Abstract
Sunflower oil is one of the major sources of edible oil. As the second largest hybrid crop in the world, hybrid sunflowers are developed by using the PET1 cytoplasmic male sterility system that contributes to a 20 % yield advantage over the open-pollinated varieties. However, sunflower production in North America has recently been threatened by the evolution of new virulent pathotypes of sunflower rust caused by the fungus Puccinia helianthi Schwein. Rf ANN-1742, an ‘HA 89’ backcross restorer line derived from wild annual sunflower (Helianthus annuus L.), was identified as resistant to the newly emerged rust races. The aim of this study was to elucidate the inheritance of rust resistance and male fertility restoration and identify the chromosome location of the underlying genes in Rf ANN-1742. Chi-squared analysis of the segregation of rust response and male fertility in F2 and F3 populations revealed that both traits are controlled by single dominant genes, and that the rust resistance gene is closely linked to the restorer gene in the coupling phase. The two genes were designated as R 11 and Rf5, respectively. A set of 723 mapped SSR markers of sunflower was used to screen the polymorphism between HA 89 and the resistant plant. Bulked segregant analysis subsequently located R 11 on linkage group (LG) 13 of sunflower. Based on the SSR analyses of 192 F2 individuals, R 11 and Rf5 both mapped to the lower end of LG13 at a genetic distance of 1.6 cM, and shared a common marker, ORS728, which was mapped 1.3 cM proximal to Rf5 and 0.3 cM distal to R 11 (Rf5/ORS728/R 11 ). Two additional SSRs were linked to Rf5 and R 11 : ORS995 was 4.5 cM distal to Rf5 and ORS45 was 1.0 cM proximal to R 11 . The advantage of such an introduced alien segment harboring two genes is its large phenotypic effect and simple inheritance, thereby facilitating their rapid deployment in sunflower breeding programs. Suppressed recombination was observed in LGs 2, 9, and 11 as it was evident that no recombination occurred in the introgressed regions of LGs 2, 9, and 11 detected by 5, 9, and 22 SSR markers, respectively. R 11 is genetically independent from the rust R-genes R 1 , R 2 , and R 5 , but may be closely linked to the rust R-gene R adv derived from wild Helianthus argophyllus, forming a large rust R-gene cluster of R adv /R 11 /R 4 in the lower end of LG13. The relationship of Rf5 with Rf1 is discussed based on the marker association analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sunflower oil provides about 13 % of the world’s edible oil. The high proportion of polyunsaturated fatty acids renders sunflower oil as a popular source of essential fatty acids in the diet. The discovery of cytoplasmic male sterility (CMS) and the gene for male fertility restoration in the early 1970s allowed for the production of hybrid sunflower, demonstrating a 20 % yield advantage over the open-pollinated varieties (Leclercq 1969; Kinman 1970). The first PET1-type CMS derived from Helianthus petiolaris subsp. petiolaris Nutt. and the restorer gene Rf1 have been extensively utilized in the commercial seed production of hybrid sunflower worldwide, which raises questions about the potential threat of genetic vulnerability of sunflower hybrids. Developing several CMS/Rf systems and elucidating their molecular mechanisms will broaden the genetic diversity of sunflower hybrids. In addition to Rf1, the restorer genes Msc1 and Rf3 that was recently reported in RHA 340 and RHA 280 lines also are able to restore CMS PET1 (Gentzbittel et al. 1999; Jan and Vick 2007; Abratti et al. 2008; Liu et al. 2011), whereas the Rf4 gene is specific for male fertility restoration of CMS GIG2, a system different from CMS PET1 (Feng and Jan 2008).
The germplasm line Rf ANN-1742 was released as a male fertility restorer in 1997. However, genetics of male fertility restoration in this line has not been investigated (Seiler and Jan 1997). Recent evaluation of rust resistance identified the Rf ANN-1742 line as resistant to the newly emerged rust races of sunflower (Qi et al. 2011a).
Rust, caused by the fungus Puccinia helianthi Schwein., is a disease of sunflower that can cause significant losses in both yield and seed quality on susceptible hybrids when conditions are favorable for disease development. In the northern Great Plains area of North America, sunflower production has recently been threatened by the evolution of new virulent pathotypes of sunflower rust. Thirty-nine North American (NA) rust races were identified in 2008 with races 334 and 336 being dominant (Gulya and Markell 2009). A newly evolved NA rust race 777 was collected in Texas and Kansas in 1995 (Miller and Gulya 2001), and was able to infect all nine differentials (7350, MC 90, MC 29, P386, HA-R1, HA-R2, HA-R3, HAR-4, and HAR5) (Gulya and Viranyi 1994; Rashid 2006; Gulya and Markell 2009). The majority of commercial hybrids are susceptible to the new predominant and virulent races (Gulya 2006; Gulya and Markell 2009). Presently, race 777 remains at low frequency in the rust populations, but can potentially cause serious epidemics if it becomes prevalent in the sunflower growing areas in North America. The limited durability of single R-genes made it necessary to continue the discovery and introgression of new R-genes. Rf ANN-1742 is an important source of resistance to sunflower rust race 777, and utilization of this novel source along with other resistance genes in commercial sunflower hybrids in North America will significantly reduce the occurrence of new pathotypes and consequent yield losses due to the disease.
The sunflower rust pathosystem follows the classical gene-for-gene concept. Genetic studies of resistance to rust have indicated that nearly all sources of rust resistance in sunflower are controlled by single, usually dominant, genes. Currently, eight genes, R 1 –R 5 , R 10 , P u6 , and R adv , have been postulated to confer resistance to different rust races, and a novel gene, R 11 , is described for the first time in the present study (Putt and Sackston 1963; Miah and Sackston 1970; Miller et al. 1988; Yang et al. 1989; Goulter 1990; Lambrides and Miller 1994; Lawson et al. 1998; Radwan 2010; Bachlava et al. 2011). New virulent races are able to overcome the rust resistance genes R 1 , R 3 , P u6 , and R adv . The genes R 2 in line MC 29, R 4 in HA-R3, and R 5 in HA-R2 remain resistant to the predominant race 336, but all are susceptible to the most virulent race 777 (Rashid 2006; Gulya and Markell 2009; Qi et al. 2011a). The gene R 10 was reported as a second resistance gene present in an Australia selection of MC 29 (AUS) that harbors the R 2 gene (Lambrides and Miller 1994). Line MC 29 (AUS) was also susceptible to race 777 (Qi unpublished data). However, the gene R 11 in line Rf ANN-1742 was recently identified as resistant to the predominant and virulent races, 336 and 777, respectively (Qi et al. 2011a).
Molecular mapping of both rust resistance genes and male fertility restorer genes has been carried out to accelerate the introgression of these genes into elite cultivars and R-gene pyramiding in sunflower. The rust resistance genes, R 1 and R 2 , were mapped to linkage groups (LGs) 8 and 9 of sunflower, respectively (Lawson et al. 1998, 2011; Slabaugh et al. 2003; Yu et al. 2003). Both R adv and R 4 genes were mapped to LG13 (Lawson et al. 1998; Yu et al. 2003; Radwan 2010; Bachlava et al. 2011; Qi et al. 2011b), whereas the R 5 gene was mapped to LG2 (Qi et al. 2011c). The male fertility restorer gene Rf1 was also mapped to LG13 (Gentzbittel et al. 1995; Berry et al. 1997; Horn et al. 2003; Yu et al. 2003; Kusterer et al. 2005; Yue et al. 2010), whereas the Msc1 and Rf3 restorer genes were mapped to LG7 (Mazeyrat et al. 1998; Gentzbittel et al. 1999; Abratti et al. 2008; Liu et al. 2011) and Rf4 was mapped to LG3, respectively (Feng and Jan 2008). Here, we report the genetic mapping of a novel rust resistance gene, R 11 , tightly linked to a restorer gene, Rf5, in LG13.
Materials and methods
Plant materials and mapping population
The Rf ANN-1742 line was derived from a BC1F2 population by crossing cms HA 89 with a wild Helianthus annuus accession, PI 613748, which originated from Hinton, Oklahoma, US. This line was released as a CMS male fertility restorer which segregated for rust resistance (Seiler and Jan 1997; Qi et al. 2011a). One resistant plant, 09-519-1, was self-pollinated and a progeny test indicated that it was heterozygous for both rust resistance and male fertility restoration, and thus that it could serve as the equivalent of an F1 for these characters. The F2 population from this plant was sown in the greenhouse in 2010 and seeds from each plant harvested separately to provide F3 families. Progeny tests of 146 rust resistant/fertile F2:3 families for rust resistance and male fertility restoration indicated the genotypes of their F2 plants.
Six sunflower maintainer lines and 18 male fertility restorer lines were used to validate DNA markers linked to the restorer genes (Table 1). Among the 18 restorer lines, RHA 265, RHA 274, and RHA 348 were known to carry the Rf1 gene (Kinman 1970; Korell et al. 1992). Both RHA 340 and RHA 280 were reported to carry the restorer gene Rf3 (Abratti et al. 2008; Liu et al. 2011), and Rf GIG2 and Rf ANN-1742 lines harbor Rf4 and Rf5, respectively (Feng and Jan 2008). The remaining 11 lines were selected from diverse sources, and the Rf genes in these lines are unknown (Korell et al. 1992).
Evaluation of rust resistance
Sunflower rust race 336 was collected originally from cultivated plants in North Dakota in 2009, and is the predominant race in North America (Gulya and Markell 2009). A total of 207 F2 seeds and 20 seeds of parental line HA 89 were planted in 36-cell plastic flats (one seed per cell of 4.6 cm × 5.4 cm) filled with Sunshine SB 100B potting mixture (SunGro Horticulture, Bellevue, WA) in May 2010. The F2 plants and HA 89 plants were inoculated with race 336 of P. helianthi. Urediniospores from a liquid N2 tank were heat shocked at 45 °C for 1 min before use. Spores were then suspended in SOLTROL 170 isoparaffin (Chevron Phillips Chemical Co., The Woodlands, TX) at 5–10 mg spores/10 ml and sprayed onto four-leaf stage seedlings using the procedure previously described (Gulya and Masirevic 1996; Qi et al. 2011b). After inoculation, seedling plants were allowed to dry for 15–30 min and then incubated in a dew chamber equipped with automated ultrasonic humidifiers to provide continuous leaf wetness, and held 16–20 h at 20 °C in the dark. Seedlings were placed on a greenhouse bench maintained at 22 ± 2 °C with a photoperiod of 16 h after incubation. Infection type (IT), described by Yang et al. (1986), combined with percentage of leaf area covered with pustules (severity), described by Gulya et al. (1990), were assessed 12–14 days post-inoculation. Infection type 0, 1, and 2 combined with pustule coverage of 0–0.5 % were classified as resistant, and IT 3 and 4 with pustule coverage more than 0.5 % were considered susceptible.
After scoring for rust infection, the 207 F2 plants (49 rust susceptible and 158 rust resistant plants) were transferred to 2-gallon pots for male fertility evaluation in the greenhouse. Forty-nine rust susceptible plants and 3 of 158 rust resistant plants were found to be completely male sterile and did not produce any seeds. The remaining 155 F2 rust resistant plants were grown to obtain F3 seeds. A total of 146 resistant/fertile F3 families were subjected to progeny test. Twenty seeds of each F3 family were planted in 36-cell plastic flats in October 2010 and April 2011, respectively, and were inoculated with race 336 at the four-leaf stage. The F3 families were classified as homozygous resistant if all seedlings had low IT and severity, or segregating if seedlings varied for low and high IT and severity.
Evaluation of male fertility restoration
The F2 and F3 plants were visually scored for the presence or absence of pollen. Plants that produced anthers and shed pollen were considered fertile, whereas those without anthers or pollen were considered sterile. The F2 population was evaluated for male fertility and sterility in the greenhouse in May 2010. One hundred and forty-six fertile F2:3 families were grown in rows of 30 plants sown in the field in June 2011. Evaluation of male fertility was conducted at the flowering stage. The results of the F3 family test were used to infer the genotypes of F2 plants for the restorer gene. A Chi-squared (χ2) analysis was performed to verify whether the observed ratios of segregation for rust resistance and male fertility in F2 and F3 populations fit expected models.
DNA extraction and PCR conditions
Genomic DNA was isolated from young leaves of the parents and F2 individuals using the Qiagen DNeasy 96 plant kit with a modified protocol described by Horne et al. (2004) (Qiagen, Valencia, CA). The quantity and the quality of DNA were determined with a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE).
Polymerase chain reaction (PCR) was conducted on a Peltier thermocycler (Bio-Rad Lab, Hercules, CA, USA) with a touchdown program as described by Qi et al. (2011a). The PCR reaction mixture (15 μl) contained 1× Taq DNA polymerase buffer, 2 mM MgCl2, 200 μM of each dNTP, 0.02 μM forward primer with an M13 tail (CACGACGTTGTAAAACGAC) at the 5′ end, 0.1 μM reverse primer, 0.1 μM fluorescently labeled M13 primer, 0.6× PVP (polyvinylpyrrolidone), 0.5 units Taq polymerase (Bioline, Randolph, MA, USA), and 10–20 ng of genomic DNA. PCR products were diluted 20- to 120-fold before analysis. SSR fragments were size separated by using an IR2 4300 DNA Analyzer (Li-COR, Lincoln, Nebraska). The PCR conditions for SCAR markers SCX20 (Lawson et al. 1998), HRG01 and HRG02 (Horn et al. 2003), and STS marker STS115 (Yue et al. 2010) were previously described.
Bulked segregant analysis (BSA) and genetic mapping
For marker screening of parents, we selected an F3 plant (10-275-2) homozygous for both rust resistance and fertility restoration as the resistant parent versus susceptible parent HA 89. DNAs of the ten homozygous resistant F2 plants, based on the F3 progeny test, were pooled in an equal amount to create a resistant (R) bulk. Likewise, DNAs of the 10 homozygous susceptible F2 plants were pooled to produce a susceptible (S) bulk. Genomic DNAs of HA 89 and 10-275-2 were first screened with a set of 723 sunflower SSR primers to detect polymorphic markers between parents. The BSA (Michelmore et al. 1991) was performed with polymorphic SSRs to determine the chromosome region containing the R 11 gene. A total of 192 F2 plants were used for R 11 and Rf5 mapping.
The phenotype and SSR data were combined for linkage analysis. Marker order and map distance were estimated using MapMaker software for the Macintosh with default parameters of LOD = 3.0 (Lander et al. 1987). The recombination fractions were transformed by the Kosambi mapping function to estimate the map distance (Kosambi 1944).
Results
Inheritance of the rust resistance gene R 11 and male fertility restorer gene Rf5
The inbred line HA 89 was highly susceptible to rust race 336, a predominant race in North America, with IT 4 and more than 20 % of the leaves covered with pustules, whereas the selected homozygous plant 10-275 was resistant, showing localized necrosis at infection sites with an IT 2 and 0.1–0.5 % of the leaves covered with pustules (Fig. 1). The 207 F2 individuals segregated at a ratio of 158R:49S, which did not differ significantly from the expected 3:1 ratio (χ 2 = 0.13, df = 1, P = 0.718), indicating a dominant gene governs rust resistance in the line Rf ANN-1742, and this gene is designated as R 11 .
Forty-nine rust susceptible F2 plants were also completely male sterile, whereas only 3 of 158 rust resistant F2 plants were male sterile (155MF:52MS), indicating that the rust resistance and male-fertility restoration were closely linked in the coupling phase in this population. Fifty-two male-sterile plants did not produce any seeds. Segregation of male-fertile and male-sterile plants fit an expected 3:1 ratio (χ 2 = 0.0008, df = 1, P = 0.977), indicating that one dominant gene segregated in the F2 population and is responsible for male fertility restoration of the male-sterile PET1 cytoplasm. This gene was named Rf5.
Rust phenotyping of 146 resistant/fertile F2:3 families (20 plants per family) showed that the F2 population had 45 homozygous resistant and 101 heterozygous resistant plants. A Chi-square test indicated that this fits a 1RR: 2Rr segregation ratio (χ 2 = 0.303, df = 1, P = 0.657), which would be expected for a single gene trait segregating 1 homozygous resistant: 2 heterozygous resistant: 1 homozygous susceptible. The F3 family data for fertility from 146 fertile F2:3 families (30 plants per family) evaluated in the field were also consistent with a segregation of 1:2. Forty-seven F3 families were nonsegregating, whereas 99 were segregating (χ 2 = 0.040, df = 1, P = 0.841). The results confirm that both genes, Rf5 and R 11 , fit a single gene model (Seiler and Jan 1994; Qi et al. 2011a).
Bulked segregant analysis
Out of 723 SSR primer pairs used, 73 showed polymorphism between the susceptible HA 89 and resistant 10-275-2, an average of ~10 % polymorphism. Fifteen of 17 linkage groups of sunflower had at least some polymorphic marker coverage, the lone exceptions being LGs 4 and 15. The minimum number of polymorphic SSRs on a linkage group was 1 (LGs 1, 5, and 6) and maximum number was 24 (LG11) (Table 2).
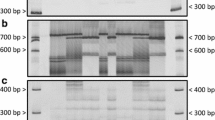
The parents, two bulks, and two F2 individual plants selected, 10-149-45 homozygous susceptible and 10-149-68 homozygous resistant, were screened with 50 polymorphic SSRs from LGs 2 (8 SSRs), 9 (10 SSRs), 11 (24 SSRs), and 13 (8 SSRs). Only SSR primers from LG13 generated polymorphic DNA fragments between the two bulks, indicating the location of the gene conferring resistance to rust in LG13. Of the eight polymorphic SSRs of LG13, six showed the HA 89 allele in the S-bulk and susceptible F2 plants, whereas the R-bulk shared the same PCR pattern as the resistant line 10-275-2 and the resistant F2 plant selected (Fig. 2).
PCR pattern of parental and bulked DNA samples with SSR primers of ORS229, ORS713, ORS686, and ORS45. ORS229 detected HA 89 allele in the F2 population, but did not show polymorphism between two bulks, whereas ORS713 and ORS686 did not detect HA 89 allele in the population. ORS45 detected polymorphism between bulks. 10-149-45: homozygous susceptible F2 plant; HA 89: susceptible parent; S-bulk from the 10 homozygous susceptible F2 plants; 10-275-2: F2:3 homozygous resistant plant; R-bulk from the 10 homozygous resistant F2 plants; 10-149-68: homozygous resistant F2 plant. M 50- to 700-bp DNA ladder (Li-COR. Inc. Lincoln, Nebraska, US). PCR products were diluted 20-fold and were size separated in an IR2 4300 DNA Analyzer (Li-COR, Lincoln, Nebraska). The PCR fragment size amplified by SSR primers included a 19-bp M13 tail primer
Surprisingly, out of 24 polymorphic SSRs in LG11, 22 showed no HA 89, a recurrent parent used to develop Rf ANN-1742, allele present in the mapping population. Both the susceptible F2 plant and the S-bulk had the same allele as the resistant plants and the R-bulk (Fig. 2). These markers detected a segment of donor DNA that showed no evidence of recombination in this region. Similarly, no HA 89 allele was detected in the F2 population with ten polymorphic SSR markers from LG9. Nine of these ten markers are located on the upper end based on the public SSR genetic map (Fig. 3, Tang et al. 2003). Of eight polymorphic SSRs of LG2, only three, CRT313, ORS229, and ORS342, detected HA 89 allele in the population. These markers were positioned to the lower end of LG2, a region of higher recombination (Fig. 3).
Diagram of inferred suppression of recombination in linkage groups 2, 9, and 11 in Rf ANN-1742. HA 89 segments are white, wild donor segments are gray, and segments possessing recombination events are crisscross. Genetic maps of linkage groups 2, 9, and 11 were taken from Tang et al. (2003) as a cross reference of marker order, and common SSR markers were aligned between a and b. Possible order of other markers on LGs 2, 9, and 11 of Rf ANN-1742 were referenced to Burke et al. (2002), Yu et al. (2003), and the sunflower CMap database (http://sunflower.uga.edu/cmap/)
Genetic mapping of the genes R 11 and Rf5
Eight polymorphic SSRs in LG13 were assayed across the mapping population of 192 F2 progenies to confirm linkage with R 11 . Recombination mapping showed that these eight polymorphic markers (5 co-dominant and three dominant) along with the R 11 and Rf5 loci were all located in the lower end of LG13 encompassing a genetic distance of 17.1 cM, an average of 1.94 cM per locus (Fig. 4a). The marker order was found to be in good agreement with those of Tang et al. (2003) and Qi et al. (2011b) (Fig. 4a, c, d). The rust resistance gene R 11 was found to be closely linked to the restorer gene Rf5. The interval between Rf5 and R 11 covered ~1.6 cM of genetic distance. Both genes were closely linked to the marker ORS728, which is 1.3 cM proximal to Rf5 and 0.3 cM distal to R 11 (Rf5/ORS728/R 11 ) (Fig. 4a). ORS728 was previously mapped to two LGs, 1 and 13 (Tang et al. 2002; Yu et al. 2003). The primers amplified two fragments in both HA 89 and 10-275-2. The top fragment showed no polymorphism between the two lines, whereas the bottom one was polymorphic generating a co-dominant marker linked to the Rf5 and R 11 genes, respectively (Fig. 5c). Two additional SSR markers were linked to Rf5 and R 11 ; ORS995 was 4.5 cM distal to Rf5 and ORS45 was 1.0 cM proximal to R 11 (Fig. 4a).
Genetic maps of sunflower linkage group (LG) 13. a Rf5 and R 11 map. b Rf1 map. c linkage group 13 genetic map, showing the positions of SCAR marker SCX20 and the R 4 locus. d public genetic map of linkage group 13 (Tang et al. 2003)
Detection of Rf loci with the PCR-based markers, STS115, HRG02, and ORS728. The Rf1 markers, STS115 (a) and HRG02 (b), were present in 14 restorer lines in which it was assumed that all carried the Rf1 gene, whereas they were absent in 6 maintainer lines and 4 restorer lines harboring different restorer genes, 10-275-2 (Rf5), RHA 340 (Rf3), RHA 280 (Rf3), and RfGIG2 (Rf4). The Rf5 marker, ORS728-specific fragment (c), is only present in 10-275-2 possessing the Rf5 gene and absent in all maintainer and restorer lines. Arrow points to the ORS728-polymorphic fragment in the 10-275-2 plant. PCR products of primers STS115 and HRG02 were separated on a 1.5 % agarose gel, and the molecular weight marker is from GeneRuler 1 kb Plus DNA Ladder (Fermentas Inc. Maryland, US). The PCR product of ORS728 was diluted 80-fold and was size separated in an IR2 4300 DNA Analyzer (Li-COR, Lincoln, Nebraska), and the molecular weight marker is a 50- to 700-bp DNA ladder (Li-COR. Inc. Lincoln, Nebraska, US)
Validation of DNA markers linked to Rf genes
Three DNA markers, including STS marker STS115 and two SCAR markers HRG01 and HRG02, linked to the Rf1 gene (Horn et al. 2003; Yue et al. 2010), along with ORS728, a marker closely linked to Rf5, were tested in 6 maintainer lines and 18 restorer lines. No PCR product with primers of the three Rf1 markers was observed in all maintainer lines and four restorer lines, RHA 340, RHA 280, Rf GIG2, and 10-275-2 (a selection of Rf ANN-1742), each harboring different Rf gene from Rf1. In contrast, the polymorphic fragments of the Rf1 markers were present in 14 other restorer lines, suggesting these lines may all have the Rf1 gene (Fig. 5; Table 3). The ORS728 primers amplified a unique fragment only from 10-275-2, but not from any other lines tested (Fig. 5).
Discussion
In this study, we mapped two genes derived from wild H. annuus, a rust resistance gene R 11 and a male restorer gene Rf5, to LG13 in sunflower. The two genes are tightly linked with a genetic distance of 1.6 cM in the coupling phase. To our knowledge, this is the first male fertility restorer gene which is closely linked to a rust resistance gene in sunflower. The advantage of such an introduced alien segment harboring both Rf5 and R 11 is its large phenotypic effect and simple inheritance, thereby facilitating their rapid deployment in sunflower breeding programs. It will be also of special interest for studying gene evolution and structure in this region in the future.
Out of 68 markers in LG13 screened in the study, only 8 SSRs detected wild donor alleles in the population. All of them were mapped to the lower end of LG13, indicating that an alien chromosome segment carrying the Rf5 and R 11 genes represents a coherent linkage block in the cultivated sunflower background. Two co-dominant SSR markers, ORS728 and ORS45, flank R 11 at 0.3 and 1.0 cM of genetic distances, respectively, and are well suited for marker-assisted selection. The marker ORS728 is also closely linked to the Rf5 gene at 1.3 cM, providing an additional selection for male fertility restoration.
Plant resistance genes that tend to cluster in genomes have been reported in diverse plant species (Saxena and Hooker 1968, 1974; Islam and Shepherd 1991; Jones et al. 1993; Song et al. 1997; Salmeron et al. 1996; Ellis et al. 1997; Michelmore and Meyers 1998; Richter and Ronald 2000; Hulbert et al. 2001; Wei et al. 1999, 2002). The lower end of sunflower LG13 was reported to harbor the second largest cluster of nucleotide binding site-leucine-rich repeat (NBS-LRR) encoded by plant R-genes identified in sunflower, and is considered as a large R-gene cluster that harbors downy mildew R-gene Pl 5 /Pl 8 and rust R-genes R adv , R 11 , and R 4 (Lawson et al. 1998; Bert et al. 2001; Yu et al. 2003; Radwan et al. 2003, 2008; Radwan 2010; Qi et al. 2011b). Tracing the origin of three rust R-genes indicated that they were derived from diverse sources. R 4 originated from an interspecific pool of crosses between Russian varieties and wild sunflower species, including H. annuus, Helianthus argophyllus, and H. petiolaris (Gulya 1985; De Romano and Vázquez 2003). R adv in lines P2 and RHA 340 was derived from wild H. argophyllus (Kong personal communication; Miller and Gulya 1988). R 11 was transferred from wild H. annuus into an HA 89 background (Seiler and Jan 1997). Rust tests reveal that each gene encodes different rust resistance specificities. R adv is resistant to race 700 (NA race 4, Miller and Gulya 1988), but is not effective against the new virulent races of 336 and 777 (Qi et al. 2011a). Tests with race 777 distinguished R 4 and R 11 ; the first being susceptible, the second resistant, but both these genes are resistant to race 336 (Rashid 2006; Gulya and Markell 2009; Qi et al. 2011a).
In spite of the location of three rust R-genes on the lower end of LG13, there are no markers in common (Fig. 4). The markers, ZVG61 and ORS581, are closely linked to the R 4 gene, but did not show polymorphism in the Rf ANN-1742 line, and were mapped to the distal end of LG13 (Fig. 4c). The polymorphic fragment of SCX20600 linked to R adv /P2 was also not present in the Rf ANN-1742 line. Sendall et al. (2006) reported that R adv /P2 was also linked to SSR markers ORS995 at 1.3 cM and ORS45 at 8.0 cM. Qi et al. (2011b) mapped SCX20600 (R adv /P2) to LG13 at 13.9 cM from the R 4 locus and 3.2 cM distal to ORS995 (Fig. 4c). In the present study, R 11 was mapped at a position 6.4 cM proximal to ORS995 and 1.1 cM distal to ORS45 (Fig. 4a). Overall, by combining pedigree information, resistance specificity, and molecular mapping, our data suggested that R 11 is closely linked to R adv forming a large rust R-gene cluster of R adv /R 11 /R 4 in the lower end of LG13, although we cannot exclude the possibility that R 11 is an allele of R adv .
Interestingly, the lower end of sunflower LG13 also harbors both Rf1 and male fertility restoration from Rf ANN-1742, denoted Rf5, the latter together with R 11 locus that confers resistance to rust (Gentzbittel et al. 1995; Berry et al. 1997; Horn et al. 2003; Yu et al. 2003; Kusterer et al. 2005; Yue et al. 2010). We were not able to precisely position Rf1 and Rf5 relative to one another on the map because the polymorphic fragments that were amplified by three markers HRG01, HRG02, and STS115 linked to Rf1 were not present in our mapping population. Meanwhile, the ORS728-specific fragment linked to Rf5 was also absent in all the restorer lines carrying the Rf1 gene, indicating that the Rf5 gene may not be an allele at the Rf1 locus (Fig. 5). In the map of Yue et al. (2010), the closest SSR marker linked to Rf 1 is ORS511, about 4 cM distal to this gene (Fig. 4b). Although ORS511 is not mapped in the present study, the marker falls in the region between ORS995 and ORS45 where Rf5 resides (Fig. 4). Thus, we propose that Rf5 may be closely linked to Rf1. Allelic tests of these two genes and its ability to restore different sunflower CMS lines of the Rf5 gene are under investigation.
Suppressed recombination was observed in LGs 2, 9, and 11. The introgressed chromosome segments in these three linkage groups are mostly inherited as large blocks of chromatin, and no recombination occurred in specific regions of LG2 (span 5 SSR markers), 9 (9 SSR markers), and 11 (22 SSR markers), respectively. During the development of Rf ANN-1742, selection was performed against male fertility. Therefore, retention of large blocks of introgressed segments in LGs 2, 9, and 11 could be due to gamete selection, indicating that the chromosomes carrying wild donor segments may be preferentially transmitted in progeny. Variation of chromosome structure between cultivated and wild H. annuus was not detected (Heiser 1954; Chandler et al. 1986; Burke et al. 2002, 2004). Therefore, suppressed recombination observed in LGs 2, 9, and 11 could be due to low levels of homology between wild and cultivated sunflower chromosomes. DNA markers that detected the large blocks of introgressed segments in the present study can be used to select genotypes without wild donor chromatins in LGs 2, 9, and 11 in breeding programs, consequently reducing the introgressed chromosome segments from wild H. annuus.
It has become abundantly clear that cultivars with single genes for resistance are of limited value because race-specific R-genes can obviously be overcome by new pathotypes relatively rapidly.
Until now, six of nine sunflower rust resistance genes were genetically mapped to LGs 2 (R 5 ), 8 (R 1 ), 9 (R 2 ), and 13 (R adv /R 11 /R 4 ), providing an opportunity to combine more rust genes in an inbred line (Lawson et al. 1998, 2011; Slabaugh et al. 2003; Yu et al. 2003; Qi et al. 2011b, c). The genes R 2 , R 4 , and R 5 have been thoroughly studied for their reaction to 300 NA rust isolates in the years 2007–2008 (Gulya and Markell 2009). Any combination of these genes with R 11 would give resistance to a majority of rust races and provide protection against the spread of new rust pathotypes. The fact that rust resistance is conferred by single genes will also facilitate pyramiding them together with other R-genes. For example, Pl 8 /R adv + R 11 would give resistance to all NA downy mildew races and the most virulent rust races. The molecular markers closely linked to the different resistance genes should make this task more feasible, and will allow breeders to effectively select disease-resistant progeny in early segregating generations.
References
Abratti G, Bazzalo ME, León A (2008) Mapping a novel fertility restoration gene in sunflower. In: Proceedings of the 17th international sunflower conference, Córdoba, 8–12 June 2008, pp 617–621
Bachlava E, Radwan OE, Abratti G, Tang S, Gao W, Heesacker AF, Bazzalo ME, Zambelli A, Leon AJ, Knapp SJ (2011) Downy mildew (Pl 8 and Pl 14 ) and rust (R Adv ) resistance genes reside in close proximity to tandemly duplicated clusters of non-TIR-like NBS-LRR-encoding genes on sunflower chromosomes 1 and 13. Theor Appl Genet 122:1211–1221
Berry ST, Leon AJ, Peerbolte R, Challis C, Livini C, Jones R, Feingold S (1997) Presentation of the Advanta sunflower RFLP linkage map for public research. In: Proceedings of the 19th sunflower research workshop, Fargo, 9–10 Jan 1997, pp 113–118
Bert P, Tourvieille de Labrouhe D, Philippon J, Mouzeyar S, Jouan I, Nicolas P, Vear F (2001) Identification of a second linkage group carrying genes controlling resistance to downy mildew (Plasmopara halstedii) in sunflower (Helianthus annuus L.). Theor Appl Genet 103:992–997
Burke JM, Tang S, Knapp SJ, Rieseberg LH (2002) Genetic analysis of sunflower domestication. Genetics 161:1257–1267
Burke JM, Lai Z, Salmaso M, Nakazato T, Tang S, Heesacker A, Knapp SJ, Rieseberg LH (2004) Comparative mapping and rapid karyotypic evolution in the genus Helianthus. Genetics 167:449–457
Chandler JM, Jan CC, Beard BH (1986) Chromosomal differentiation among the annual Helianthus species. Syst Bot 11:354–371
de Romano AB, Vázquez AN (2003) Origin of the Argentine sunflower varieties. Helia 26:127–136
Ellis JG, Lawrence G, Ayliffe M, Anderson P, Collins N et al (1997) Advances in the molecular genetic analysis of the flax–flax rust interaction. Annu Rev Phytopathol 35:271–291
Feng J, Jan CC (2008) Introgression and molecular tagging of Rf4, a new male fertility restoration gene from wild sunflower Helianthus maximiliani L. Theor Appl Genet 117:241–249
Gentzbittel L, Vear F, Zhang YX, Berville A (1995) Development of a consensus linkage RFLP map of cultivated sunflower (Helianthus annuus L.). Theor Appl Genet 90:1079–1086
Gentzbittel L, Mestries E, Mouzeyar S, Mazeyrat F, Badaoui S, Vear F, Tourvieille De Labrouhe D, Nicolas P (1999) A composite map of expressed sequences and phenotypic traits of the sunflower (Helianthus annuus L.) genome. Theor Appl Genet 99:218–234
Goulter KC (1990) Breeding of a rust differential sunflower line. In: Proceedings of the 8th Australian sunflower association workshop, Toowoomba, 19–22 Mar 1990, pp 120–124
Gulya TJ (1985) Registration of five disease-resistant sunflower germplasms. Crop Sci 25:719–720
Gulya TJ (2006) The sunflower rust situation: current races in the northern and central Great Plains, and resistance in oilseed and confection hybrids. In: Proceedings of the 28th sunflower research workshop, Fargo, 11–12 Jan 2006. http://www.sunflowernsa.com/research/research-workshop/documents/Gulya_Rust_06.pdf
Gulya TJ, Markell S (2009) Sunflower rust status—2008. Race frequency across the midwest and resistance among commercial hybrids. http://www.sunflowernsa.com/uploads/Gulya_RustStatus_09.pdf
Gulya TJ, Masirevic S (1996) Inoculation and evaluation methods for sunflower rust. In: Proceedings of the 18th sunflower research workshop, Fargo, 11–12 Jan 1996, pp 31–38
Gulya TJ, Viranyi F (1994) Virulent new races of sunflower rust (Puccinia helianthi) from the southern Great Plains. In: Proceedings of the 16th sunflower research workshop, Fargo, 13–14 Jan 1994, pp 94–98
Gulya TJ, Venette R, Venette JR, Lamey HA (1990) Sunflower rust. NDSU Ext. Ser. Bull., Fargo, p 998. http://www.ag.ndsu.edu/pubs/plantsci/rowcrops/pp998w.htm
Heiser CB Jr (1954) Variation and subspeciation in the common sunflower, Helianthus annuus. Am Midl Nat 51:287–305
Horn R, Kusterer B, Lazarescu E, Prufe M, Friedt W (2003) Molecular mapping of the Rf1 gene restoring pollen fertility in PET1-based F1 hybrids in sunflower (Helianthus annuus L.). Theor Appl Genet 106:599–606
Horne EC, Kumpatla SE, Patterson KA, Gupta M, Thompson SA (2004) Improved high-throughput sunflower and cotton genomic DNA extraction and PCR fidelity. Plant Mol Biol Rep 22:83–84
Hulbert SH, Webb CA, Smith SM, Sun Q (2001) Resistance gene complexes: evolution and utilization. Ann Rev Phytopathol 39:285–312
Islam MR, Shepherd KW (1991) Present status of genetics of rust resistance in flax. Euphytica 55:255–267
Jan CC, Vick BA (2007) Inheritance and allelic relationships of fertility restoration genes for seven new sources of male-sterile cytoplasm in sunflower. Plant Breed 126:213–217
Jones DA, Dickinson MJ, Balint-Kurti PJ, Dixon MS, Jones JDG (1993) Two complex resistance loci revealed in tomato by classical and RFLP mapping of the Cf-2, Cf-4, Cf-5, and Cf-9 genes for resistance to Cladosporium fulvum. Mol Plant Microbe Int 6:348–357
Kinman ML (1970) New development in the USDA and state experiment station sunflower breeding programs. In: Proceedings of the 4th international sunflower conference, Memphis, 23–25 June 1970, pp 181–183
Korell M, Mösges G, Friedt W (1992) Construction of a sunflower pedigree map. Helia 15:7–16
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Kusterer B, Horn R, Friedt W (2005) Molecular mapping of the fertility restoration locus Rf1 in sunflower and development of diagnostic markers for the restorer gene. Euphytica 143:35–42
Lambrides CJ, Miller JF (1994) Inheritance of rust resistance in a source of MC29 sunflower germplasm. Crop Sci 34:1225–1230
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic maps of experimental and natural population. Genomics 1:174–181
Lawson WR, Goulter KC, Henry RJ, Kong GA, Kochman JK (1998) Marker assisted selection for two rust resistance genes in sunflower. Mol Breed 4:227–234
Lawson WR, Jan CC, Shatte T, Smith L, Kong GA, Kochman JK (2011) DNA markers linked to the R 2 rust resistance gene in sunflower (Helianthus annuus L.) facilitate anticipatory breeding for this disease variant. Mol Breed 28:569–576. doi:10.1007/s11032-010-9506-1
Leclercq P (1969) Une stérilité male cytoplasmique chez letournesol. Ann Amélior Plant 19:99–106
Liu Z, Mulpuri S, Feng J, Vick BA, Jan CC (2011) Molecular mapping of the Rf3 fertility restoration gene to facilitate its utilization in breeding confection sunflower. Mol Breed. doi:10.1007/s11032-011-9563-0
Mazeyrat F, Mouzeyar S, Nicolas P, Tourvieille de Labrouhe D, Ledoigt G (1998) Cloning, sequence and characterization of a sunflower (Helianthus annuus L.) pathogen-induced gene showing sequence homology with auxin-induced genes from plants. Plant Mol Biol 38:899–903
Miah MAJ, Sackston WE (1970) Genetics of host-pathogen interaction in sunflower. Phytoprotection 51:1–16
Michelmore RW, Meyers BC (1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res 8:1113–1130
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Miller JF, Gulya TJ (1988) Registration of six downy mildew resistant sunflower germplasm lines. Crop Sci 28:1040–1041
Miller JF, Gulya TJ (2001) Registration of three rust resistant sunflower germplasm populations. Crop Sci 41:601
Miller JF, Rodriguez RH, Gulya TJ (1988) Evaluation of genetic materials for inheritance of resistance to race 4 rust in sunflower. In: Proceedings of the 12th international sunflower conference, Novi Sad, 25–29 July 1988, pp 361–365
Putt ED, Sackston WE (1963) Studies on sunflower rust. IV. Two genes, R 1 and R 2 for resistance in the host. Can J Plant Sci 43:490–496
Qi LL, Gulya TJ, Seiler GJ, Hulke BS, Vick BA (2011a) Identification of resistance to new virulent races of rust in sunflowers and validation of DNA markers in the gene pool. Phytopathol 101:241–249
Qi LL, Hulke BS, Vick BA, Gulya TJ (2011b) Molecular mapping of the rust resistance gene R 4 to a large NBS-LRR cluster on linkage group 13 of sunflower. Theor Appl Genet 123:351–358. doi:10.1007/s00122-011-1588-6
Qi LL, Gulya TJ, Hulke BS, Vick BA (2011c) Chromosome location, DNA markers and rust resistance of the sunflower gene R 5 . Mol Breed. doi:10.1007/s11032-011-9659-6
Radwan O (2010) Isolation and expression of an NBS-LRR protein-encoding resistance gene candidate that segregates with a rust resistance gene in sunflower. J Phytopathol 158:433–443
Radwan O, Bouzidi MF, Vear F, Philippon J, de Labrouhe DT, Nicolas P, Mouzeyar S (2003) Identification of non-TIR-NBS-LRR markers linked to Pl5/Pl8 locus for resistance to downy mildew in sunflower. Theor Appl Genet 106:1438–1446
Radwan O, Gandhi S, Heesacker A, Whitaker B, Taylor C, Plocik A, Kesseli R, Kozik A, Michelmore RW, Knapp SJ (2008) Genetic diversity and genomic distribution of homologs encoding NBS-LRR disease resistance proteins in sunflower. Mol Genet Genomics 280:111–125
Rashid KY (2006) Sunflower rust races in Manitoba. http://www.umanitoba.ca/afs/agronomists_conf/proceedings/2006/Rashid_sunflower_rust_races.pdf
Richter TE, Ronald PC (2000) The evolution of disease resistance genes. Plant Mol Biol 42:195–204
Salmeron JM, Oldroyd GE, Rommens CM, Scofield SR, Kim HS et al (1996) Tomato Prf is a member of the leucine-rich repeat class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster. Cell 86:123–133
Saxena KMS, Hooker AL (1968) On the structure of a gene for disease resistance in maize. Proc Natl Acad Sci USA 61:1300–1305
Saxena KMS, Hooker AL (1974) A study on the structure of gene Rp3 for rust resistance in Zea mays. Can J Genet Cytol 16:857–860
Seiler GJ, Jan CC (1994) New fertility restoration genes from wild sunflowers for sunflower PET1 male-sterile cytoplasm. Crop Sci 34:1526–1528
Seiler GJ, Jan CC (1997) Registration of 10 interspecific germplasm fertility restoration populations for sunflower PET1 male-sterile cytoplasm. Crop Sci 37:1989–1991
Sendall BC, Kong GA, Goulter KC, Aitken EAB, Thompson SM, Mitchell JHM, Kochman JK, Lawson W, Shatte T, Gulya TJ (2006) Diversity in the sunflower: Puccinia helianthi pathosystem in Australia. Australas Plant Pathol 35:657–670
Slabaugh MB, Yu JK, Tang SX, Heesacker A, Hu X, Lu GH, Bidney D, Han F, Knapp SJ (2003) Haplotyping and mapping a large cluster of downy mildew resistance gene candidates in sunflower using multilocus intron fragment length polymorphisms. Plant Biotech J 1:167–185
Song WY, Pi LY, Wang GL, Gardner J, Holsten T et al (1997) Evolution of the rice Xa21 disease resistance gene family. Plant Cell 9:1279–1287
Tang S, Yu JK, Slabaugh MB, Shintani DK, Knapp SJ (2002) Simple sequence repeat map of the sunflower genome. Theor Appl Genet 105:1124–1136
Tang S, Kishore VK, Knapp SJ (2003) PCR-multiplexes for a genome-wide framework of simple sequence repeat marker loci in cultivated sunflower. Theor Appl Genet 107:6–19
Wei F, Gobelman-Werner K, Morroll SM, Kurth J, Mao L, Wing R, Leister D, Schulze-Lefert P, Wise RP (1999) The Mla (powdery mildew) resistance cluster is associated with three NBS-LRR gene families and suppressed recombination within a 240-kb DNA interval on chromosome 5S (1HS) of barley. Genetics 153:1929–1948
Yang SM, Antonelli EF, Luciano A, Luciani ND (1986) Reactions of Argentine and Australian sunflower rust differentials to four North American cultures of Puccinia helianthi from North Dakota. Plant Dis 70:883–886
Yang SM, Dowler WM, Luciano A (1989) Gene Pu 6 : a new gene in sunflower for resistance to Puccinia helianthi. Phytopathology 79:474–477
Yu JK, Tang S, Slabaugh MB, Heesacker A, Cole G et al (2003) Towards a saturated molecular genetic linkage map for cultivated sunflower. Crop Sci 43:367–387
Yue B, Vick BA, Cai X, Hu J (2010) Genetic mapping for the Rf1 (fertility restoration) gene in sunflower (Helianthus annuus L.) by SSR and TRAP markers. Plant Breed 129:24–28
Acknowledgments
We thank Drs. Xiwen Cai and Zengcui Zhang for critical review of the manuscript, and Angelia Hogness and Hannah Worral for technical assistance. This project was supported by the USDA-ARS CRIS Project No. 5442-21000-034-00D.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Bervillé.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Rights and permissions
About this article
Cite this article
Qi, L.L., Seiler, G.J., Vick, B.A. et al. Genetics and mapping of the R 11 gene conferring resistance to recently emerged rust races, tightly linked to male fertility restoration, in sunflower (Helianthus annuus L.). Theor Appl Genet 125, 921–932 (2012). https://doi.org/10.1007/s00122-012-1883-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-012-1883-x