298 was homozygous for the resistant alleles at both QLr.cau-1AS and Lr34 (as represented by their corresponding marker loci gpw2246 and cssfr5), but homozygous for the susceptible alleles at the other four QTLs (represented by gdm72, wmc468, BE40413, and barc196, respectively). The resistant alleles at the latter four loci were excluded so that they would not interfere with QLr.cau-1AS and Lr34 in LX × KA298. Such an exclusion should create a clear genetic background for simplifying the comparison of QLr.cau-1AS with Lr34 (see “Discussion” section). In the second step, 900 F2 seedlings of LX × KA298 were screened on the basis of gpw2246 and cssfr5. Four DNA marker classes were selected, i.e., the seedlings in homozygous state for resistant alleles at both QLr.cau-1AS and Lr34 (denoted with +, +), for resistant allele at QLr.cau-1AS but susceptible allele at Lr34 (+, −), for susceptible allele at QLr.cau-1AS but resistant allele at Lr34 (−, +), and for susceptible alleles at both QLr.cau-1AS and Lr34 (−, −). From each class, 20 F2 seedlings were sampled and advanced to F2:3 families that were then evaluated for leaf rust development. The temporal progress of leaf rust was depicted for each class in Fig. 4. It can be seen that the disease developed as slowly on the QLr.cau-1AS plants (+, −) as on the Lr34 plants (−, +) with no substantial difference between the two marker classes. This was consistent between the greenhouse (Fig. 4a) and field (Fig. 4b) trials. These results indicate that selection for QLr.cau-1AS based on gpw2246 was as effective as selection for Lr34 based on cssfr5. As shown in Table 4, AUDPC of (−, −) was not significantly different from that of the susceptible control MX, suggesting that no significant leaf rust-resistance QTL other than QLr.cau-1AS and Lr34 likely existed in LX × KA298. AUDPC of (+, +) was significantly lower (P < 0.001) than those of (+, −) and (−, +), illustrating that the simultaneous selection based on gpw2246 and cssfr5 yielded higher level of resistance compared with individual selection based on either gpw2246 or cssfr5. R 2 % value, a measurement of effect size, was calculated for LX × KA298 on AUDPC data using the SAS statistics package (SAS Institute Inc., Cary, NC, USA). To examine the independent effect of either QLr.cau-1AS or Lr34, the calculation was based on the 40 F2:3 families of (+, −) and (−, −) for QLr.cau-1AS, and based on the 40 F2:3 families of (−, +) and (−, −) for Lr34. These R 2 % values were listed in Table 3. It can be seen that the R 2 % values of QLr.cau-1AS (55.2 and 48.5 %) were similar to those of Lr34 (53.7 and 47.7 %).
A comparison between the selection for QLr.cau-1AS based on the DNA marker gpw2246 and the selection for Lr34 based on cssfr5 with respect to their effect on slowing down leaf rust progress as observed at five dates in the greenhouse (a) and in the field (b). The tests were done, using the Liangxing 66 × KA298 population in which QLr.cau-1AS and Lr34 segregated with other leaf rust-resistance QTLs being excluded, on the four marker classes (+, +), (+, −), (−, +), and (−, −). The “+” and “−” before comma stand for the plants that were homozygous for resistant and susceptible allele, respectively, at QLr.cau-1AS, while those after comma refer to Lr34. Vertical bar indicates standard deviation. Bar height is calculated on the basis of 20 F2:3 families of each marker class
Detection of the gene Lr10 in AL, AQ, and Luke
The gene Lr10 has been cloned by Feuillet et al. (1997, 2003). On the basis of the cloned sequence, Schachermayr et al. (1997) designed a set of primers that amplify a 282 bp fragment specific to the resistant allele at Lr10, and mapped it on 1AS. This 282 bp fragment can be readily used to diagnose the resistant allele at Lr10. With these primers, we amplified the 282 bp fragment from both AQ and Luke as well as from the near-isogenic line for Lr10 in the genetic background of Thatcher, but not from AL (Fig. 5). This result indicates that both AQ and Luke harbor Lr10, and thus the Luke × AQ population did not segregate for Lr10. However, the Luke × AL population was segregating for Lr10. QLr.cau-1AS was distant from Lr10 by 30.4 cM (Fig. 2a). These results indicate that QLr.cau-1AS is different from Lr10.
Detection of the Lr10 resistance gene in the parental wheat lines Aquileja, AQ24788-83, and Luke, with ThLr10 as control. ThLr10 denotes the near-isogenic line for Lr10 in the genetic background of Thatcher. Arrow indicates the 282 bp fragment which is specifically amplified from the wheat lines containing resistant allele at Lr10
Discussion
In this study, we identified a major QTL (QLr.cau-1AS) for reducing leaf rust severity at the adult plant stage. QLr.cau-1AS was mapped on chromosome arm 1AS with the resistant allele being contributed by Luke. Currently, 73 formally and more than 10 temporarily designated Lr genes have been described for hexaploid wheat (McIntosh et al. 2013; Buerstmayr et al. 2014; Li et al. 2014; Park et al. 2014), and more than 80 leaf rust-resistance QTLs have been mapped (e.g., Buerstmayr et al. 2014; Li et al. 2014; Singh et al. 2014; Tsilo et al. 2014; Zhou et al. 2014). Of these genes and QTLs, two are located on 1AS, namely, the gene Lr10 (Schachermayr et al. 1997) and a QTL reported by Singh et al. (2009). QLr.cau-1AS is different from Lr10. The marker (Glu-3)-1A on 1AS is common for the Luke × AL and Luke × AQ populations in the present study and the Frisal × Thatcher Lr10 population in Schachermayr et al. (1997). With (Glu-3)-1A as reference, Lr10 was located approximately 7 cM distal of (Glu-3)-1A in both Luke × AL and Frisal × Thatcher Lr10 (Schachermayr et al. 1997), while QLr.cau-1AS was located approximately 20–25 cM proximal of (Glu-3)-1A (Fig. 2a, b). Singh et al. (2009) detected a QTL for reducing leaf rust severity on 1AS using a doubled-haploid population derived from the cross Beaver/Soissons (B/S), two European cultivars. The 1AS QTL of B/S was located at 13.7 ± 10.8 cM distant from the telomere of 1AS with psp3027 being the nearest DNA marker (Singh et al. 2009). No common marker is available for the 1AS maps of the present study and that of B/S. The position of QLr.cau-1AS could not be precisely compared with that of the 1AS QTL of B/S. Nevertheless, with the wheat SSR consensus map of Somers et al. (2004) as reference, the 1AS QTL of B/S seems to be rather close to Lr10, but distant from QLr.cau-1AS. Moreover, Singh et al. (2009) indicated that the 1AS QTL of B/S was inconsistent because it was significant (P = 0.041) in only one of three test years. This suggests that QLr.cau-1AS is highly likely to be a previously un-reported/novel QTL for reducing leaf rust severity, though further studies are required for conclusively distinguishing QLr.cau-1AS from the B/S QTL.
Effectiveness of QLr.cau-1AS across different genetic backgrounds and diverse environments
QLr.cau-1AS was significant (P < 0.001; Table 3) in all the three populations of Luke × AL, Luke × AQ, and LX × KA298. AL, AQ, and LX were chosen for this study because they were different from one another in genetic background as illustrated by their pedigree data (see “Materials and methods” section). These three wheat lines have no common ancestor in their pedigrees, and thus could represent a substantial diversity of genetic background. Effect size was measured in the form of R 2 % value. The overall R 2 % range of QLr.cau-1AS (22.3–55.2 %) was attributable to differences in both populations and environments. The variation due to populations can be examined using the greenhouse trial where all the three populations were tested in the same greenhouse with a single race (THTT) as inoculums. This trial provided a homogeneous environment for comparing the effect sizes of QLr.cau-1AS among different populations. The R 2 % value in Luke × AQ (32.8 %) was much smaller than those in Luke × AL (52.6 %) and LX × KA298 (55.2 %). Also, the effect size of Lr34 was greater in LX × KA298 than in Luke × AQ. This might be caused mainly by the presence of other slow leaf-rusting QTLs in Luke × AQ. In addition to QLr.cau-1AS and Lr34, four QTLs segregated in Luke × AQ. These QTLs might dilute or bias the effect of QLr.cau-1AS, or also might partially neutralize the expression of QLr.cau-1AS through QTL × QTL interaction in Luke × AQ. In LX × KA298, however, these four QTLs were excluded and thus the effect of QLr.cau-1AS could be observed to a greater extent (55.2 %). Similarly, the effect of QLr.cau-1AS could be better displayed in Luke × AL (52.6 %) where no other significant QTL likely existed. These data and results support the conclusion that QLr.cau-1AS was effective across different genetic backgrounds, and its effect size could be better revealed when no other significant slow leaf-rusting QTL was present.
QLr.cau-1AS was significant (P < 0.001) in all four locations (Beijing, Gansu, Shandong, and greenhouse) and all five crop seasons (2009–10 to 2013–14). These test environments were considerably diverse. For instance, a severe drought happened in 2010–11, whereas rainfall was extraordinarily frequent in 2011–12 in Beijing, Gansu, and Shandong. Agro-ecologically speaking, Gansu and Shandong are representatives of the extreme wheat-growing regions in North and Northwest China (the main wheat-production areas). The field trials of Gansu and Shandong are geographically away from each other by a distance of approximately 1100 km. In Gansu, the trial plots were located on a high land terrace with an elevation of about 1680 m, and the winter wheat season lasted from mid-September to the coming late July. In Shandong, the plots were located in the field with an elevation of about 90 m and the crop season was between early October and the coming mid-June. Gansu was more favorable for P. triticina to infect wheat seedlings in autumn, while Shandong was more favorable for P. triticina to overwinter. In the wheat-growing months of spring and summer, the monthly mean temperatures ranged from 6.7 to 19.3 °C in the plots in Gansu and from 10.3 to 28.2 °C in Shandong. Effect sizes, measured by R 2 %, of QLr.cau-1AS can be compared among these environments using Luke × AQ. This population was tested in nine environments involving all locations (Beijing, Gansu, Shandong, and greenhouse) and all seasons (2009–10 to 2013–14). The R 2 % value of QLr.cau-1AS varied from 22.3 % (Gansu field in 2012–13) to 32.8 % (Greenhouse in 2013–14) within the Luke × AQ population. This variation might be caused mainly by the difference in spatial uniformity of disease pressure within individual trials. For instance, in the greenhouse, temperature and humidity were controlled to be suitable for leaf rust, and fans were frequently used to facilitate even distribution of urediniospores for re-infection. Leaf rust developed quite evenly both within and among replicates. This could increase test accuracy and the effect of QLr.cau-1AS could be better observed compared with the trial in Gansu during 2012–13. In the latter case, rainfall was not frequent, and urediniospores were not adequate enough for establishing a good spatially uniform distribution and re-infection. The lower uniformity might increase experimental error and consequently the effect of QLr.cau-1AS could not be fully displayed. According to these results and observations, it can be concluded that QLr.cau-1AS was effective across diverse environments, and its effect size could be better revealed in a trial of higher uniformity.
Comparison of QLr.cau-1AS with Lr34 and combination of them based on DNA markers
The Luke × AQ population segregated for Lr34 as well as for QLr.cau-1AS. Lr34 has been extensively studied worldwide and well proven to have major effect on limiting leaf rust in a slow-rusting nature (e.g., Dyck and Samborski 1982; Singh and Gupta 1992; Schnurbusch et al. 2004; Krattinger et al. 2009; Risk et al. 2012). Lr34 is, therefore, an excellent reference or baseline with which QLr.cau-1AS can be compared in effect size. In Luke × AQ, 22.3–32.8 and 20.6–32.5 % of the leaf rust phenotypic variation were explained by QLr.cau-1AS and Lr34, respectively (Table 3), illustrating that the effect size of QLr.cau-1AS was nearly equal to that of Lr34. This also held true in LX × KA298 where no significant difference in resistance level was detected between QLr.cau-1AS and Lr34 (Table 4). With Lr34 as reference, QLr.cau-1AS could also be indirectly compared with some other leaf rust-resistance genes/QTLs. For example, the effect size of QLr.cau-1AS appears larger than those of Lr67 (Hiebert et al. 2010; Herrera-Foessel et al. 2011) and LrP.sfr-1BS (Schnurbusch et al. 2004), further larger than that of Lr46 (William et al. 1997; Singh et al. 1998; Suenaga et al. 2003), and still further larger than that of Lr68 (Herrera-Foessel et al. 2012).
Selection for QLr.cau-1AS based on the DNA marker gpw2246 was compared with the selection for Lr34 based on cssfr5 using the LX × KA298 population. Although Lr34 may not provide adequate resistance under high disease pressure when present alone, it can be a major contribution to achieving high level resistance when being combined with other genes/QTLs (Singh and Gupta 1992; Suenaga et al. 2003). The DNA marker cssfr5 (Lagudah et al. 2009) is completely applicable to precise marker-based selection for Lr34 and thus is a powerful tool for combining Lr34 with other QTL. QLr.cau-1AS could be well represented by the SSR marker gpw2246 because this marker is located rightly at the peaks of the LOD curves of QLr.cau-1AS in both Luke × AL and Luke × AQ (Fig. 2a, b). In LX × KA298, KA298 contributed the resistant alleles at QLr.cau-1AS and Lr34, whereas LX is as susceptible to leaf rust as the susceptible control MX. LX was developed by the Shandong Liangxing Seed Co. Ltd., Shandong Province, P. R. China (http://www.chinaseed114.com/seed/8/seed_36812.html), and it has been one of the wheat cultivars commercially planted over a large acreage in China in recent years. This cultivar displays high resistance to powdery mildew (Huang et al. 2012) and slow-rusting resistance to yellow rust (http://www.chinaseed114.com/seed/8/seed_36812.html). It is reasonable to choose LX as a parent because breeders generally introduce disease resistance QTLs into an elite material. According to Table 4, no significant QTL other than QLr.cau-1AS and Lr34 likely existed in LX × KA298, thus the comparison of QLr.cau-1AS with Lr34 would not be complicated by other QTL. The selection based on gpw2246 for QLr.cau-1AS resulted in virtually the same level of effect on reducing leaf rust as the selection based on cssfr5 for Lr34 (Fig. 4). The simultaneous selection based on gpw2246 and cssfr5 yielded an elevated level of resistance. Additionally, the SSR primer pair gpw2246 amplifies clearly distinct and readily repeatable DNA bands of co-dominance (data not shown) that are favorable for practical selection. These results support the conclusion that the selection based on gpw2246 for QLr.cau-1AS was as effective as the selection based on csffr5 for Lr34.
According to Fig. 4, QLr.cau-1AS and Lr34 could reduce final leaf rust severity by 55.5 and 51.5 % on average, respectively, and by 78.5 % cumulatively. This can be comparable to the combination of Lr46 with Lr34 as reported by Suenaga et al. (2003) where Lr46 and Lr34 reduced leaf rust severity by 25.5 and 40.1 % on average, respectively, and by 61.6 % cumulatively. Both Lr46 and Lr34 pleiotropically confer a leaf tip necrosis (Suenaga et al. 2003) and thus they likely share common defense mechanism. Luke, the contributor of the resistant allele at QLr.cau-1AS, showed no leaf tip necrosis (Zhang and Du, unpublished data). Hence, QLr.cau-1AS may have some defense mechanism different from that of Lr34 and Lr46. The combination of QLr.cau-1AS with Lr34 should enhance the diversity of leaf rust-resistance in the sense of defense mechanism.
Effectiveness of QLr.cau-1AS against likely a wide range of P. triticina races
The slow leaf-rusting resistance of Luke (conferred by QLr.cau-1AS) appeared effective against a wide range of P. triticina races for the three following reasons. First, the nine races identified from 60 single P. triticina isolates, derived from the collections made in the field plots during the course of this study, can be considered as representative of the prevalent races in the field plots. In the presence of these races in Gansu and Shandong, Luke consistently showed reduced leaf rust severity during the course of this study. This implies that QLr.cau-1AS was effective against at least the nine races. QLr.cau-1AS might be also effective against other races that were not included in the 60 analyzed isolates. Second, we have used Luke as a control in our wheat nurseries for screening for quantitative resistance to diseases in Gansu since 1990 and in Shandong since 2011. Luke stably exhibited slow leaf-rusting (Zhang and Du, unpublished data), implying that QLr.cau-1AS was effective against all the races occurring during this long period. Indeed, the P. triticina populations consisted of diverse virulence races in the major wheat-production regions including Gansu and Shandong during the last 25 years (Chen et al. 1994; Wan et al. 2010). Last, Milus and Line (1980b) reported that Luke could restrict lesion size of leaf rust at the adult plant stage. Such a disease reduction was observed for each of the tested pathogen races and in each of the tested environments (Milus and Line 1980b).
In summary, QLr.cau-1AS is highly likely to be a previously un-reported/novel QTL for reducing leaf rust severity. The effect size of QLr.cau-1AS was nearly equal to that of Lr34. QLr.cau-1AS was effective consistently/stably across different genetic backgrounds, diverse environments, and likely a wide range of P. trticina races. The selection based on the DNA marker gpw2246 for QLr.cau-1AS was as effective as the selection based on csffr5 for Lr34. The combination of QLr.cau-1AS with Lr34 yielded an elevated level of resistance. The defense mechanism of QLr.cau-1AS may be different from that of Lr34 and other leaf tip necrosis-associated genes/QTLs. QLr.cau-1AS is potentially helpful for increasing the genetic diversity of slow leaf-rusting resistance in wheat breeding programs.
Author contribution statement
Z. D. and Z. Z. conceived, designed, and managed the experiments. Z. D., M. C., G. L., J. C., W. Q., Y. G., Z. W., J. R., H. Z., and Z. Z. performed the experiments. Z. D., G. L., and Z. Z. analyzed the data. Z. D. and Z. Z. prepared the manuscript.
References
Akbari M, Wenzl P, Caig V, Carling J, Xia L, Yang S, Uszynski G, Mohler V, Lehmensiek A, Kuchel H, Hayden MJ, Howes N, Sharp PJ, Vaughan P, Rathmell B, Huttner E, Kilian A (2006) Diversity array technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor Appl Genet 113:1409–1420
Bassam BJ, Caetano-Anolles G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Bjarko ME, Line RF (1988) Quantitative determination of the gene action of leaf rust resistance in four cultivars of wheat, Triticum aestivum. Phytopathology 78:451–456
Bolton M, Kolmer J, Garvin D (2008) Wheat leaf rust caused by Puccinia triticina. Mol Plant Pathol 9:563–575
Buerstmayr M, Matiasch L, Mascher F, Vida G, Ittu M, Robert O, Holdgate S, Flath K, Neumayer A, Buerstmayr H (2014) Mapping of quantitative adult plant field resistance to leaf rust and stripe rust in two European winter wheat populations reveals co-location of three QTL conferring resistance to both rust pathogens. Theor Appl Genet 127:2011–2028
Caldwell RM (1968) Breeding for general and/or specific plant disease resistance. In: Findlay KW, Shepherd KW (eds) Proc 3th Int Wheat Genetics Symp. Australian Academy of Science, Canberra, pp 263–272
Caldwell RM, Schafer JF, Compton LE, Patterson FL (1957) A mature plant type of wheat leaf-rust resistance of composite origin. Phytopathology 47:691–692
Chen WQ, Yan SB, Hu CC, Xie SX (1994) Physiological race and pathogenicity of Puccinia recondita f. sp. tritici in China during the period from 1990 to 1993. (In Chinese with English abstract). Acta Phytophylacica Sinica 21:289–294
Chen J, Li GH, Du ZY, Quan W, Zhang HY, Che MZ, Wang Z, Zhong ZJ (2013) Mapping of QTL conferring resistance to sharp eyespot (Rhizoctonia cerealis) in bread wheat at the adult plant growth stage. Theor Appl Genet 126:2865–2878
Devos KM, Bryan GJ, Collins AJ, Stephenson P, Gale MD (1995) Application of two microsatellite sequences in wheat storage proteins as molecular markers. Theor Appl Genet 90:247–252
Dubin HJ, Torres E (1981) Causes and consequences of the 1976–1977 wheat leaf rust epidemic in Northwest Mexico. Annu Rev Phytopathol 19:41–49
Dyck PL, Samborski DJ (1982) The inheritance of resistance to Puccinia recondita in a group of common wheat cultivars. Can J Genet Cytol 24:273–283
Feuillet C, Schachermayr G, Keller B (1997) Molecular cloning of a new receptor-like kinase gene encoded at the Lr10 disease resistance locus of wheat. Plant J 11:45–52
Feuillet C, Travella S, Stein N, Albar L, Nublat A, Keller B (2003) Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploid wheat (Triticum aestivum L.) genome. Proc Natl Acad Sci USA 100:15253–15258
Flor HH (1971) Current status of the gene-for-gene concept. Annu Rev Phytopathol 9:275–296
Guo Q, Zhang ZJ, Xu YB, Li GH, Feng J, Zhou Y (2008) Quantitative trait loci for high-temperature adult-plant and slow-rusting resistance to Puccinia striiformis f. sp. tritici in wheat cultivars. Phytopathology 98:803–809
Heath M (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Herrera-Foessel SA, Lagudah ES, Huerta-Espino J, Hayden MJ, Bariana HS, Singh D, Singh RP (2011) New slow-rusting leaf rust and stripe rust resistance genes Lr67 and Yr46 in wheat are pleiotropic or closely linked. Theor Appl Genet 122:239–249
Herrera-Foessel SA, Singh RP, Huerta-Espino J, Rosewarne GM, Periyannan SK, Viccars L, Calvo-Salazar V, Lan CX, Lagudah ES (2012) Lr68: a new gene conferring slow rusting resistance to leaf rust in wheat. Theor Appl Genet 124:1475–1486
Hiebert CW, Thomas JB, McCallum BD, Humphreys DG, DePauw RM, Hayden MJ, Mago R, Schnippenkoetter W, Spielmeyer W (2010) An introgression on wheat chromosome 4DL in RL6077 (Thatcher × 6/PI 250413) confers adult plant resistance to stripe rust and leaf rust (Lr67). Theor Appl Genet 121:1083–1091
Huang J, Zhao Z, Song F, Wang X, Xu H, Huang Y, An D, Li H (2012) Molecular detection of a gene effective against powdery mildew in the wheat cultivar Liangxing 66. Mol Breed 30:1737–1745
Ingala L, López M, Darino M, Pergolesi MF, Diéguez MJ, Sacco F (2012) Genetic analysis of leaf rust resistance genes and associated markers in the durable resistant wheat cultivar Sinvalocho MA. Theor Appl Genet 124:1305–1314
Keen NT (1990) Gene-for-gene complementarity in plant-pathogen interactions. Annu Rev Genet 24:447–473
Khan M, Bukhari A, Dar Z, Rizvi S (2013) Status and strategies in breeding for rust resistance in wheat. Agric Sci 4:292–301
Kolmer JA (1996) Genetics of resistance to wheat leaf rust. Annu Rev Phytopathol 34:435–455
Kolmer JA (2005) Tracking wheat rust on a continental scale. Curr Opin Plant Biol 8:441–449
Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL, Keller B (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323:1360–1363
Lagudah ES, Krattinger SG, Herrera-Foessel S, Singh RP, Huerta-Espino J, Spielmeyer W, Brown-Guedira G, Selter LL, Keller B (2009) Gene-specific markers for the wheat gene Lr34/Yr18/Pm38 which confers resistance to multiple fungal pathogens. Theor Appl Gent 119:889–898
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer pack-age for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Li ZF, Xia XC, He ZH, Li X, Zhang LJ, Wang HY, Meng QF, Yang WX, Li GQ, Liu DQ (2010) Seedling and slow rusting resistance to leaf rust in Chinese wheat cultivars. Plant Dis 94:45–53
Li ZF, Lan CX, He ZH, Singh RP, Rosewarne GM, Chen XM, Xia XC (2014) Overview and application of QTL for adult plant resistance to leaf rust and powdery mildew in wheat. Crop Sci 54:1907–1925
Line RF (2002) Stripe rust of wheat and barley in North America: a retrospective historical review. Annu Rev Phytopathol 2002(40):75–118
Long DL, Kolmer JA (1989) A North American system of nomenclature for Puccinia recondita f. sp. tritici. Phytopathology 79:525–529
Maccaferri M, Mantovani P, Tuberosa R, DeAmbrogio E, Giuliani S, Demontis A, Massi A, Sanguineti MC (2008) A major QTL for durable leaf rust resistance widely exploited in durum wheat breeding programs maps on the distal region of chromosome arm 7BL. Theor Appl Genet 117:1225–1240
McDonald BA, Linde C (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol 40:349–379
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers WJ, Morris C, Appels R, Xia XC (2013) Catalogue of gene symbols for wheat: 2013 supplement. www.shigen.nig.ac.jp/wheat/komugi/genes/macgene/2013/GeneCatalogueIntroduction.pdf. Accessed 30 June 2014
Messmer MM, Seyfarth R, Keller M, Schachermayr G, Winzeler M, Zanetti S, Feuillet C, Keller B (2000) Genetic analysis of durable leaf rust resistance in winter wheat. Theor Appl Genet 100:419–431
Michelmore RW, Christopoulou M, Caldwell KS (2013) Impacts of resistance gene genetics, function, and evolution on a durable future. Annu Rev Phytopathol 51:291–319
Milus EA, Line RF (1980a) Virulence of Puccinia recondita in the pacific Northwest. Plant Dis 64:78–80
Milus EA, Line RF (1980b) Characterization of resistance to leaf rust in Pacific Northwest wheats. Phytopathology 70:167–172
Milus EA, Line RF (1986) Number of genes controlling high temperature, adult-plant resistance to stripe rust in wheat. Phytopathology 76:93–96
Ohm HW, Shaner GE (1976) Three components of slow leaf-rusting at different growth stages in wheat. Phytopathology 66:1356–1360
Paillard S, Schnurbusch T, Winzeler M, Messmer M, Sourdille P, Abderhalden O, Keller B, Schachermayr G (2003) An integrative genetic linkage map of winter wheat (Triticum aestivum L.). Theor Appl Genet 107:1235–1242
Park RF, McIntosh RA (1994) Adult plant resistances to Puccinia recondita f. sp. tritici in wheat. N Z J Crop Hortic Sci 22:151–158
Park RF, Mohler V, Nazari K, Singh D (2014) Characterization and mapping of gene Lr73 conferring seedling resistance to Puccinia triticina in common wheat. Theor Appl Genet 127:2041–2049
Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity of leaves and stems of cereals. Can J Res 26:496–500
Qayoum A, Line RF (1985) High-temperature, adult plant resistance to stripe rust of wheat. Phytopathology 75:121–1125
Quan W, Hou GL, Chen J, Du ZY, Lin F, Guo Y, Liu S, Zhang ZJ (2013) Mapping of QTL lengthening the latent period of Puccinia striiformis in winter wheat at the tillering growth stage. Eur J Plant Pathol 136:715–727
Risk JM, Selter LL, Krattinger SG, Viccars LA, Richardson TM, Buesing G, Herren G, Lagudah ES, Keller B (2012) Functional variability of the Lr34 durable resistance gene in transgenic wheat. Plant Biotechnol J 10:477–487
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal M (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Roelfs AP, Singh RP, Saari EE (1992) Rust diseases of wheat: Concepts and methods of disease management. CIMMYT, Mexico
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Schachermayr G, Feuillet C, Keller B (1997) Molecular markers for the detection of the wheat leaf rust resistance gene Lr10 in diverse genetic backgrounds. Mol Breed 3:65–74
Schnurbusch T, Paillard S, Schori A, Messmer M, Schachermayr G, Winzeler M, Keller B (2004) Dissection of quantitative and durable leaf rust resistance in Swiss winter wheat reveals a major resistance QTL in the Lr34 chromosomal region. Theor Appl Genet 108:477–484
Singh RP (1992) Genetic association of leaf rust resistance gene Lr34 with adult plant resistance to stripe rust in bread wheat. Phytopathology 82:835–838
Singh RP, Gupta AK (1992) Expression of wheat leaf rust resistance gene Lr34 in seedlings and adult plants. Plant Dis 76:489–491
Singh RP, Mujeeb-Kazi A, Huerta-Espino J (1998) Lr46: a gene conferring slow-rusting resistance to leaf rust in wheat. Phytopathology 88:890–894
Singh RP, Chen WQ, He ZH (1999) Leaf rust resistance of spring, facultative, and winter wheat cultivars from China. Plant Dis 83:644–651
Singh D, Simmonds J, Park RF, Bariana HS, Snape JW (2009) Inheritance and QTL mapping of leaf rust resistance in the European winter wheat cultivar ‘Beaver’. Euphytica 169:253–261
Singh A, Knox RE, DePauw RM, Singh AK, Cuthbert RD, Campbell HL, Shorter S, Bhavani S (2014) Stripe rust and leaf rust resistance QTL mapping, epistatic interactions, and co-localization with stem rust resistance loci in spring wheat evaluated over three continents. Theor Appl Genet 127:2465–2477
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, Gill BS, Dufour P, Murigneux A, Bernard M (2004) Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomics 4:12–25
Spielmeyer W, McIntosh RA, Kolmer J, Lagudah ES (2005) Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust cosegregate at a locus on the short arm of chromosome 7D of wheat. Theor Appl Genet 111:731–735
Suenaga K, Singh RP, Huerta-Espino J, William HM (2003) Microsatellite markers for genes Lr34/Yr18 and other quantitative trait loci for leaf rust and stripe rust resistance in bread wheat. Phytopathology 93:881–890
Tsilo TJ, Kolmer JA, Anderson JA (2014) Molecular mapping and improvement of leaf rust resistance in wheat breeding lines. Phytopathology 104:865–870
Van der Hoorn RAL, Kamoun S (2008) From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell 20:2009–2017
Wan JJ, Meng QF, Yang WX, Wen XL, Liu DQ (2010) Study on virulence of Puccininia triticina on wheat in Shandong province. (In Chinese with English abstract). J Anhui Agri Sci 38:16929–16930
Wang S, Basten JC, Zeng ZB (2010) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm. Accessed 27 Dec 2014
Wenzl P, Kudrna D, Jaccoud D, Huttner E, Kleinhofs A, Kilian A (2004) Diversity arrays technology (DArT) for whole genome profiling of barley. Proc Natl Acad Sci USA 101:9915–9920
William HM, Hoisington D, Singh RP, Gonzalez-de-Leon D (1997) Detection of quantitative trait loci associated with leaf rust resistance in bread wheat. Genome 40:253–260
William HM, Singh RP, Huerta-Espino J, Palacios G, Suenaga K (2006) Characterization of genetic loci conferring adult plant resistance to leaf rust and stripe rust in spring wheat. Genome 49:977–990
Xu X, Bai G, Carver BF, Shaner GE, Hunger RM (2005) Molecular characterization of slow leaf-rusting resistance in wheat. Crop Sci 45:758–765
Zhang ZJ (1995) Evidence of durable resistance in nine Chinese landraces and one Italian cultivar of Triticum aestivum to Puccinia striiformis. Eur J Plant Pathol 101:405–409
Zhou HX, Xia XC, He ZH, Li X, Wang CF, Li ZF, Liu DQ (2013) Molecular mapping of leaf rust resistance gene LrNJ97 in Chinese wheat line Neijiang 977671. Theor Appl Genet 126:2141–2147
Zhou Y, Ren Y, Lillemo M, Yao ZJ, Zhang PP, Xia XC, He ZH, Li ZF, Liu DQ (2014) QTL mapping of adult-plant resistance to leaf rust in a RIL population derived from a cross of wheat cultivars Shanghai 3/Catbird and Naxos. Theor Appl Genet 127:1873–1883
Acknowledgments
We gratefully thank Professors W. Q. Chen and W. X. Yang for their supports with the purification of P. triticina race and some wheat lines. This study was supported by the National Natural Science Foundation of China (30871612), the National Basic Research Program of China (2013CB127700), and the Special Fund for Agro-scientific Research in the Public Interest (201203014).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This research complies with the current laws of P. R. China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Miedaner.
M. Che and G. Li contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, Z., Che, M., Li, G. et al. A QTL with major effect on reducing leaf rust severity on the short arm of chromosome 1A of wheat detected across different genetic backgrounds and diverse environments. Theor Appl Genet 128, 1579–1594 (2015). https://doi.org/10.1007/s00122-015-2533-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2533-x