Abstract
Rust is a serious fungal disease in the sunflower growing areas worldwide with increasing importance in North America in recent years. Several genes conferring resistance to rust have been identified in sunflower, but few of them have been genetically mapped and linked to molecular markers. The rust resistance gene R 4 in the germplasm line HA-R3 was derived from an Argentinean open-pollinated variety and is still one of most effective genes. The objectives of this study were to determine the chromosome location of the R 4 gene and the allelic relationship of R 4 with the R adv rust resistance gene. A total of 63 DNA markers previously mapped to linkage group (LG) 13 were used to screen for polymorphisms between two parental lines HA 89 and HA-R3. A genetic map of LG 13 was constructed with 21 markers, resulting in a total map length of 93.8 cM and an average distance of 4.5 cM between markers. Two markers, ZVG61 and ORS581, flanked the R 4 gene at 2.1 and 0.8 cM, respectively, and were located on the lower end of LG 13 within a large NBS-LRR cluster identified previously. The PCR pattern generated by primer pair ZVG61 was unique in the HA-R3 line, compared to lines HA-R1, HA-R4, and HA-R5, which carry other R 4 alleles. A SCAR marker linked to the rust resistance gene R adv mapped to LG 13 at 13.9 cM from the R 4 locus, indicating that R adv is not an allele of the R 4 locus. The markers tightly linked to the R 4 gene will facilitate gene pyramiding for rust resistance breeding of sunflower.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rust, caused by the fungus Puccinia helianthi Schwein., is one of the most serious diseases of sunflower (Helianthus annuus L.) worldwide. The rapid changes that occur in the virulence characteristics of populations raise a continuous threat to the effectiveness of existing rust-resistant inbred lines and hybrids (Gulya and Markell 2009; Qi et al. 2011). Hence, there is an urgent need for strategies to develop inbred lines with durable resistance to the disease. The concept of combining resistance genes, i.e. incorporating multiple resistance genes (R-genes) into a single cultivar, to achieve greater durability is referred to as ‘gene pyramiding’ or ‘gene stacking’. This pyramiding approach is envisaged to make it more difficult for the pathogen to overcome these multiple resistances as, presumably, mutations in multiple pathogen genes will be required. However, the selection of genotypes with gene combinations is difficult by conventional methods. Mapping rust resistance genes and developing robust molecular markers will facilitate this breeding approach and add precision to selection.
Several genes conferring resistance to rust have been identified in sunflower including R 1 , R 2 , R 3 , R 4 , R 5 , P u6 , and R adv (Putt and Sackston 1963; Miah and Sackston 1970; Miller et al. 1988; Yang et al. 1989; Goulter 1990; Lawson et al. 1998). In addition to the already named rust resistance genes, several inbred lines and interspecific germplasm lines were reported to have resistance to different rust races of sunflower, especially to races 336, the predominant races in North America, and 777, the most virulent race in North America. These lines can be used as new rust resistance gene sources (Quresh et al. 1993; Gulya et al. 2000; Jan et al. 2004; Jan and Gulya 2006; Hulke et al. 2010; Qi et al. 2011). Lawson et al. (1998) developed two sequence characterized amplified region (SCAR) markers that co-segregated with rust resistance genes R 1 and R adv , respectively. Yu et al. (2003) mapped SCAR markers SCT06950 associated with the R 1 gene and SCX20600 associated with the R adv gene on the linkage groups (LGs) 8 and 13, respectively. However, Lawson et al. (2010) reported the mapping of SCT06950 on LG9, 17 cM away from the R 2 rust resistance gene locus. The R 1 gene is no longer effective against the predominant rust races and the R adv gene is present only in the proprietary line P2 developed by Pioneer Seeds (Pioneer Hi-Bred Australia) and is unavailable for use in public breeding programs (Lawson et al. 1998; Qi et al. 2011).
Rust resistance gene R 4 in the germplasm line HA-R3 was derived from the Argentinean open-pollinated variety ‘Charata INTA’ (Gulya 1985). The origin of its rust resistance gene can be traced to wild annual Helianthus species. Charata INTA was selected from the cross between Russian lines and wild sunflower species, including H. annuus, H. argophyllus, and H. petiolaris (de Romano and Vazquez 2003). Miller et al. (1988) reported that rust resistance genes in three germplasm lines, HA-R1, HA-R4, and HA-R5, which were also selected from Argentinean open-pollinated varieties (Gulya 1985), were allelic to the R 4 locus. These R genes express resistance to different sunflower rust isolates; therefore, each encodes different resistance specificity. The line HA-R3 confers resistance to 88% of 300 rust isolates tested in the US in the years 2007 and 2008, but it is moderately susceptible to the new virulent race 777 (Rashid 2006; Gulya and Markell 2009; Qi et al. 2011). It is possible to combine this gene with other resistance genes to attain a wide spectrum of resistance using molecular marker-assisted selection.
Many of the plant disease resistance genes that have been cloned encode proteins with a putative nucleotide binding site and leucine-rich repeats (NBS-LRR resistance genes) (Bent et al. 1994; Hammond-Kosack and Jones 1997; Hulbert et al. 2001; Huang et al. 2003; Yahiaoui et al. 2004). Previous research has identified 149 and 480 NBS-LRR genes in Arabidopsis and rice genomes, respectively (Meyers et al. 2003; Zhou et al. 2004; Yang et al. 2007). Radwan et al. (2008) identified 630 NBS-LRR homologs in the sunflower genome and mapped 167 NBS-LRR loci throughout the sunflower genome in 44 clusters or single locus. Of 44 clusters, the LG 8 cluster is the largest with 54 NBS-LRR loci in three subclusters. The second largest NBS-LRR cluster was found on LG 13 with 27 NBS-LRR loci distributed on the lower end of LG 13 which harbors downy mildew (Pl 5 and Pl 8 ) and rust (R adv ) resistance genes (Lawson et al. 1998; Radwan et al. 2003, 2008; Slabaugh et al. 2003; Yu et al. 2003). Sendall et al. (2006) also reported that the R 4 gene was located on LG 13, but no molecular marker associated with the R 4 gene and its position were reported. In the study presented here, we mapped the R 4 gene to a large NBS-LRR cluster on LG 13 of sunflower. The R 4 locus was located in the interval of 0.8–2.9 cM delimited by the INDEL marker ZVG61 and SSR marker ORS581.
Materials and methods
Plant materials
The mapping population used in this study was derived from a cross of HA 89 with HA-R3. HA 89 is a sunflower inbred line used as a susceptible parent and HA-R3 served as a resistant parent that carries the rust resistance gene R 4 (Miller et al. 1988). The population consisted of 120 F2 plants derived from a single F1 plant. A progeny test of 118 F2:3 families was performed in order to confirm the phenotype and assign the genotype of the F2 plants. Three germplasm lines, HA-R1, HA-R4, and HA-R5 previously reported as carrying the R 4 alleles (Miller et al. 1988), were chosen to identify allele-specific microsatellite patterns.
Plant growth and inoculations
Seeds were planted in 36-cell plastic flats (each cell 4.6 cm × 5.4 cm) filled with Sunshine SB 100B potting mixture (SunGro Horticulture, Bellevue, WA). The greenhouse was maintained at 24°C day/20°C night with a 16-h photoperiod and sodium vapor lighting. Plants were fertilized weekly with a water soluble 15-16-17 analysis fertilizer and sprayed weekly to runoff with B-Nine (daminozide; Chemtura USA, Middlebury, CT) growth regulator at 0.5% w/v to maintain compact growth. For phenotypic analysis of rust resistance, a total of 120 F2 plants and 118 F2:3 families (20 plants from each family, a total of 2,360 F3 individuals), along with two parental lines and F1 plants, were inoculated with P. helianthi spores of race 336 at the four-leaf stage (about 3 weeks after planting) using the procedure described by Gulya and Masirevic (1996). Race 336 was collected originally from cultivated plants in North Dakota in 2009, and is the predominant race in North America, and was increased from a single pustule. Urediniospores were collected from greenhouse-grown Mycogen hybrid 7350 plants with the aid of a cyclone collector (Trevet et al. 1951) and stored at 4°C or liquid nitrogen until needed. Spores were suspended in SOLTROL 170 isoparaffin (Chevron Phillips Chemical Co., The Woodlands, TX) at 5–10 mg spores/10 ml which was equivalent to 1.5–3.0 × 106 spores/ml. The spore suspension was atomized onto all leaf surfaces with compressed air. After allowing the SOLTROL 170 to evaporate for 15 min, the plants were incubated in sealed chambers equipped with automated ultrasonic humidifiers to provide continuous leaf wetness within a room maintained at 18–20°C in the dark for 16–24 h. Plants were then returned to the greenhouse and maintained under the conditions mentioned above.
Rust evaluation
Rust pustules started to appear in 7–10 days, and evaluations were made at 12–14 days post inoculation to allow full development of symptoms. Rust evaluations were made using both pustule size or infection type (IT) and percentage of leaf area covered with pustules (severity) on all inoculated leaves, as cited in previous papers (Qi et al. 2011). A modified Sackston’s numerical rating system (1962) described by Yang et al. (1986) was used to categorize infection type. Infection categories were as follows: 0 = immune, no uredia and no hypersensitive flecks, 1 = highly resistant, presence of hypersensitive flecks or lesions, or pustules smaller than 0.2 mm in diameter with or without chlorotic haloes; 2 = resistant, pustules smaller than 0.4 mm; 3 = susceptible, pustules 0.4–0.6 mm in diameter; 4 = highly susceptible, pustules larger than 0.6 mm. Reactions 0, 1, and 2 were classified as resistant, while reactions 3 and 4 were rated as susceptible. This categorization is similar to that observed for cereal rust (Stakman et al. 1962), but the sunflower/Puccinia helianthi pathosystem does have different symptomotology. Pustule coverage was visually assessed using the computer generated diagrams of Gulya et al. (1990), showing pustule coverage from 0.1 to 40%. Pustule coverage of 0–0.5% was classified as resistant, along with IT of 0–2. Susceptible parent HA 89 and susceptible plants in F2 and F3 populations always had IT 3 or 4 pustules with 10–20% or more pustule coverage, whereas resistant plants gave IT 1 or 2 pustules with 0.1–0.5% pustule coverage.
DNA marker analysis
Total DNA was extracted from seedlings of parental lines and individual F2 plants using the Qiagen DNeasy 96 plant kit with a modified protocol described by Horne et al. (2004) and DNA concentration was quantified on a NanoDrop 2000 Spectrophotometer (Qiagen, Valencia, CA; Thermo Fisher Scientific, Wilmington, DE). Sendall et al. (2006) reported that the rust resistance gene R 4 was located on LG 13 in sunflower. A total of 59 simple sequence repeat (SSR) markers, three INDEL markers, and one SCAR marker, SCX20600, that were previously mapped to LG 13 were used to screen HA 89 and HA-R3 (Lawson et al. 1998; Burke et al. 2002; Tang et al. 2002, 2003; Yu et al. 2003). Polymerase chain reaction (PCR) in a 15-μl volume contained 2 mM MgCl2, 200–250 μM of each dNTP, 0.02–0.06 μM forward primer with an M13 tail (CACGACGTTGTAAAACGAC) at the 5′ end, 0.1–0.3 μM reverse primer, 0.1–0.3 μM fluorescently labeled M13 primer, 1× PCR buffer, 0.5 units Taq polymerase (Bioline, Randolph, MA, USA), and 10–20 ng of genomic DNA. The PCR reactions were performed in a Peltier thermocycler (Bio-Rad Lab, Hercules, CA, USA) with a touchdown program described by Qi et al. (2011). PCR products were diluted 10- to 40-fold before analysis. SSR fragments were size separated by using an IR2 4300 DNA Analyzer (Li-COR, Lincoln, Nebraska).
The PCR conditions for SCAR marker SCX20 were previously described in Lawson et al. (1998). A multiplex PCR procedure was applied to the SSR primer ORS581, in which the SSR primer CRT504 was used as an internal control for the reactions. A 25-μl PCR mixture contained 1.25 μM of each primer of ORS581 and CRT504, 2.5 mM MgCl2, 250 μM of each dNTP, 1× PCR buffer, 1 unit Taq polymerase (Bioline, Randolph, MA, USA), and 20 ng of DNA template. The reaction was incubated at 94°C for 2 min, followed by 35 cycles of 1 min at 94°C, 1 min at 63°C, and 1 min at 72°C with a final extension at 72°C for 20 min. The PCR products were separated in 2.0% agarose gels and visualized under UV light.
Genetic mapping of the R 4 gene
The genetic linkage map of LG 13 with the R 4 gene was constructed using the population of 118 F2 plants derived from the cross HA 89 with HA-R3. This population segregating for the R 4 resistance locus was used to estimate genetic linkage between the resistance locus and closely linked DNA markers. The mapping data were analyzed with the computer program Mapmaker V2.0 for Macintosh (Lander et al. 1987) using default parameters of LOD = 3.0 and the Kosambi mapping function (Kosambi 1944). Goodness-of-fit to a 1:2:1 segregation ratio of F2 genotypes for rust reaction from the F3 families was tested by means of a chi-square analysis.
Results
Reaction of parents, F2 population, and F2:3 progenies to rust infection
The segregating F2 population was inoculated with the P. helianthi spores of race 336, which is avirulent on plants containing the gene R 4 . The rust resistance genotype of each F2 plant was determined by testing their F3 progenies. HA 89 showed a fully susceptible reaction with infection type 4 and 10–20% of the leaves covered with pustules, whereas HA-R3 was highly resistant to rust race 336 with necrotic or small pustules (IT 1) and 0–0.1% of the leaves covered with pustules. Heterozygous F1 plants gave an intermediate infection type (IT 2) and 0.1–0.5% of the leaves covered with pustules. Therefore, rust symptoms in resistant plants were clearly distinct from those in susceptible plants. Resistant and susceptible plants segregated in the F2 population in a 3:1 ratio (94 resistant and 26 susceptible). Of the 118 F2:3 families selected for molecular mapping, 31 were homozygous resistant (RR), 62 were heterozygous (Rr) segregating for resistance and susceptibility, and 25 were homozygous susceptible (rr) (Fig. 2b). The scoring results fit the expected 1:2:1 ratio of F2 genotypes (χ 2 = 0.9153, df = 2, 0.75 > P > 0.50), indicating that a single dominant gene in the HA-R3 line controls the rust resistance.
Molecular mapping of the rust resistance gene R 4
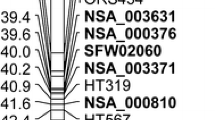
A total of 59 SSR markers, three INDELs, and one SCAR marker that previously mapped to LG 13 were selected (Lawson et al. 1998; Burke et al. 2002; Tang et al. 2002, 2003; Yu et al. 2003). Of those, 52 markers were mapped only to LG 13, 10 to two LGs, and one to three LGs. Twenty-five SSR markers (40%) were polymorphic between HA 89 and HA-R3. Among the 25 polymorphic markers, 21 were mapped to LG 13 in the present study. The genetic map of LG 13 consisted of 21 markers spanning a total of 93.8 cM for an average distance of 4.5 cM between markers. Similar to the previous LG 13 linkage map, the SSR markers were concentrated on the upper and lower ends of LG 13 with a sizable gap between marker ZVG59 and ORS317 (Fig. 1a, Tang et al. 2002, 2003). Fifteen markers concentrated on the lower end of LG13 spanned 21.4 cM in genetic length, averaging a distance of 1.4 cM between markers (Fig. 1a).
Genetic linkage map of sunflower linkage group (LG) 13. a LG 13 genetic map, showing the position of the R 4 locus and SCX20 marker. b A dense public genetic linkage map of LG 13. c LG 13 genetic map, showing the position of the SCX20 marker. d LG 13 genetic map, showing the position of 27 NBS-LRR loci on the lower end of LG 13. SSR marker ORS581 was located on the NBS-LRR cluster. Common SSR markers were aligned between a and b
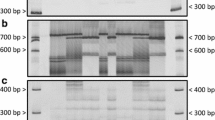
The R 4 gene showed linkage with the INDEL marker ZVG61 and the SSR marker ORS581. ZVG61 is a codominant marker that mapped 2.1 cM distal to the R 4 gene (Fig. 1a). A polymorphic fragment in HA-R3 that was amplified by ZVG61 primers was 336 bp in length compared to a 220-bp fragment in HA 89 (Fig. 2a). The SSR marker ORS316 co-segregated with ZVG61, providing another codominant marker for the R 4 gene. ORS581 is a dominant-repulsion marker that mapped 0.8 cM proximal to the R 4 gene. A 308-bp fragment amplified with ORS581 primers was present in HA 89, but absent in HA-R3 (Fig. 2c). In a multiplex PCR using SSR marker CRT504 as an internal control in the F2 population, each sample displayed the presence of the CRT504 band demonstrating that the DNA was suitable for amplification and ruling out any possible false negative of the ORS581 marker due to PCR failure (Fig. 2c).
PCR patterns of markers ZVG61 (a) and ORS581 plus CRT504 (c) and R 4 gene phenotype (rust score) and R 4 genotype (b) in an F2 segregating population (showing 46 of 118 F2 plants from lane 2 to 48). SSR primer CRT504 was used in the multiplex PCR reaction with ORS581 as an internal control to rule out the possibility that the missing ORS581 fragment is caused by PCR error. PCR pattern of ORS581 primers for the heterozygous F2 plants is the same as that of HA 89. F2 genotypes for the R 4 gene were determined by F2:3 progeny test. The symbol A represents homozygous HA 89 (genotype AA); B homozygous HA-R3 (genotype BB); H heterozygous (genotype AB); D either homozygous HA 89 (AA) or heterozygous (AB). S susceptible, R resistant. The bold capital indicates the recombination between ZVG61 and the R 4 gene. The PCR fragment size amplified by ZVG61, ORS581, and CRT504 includes a 19-bp M13 tail primer. The molecular weight marker is a 1-kb plus DNA ladder (Invitrogen, Carlsbad, CA, USA). The PCR products were separated in 2.0% agarose gels
The SCAR marker SCX20600 was previously reported to co-segregate with the rust resistance gene R adv , which is present in the proprietary line P2 and was mapped to LG 13 (Lawson et al. 1998; Yu et al. 2003). We tested this marker on HA 89 and HA-R3. The SCX20600 primers amplified a 600-bp fragment that was present in HA-R3, but absent in HA 89 (Fig. 3c). The screening of the SCX20600 marker in the F2 population from the cross of HA 89 with HA-R3 indicated that this marker is approximately 13.9 cM from the R 4 gene locus (Fig. 1a).
PCR patterns of markers ZVG61 (a) and ORS581 plus CRT504 (b), and SCX20600 (c) in the lines HA 89, HA-R1, HA-R3, HA-R4, and HA-R5. ZVG61 shows a unique PCR pattern in HA-R3 different from that in HA-R1, HA-R4, and HA-R5, whereas HA-R1, HA-R4, and HA-R5 share the same PCR pattern as HA-R3 with ORS581, missing a 308-bp fragment. In the multiplex PCR reaction with ORS581, a fragment (~154 bp) amplified by CRT504 primers was present in all lines. SCX20600 only amplified a ~600-bp fragment in HA-R3. The PCR fragment size amplified by ZVG61, ORS581, and CRT504 includes a 19-bp M13 tail primer. The PCR products were separated in 2.0% agarose gels
The two molecular markers linked to the R 4 gene, ZVG61 and ORS581, were tested in three germplasm lines, HA-R1, HA-R4, and HA-R5 that possess resistance genes which are allelic to R 4 (Miller et al. 1988). The three lines show the same PCR pattern as HA-R3 with ORS581 primers, i.e. they lack the 308-bp fragment which is present in HA 89. In contrast, they share the same PCR pattern as HA 89 with primers of ZVG61 (Fig. 3).
Discussion
The rust resistance in germplasm line HA-R3 was thought to be controlled by a single dominant gene by means of classic genetic analysis of F2 and BC1F1 progenies derived from the cross of the susceptible line S37388 with HA-R3 (Miller et al. 1988). The rust test results of F2:3 families segregating in an approximate ratio of 1:2:1 in the present study supported the hypothesis of a single dominant gene. This gene was mapped to LG 13 within a large NBS-LRR cluster, which is the first step for further research toward cloning the resistance gene through the map-based cloning method. The tightly linked markers will facilitate efficient resistance breeding in sunflower.
PCR-based SSR markers currently are among the most widely used marker systems and often the best choice because of their high information content, ease of genotyping, and codominant nature. Codominant markers are preferable to dominant markers due to the larger information content. A codominant molecular marker allows unequivocal distinction of homozygous and heterozygous genotypes on an electrophoresis gel. It is particularly useful for marker-assisted selection in breeding programs. However, a high proportion of dominant SSR markers was found in the present study. Of 21 polymorphic markers (18 SSRs, two INDELs, and one SCAR marker) mapped to LG 13, nine SSR markers (50%), ORS534, ORS418, HT1037, HT568, ORS511, ORS630, ORS191, ORS581, and ORS464, showed dominant nature. In these cases SSRs were analyzed on the basis of the presence or absence of a band on an electrophoresis gel. As a result, dominant homozygous individuals are not distinguished from heterozygous individuals, resulting in a considerable loss of information. There are two phases of dominant markers based on the amplified fragment presence or absence in a target chromosome. In a dominant-coupling phase, a fragment amplified from a given pair of primers is present in the target chromosome, whereas in a dominant-repulsion phase there is an opposite situation. In the present study, two markers, ZVG61 and ORS581, flanked the rust resistance gene R 4 within a 2.9-cM interval. As a dominant-repulsion marker, ORS581 can only detect plants homozygous for the R 4 gene as revealed by the lack of a PCR product, whereas heterozygous plants show the same PCR pattern as the susceptible parent (Fig. 2c). Combining the use of the codominant marker ZVG61 will allow an efficient selection in the disease resistance breeding in sunflower.
Clusters of genes conferring resistance to plant diseases in the host chromosomes have been identified in diverse plant species (Islam and Shepherd 1991; Jones et al. 1993; Song et al. 1997; Salmeron et al. 1996; Ellis et al. 1997; Meyers et al. 1998; Michelmore and Meyers 1998; Richter and Ronald 2000; Wei et al. 2002; Huang et al. 2003). Genes within a cluster can be allelic or closely linked as, for example, the flax rust resistance genes at the L and M loci. At the flax L locus, a single gene has 13 alleles encoding different rust resistance specificities, whereas a series of tightly linked genes with related coding sequences separated by unique regions were found at the M locus (Islam and Mayo 1990; Anderson et al. 1997; Ellis et al. 1997, 2007). The R 4 alleles have been characterized in sunflower germplasm lines HA-R1, HA-R3, HA-R4, and HA-R5 by means of classical allelic tests (Miller et al. 1988). HA-R1, HA-R3 and HA-R4 all have similar origins but differ in their reaction to rust. All were derived from an Argentinean interspecific pool with Russian open-pollinated varieties crossed with H. annuus, H. argophyllus, and H. petiolaris (Gulya 1985; de Romano and Vazquez 2003). HA-R4 (also referred to AMES 18925) was also found to be highly resistant to a Spanish isolate of P. helianthi (CO97) (Prats et al. 2007). HA-R5 was derived from a selection of the cultivar Guayacan INTA, whose rust resistance can be traced to the undomesticated form of H. annuus (de Romano and Vazquez 2003). When these lines were tested with markers ZVG61 and ORS581 linked to the R 4 gene, the same amplification pattern was found for the four lines with primer pair ORS581, all of which were absent of a 308-bp fragment. However, the amplification pattern with primer pair ZVG61 was unique in HA-R3, distinguishing the R 4 allele in HA-R3 from those in HA-R1, HA-R4, and HA-R5 (Fig. 3). Such a marker is of interest because the R 4 allele in HA-R3 is the most effective one in North America.
The SCAR marker SCX20600 is linked to the rust resistance gene R adv in the proprietary line P2 (Lawson et al. 1998). Yu et al. (2003) mapped SCX20600 to LG 13 closely linked to SSR marker ORS191 using a RIL population derived from the cross of two proprietary inbred lines PHA and PHB developed by Pioneer Hi-Bred International Inc. (Johnston, Iowa). Sendall et al. (2006) reported that the R adv gene is an allele to the R 4 locus based on linkage association data, and two additional SSR markers, ORS781 (0.0 cM) and ORS995 (1.3 cM), are linked to this gene. In the present study we mapped SCX20600 distal to ORS995 with a 3.2 cM genetic distance on LG 13 (Fig. 1a). The marker SCX20600 has a genetic distance of 13.9 cM from the R 4 locus, which does not support the conclusion that the R adv gene is an allele of the R 4 locus. The discrepancy between the mapping position of SCX20600 in our map and Yu’s map is probably due to the different segregating population used for mapping. However, the marker order in our map is identical to the RHA 280 × RHA 801 map, a dense public genetic linkage map in sunflower (Fig. 1b, Tang et al. 2003).
Interestingly, both rust resistance genes R 4 and R adv are present within a large NBS-LRR cluster corresponding to disease resistance identified in LG 13 (Radwan et al. 2008). Two RGCs (resistance gene candidates), RGC261-13 and RGC16, flank SSR marker ORS581, the closest marker linked to the R 4 gene, with 1.9 and 2.2 cM of genetic distance, respectively (Fig. 1d). Fine-mapping of the R 4 gene region with different DNA markers, especially newly developed SNP (single-nucleotide polymorphism) markers, is underway. Cloning of the gene(s) will contribute to better understanding the allelic relationships in this complex locus.
References
Anderson PA, Lawrence GJ, Morrish BC, Ayliffe MA, Finnegan EJ, Ellis JG (1997) Inactivation of the flax rust resistance gene M associated with loss of a repeated unit within the leucine-rich repeat coding region. Plant Cell 9:641–651
Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ (1994) RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265:1856–1860
Burke JM, Tang S, Knapp SJ, Rieseberg LH (2002) Genetic analysis of sunflower domestication. Genetics 161:1257–1267
de Romano AB, Vázquez AN (2003) Origin of the Argentine sunflower varieties. Helia 26:127–136
Ellis JG, Lawrence G, Ayliffe M, Anderson P, Collins N et al (1997) Advances in the molecular genetic analysis of the flax–flax rust interaction. Annu Rev Phytopathol 35:271–291
Ellis JG, Dodds PN, Lawrence GJ (2007) Flax rust resistance gene specificity is based on direct resistance-avirulence protein interactions. Annu Rev Phytopathol 45:289–306
Goulter KC (1990) Breeding of a rust differential sunflower line. In: Kochman JK (ed) Proceedings of the 8th Australian Sunflower Association workshop, 19–22 March 1990. Australian Sunflower Association, Toowoomba, pp 120–124
Gulya TJ (1985) Registration of five disease-resistant sunflower germplasms. Crop Sci 25:719–720
Gulya TJ, Markell S (2009) Sunflower rust status—2008. Race frequency across the midwest and resistance among commercial hybrids. http://www.sunflowernsa.com/uploads/Gulya_RustStatus_09.pdf
Gulya TJ, Masirevic S (1996) Inoculation and evaluation methods for sunflower rust. In: Proceedings of the 18th sunflower research workshop, Fargo, ND, 11–12 January 1996. National Sunflower Association, Bismarck, pp 31–38
Gulya TJ, Venette R, Venette JR, Lamey HA (1990) Sunflower rust. NDSU Ext. Ser. Bull. PP-998 Fargo, ND. http://www.ag.ndsu.edu/pubs/plantsci/rowcrops/pp998w.htm
Gulya TJ, Kong G, Brothers M (2000) Rust resistance in wild Helianthus annuus and variation by geographic origin. In: Proceedings of the 15th international sunflower conference, Toulouse, France, 12–15 June 2000, pp I38–42
Hammond-Kosack KE, Jones JDG (1997) Plant disease resistance genes. Ann Rev Plant Phys Plant Mol Biol 48:575–607
Horne EC, Kumpatla SE, Patterson KA, Gupta M, Thompson SA (2004) Improved high-throughput sunflower and cotton genomic DNA extraction and PCR fidelity. Plant Mol Biol Rep 22:83–84. Biology r 22:83–84
Huang L, Brooks SA, Fellers JP, Gill BS (2003) Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploidy genome of bread wheat. Genetics 164:655–664
Hulbert SH, Webb CA, Smith SM, Sun Q (2001) Resistance gene complexes: evolution and utilization. Ann Rev Phytopathol 39:285–312
Hulke BS, Miller JF, Gulya TJ (2010) Registration of the restorer oilseed sunflower germplasm RHA 464 possessing genes for resistance to downy mildew and sunflower rust. J Plant Regist 4:249–254
Islam MR, Mayo GME (1990) A compendium on host genes in flax conferring resistance to flax rust. Plant Breed 104:89–100
Islam MR, Shepherd KW (1991) Present status of genetics of rust resistance in flax. Euphytica 55:255–267
Jan CC, Gulya TJ (2006) Registration of a sunflower germplasm resistant to rust, downy mildew and virus. Crop Sci 46:1829
Jan CC, Quresh Z, Gulya TJ (2004) Registration of seven rust resistance sunflower germplasms. Crop Sci 44:1887–1888
Jones DA, Dickinson MJ, Balint-Kurti PJ, Dixon MS, Jones JDG (1993) Two complex resistance loci revealed in tomato by classical and RFLP mapping of the Cf-2, Cf-4, Cf-5, and Cf-9 genes for resistance to Cladosporium fulvum. Mol. Plant Microbe Interact 6:348–357
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic maps of experimental and natural population. Genomics 1:174–181
Lawson WR, Goulter KC, Henry RJ, Kong GA, Kochman JK (1998) Marker assisted selection for two rust resistance genes in sunflower. Mol Breed 4:227–234
Lawson WR, Jan CC, Shatte T, Smith L, Kong GA, Kochman JK (2010) DNA markers linked to the R 2 rust resistance gene in sunflower (Helianthus annuus L.) facilitate anticipatory breeding for this disease variant. Mol Breed. doi:10.1007/s11032-010-9506-1
Meyers BC, Chin DB, Shen KA, Sivaramakrishnan S, Lavelle DO et al (1998) The major resistance gene cluster in lettuce is highly duplicated and spans several megabases. Plant Cell 10:1817–1832
Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15:809–834
Miah MAJ, Sackston WE (1970) Genetics of host-pathogen interaction in sunflower. Phytoprotection 51:1–16
Michelmore RW, Meyers BC (1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res 8:1113–1130
Miller JF, Rodriguez RH, Gulya TJ (1988) Evaluation of genetic materials for inheritance of resistance to Race 4 rust in sunflower. In: Proceedings of the 12th international sunflower conference, Novi Sad, Yugoslavia, 25–29 July 1988. International Sunflower Association, Paris, pp 361-365
Prats E, Llamas MJ, Jorrin J, Rubiales D (2007) Constitutive coumarin accumulation on sunflower leaf surface prevents rust germ tube growth and appressorium differentiation. Crop Sci 47:1119–1124
Putt ED, Sackston WE (1963) Studies on sunflower rust. IV. Two genes, R 1 and R 2 for resistance in the host. Can J Plant Sci 43:490–496
Qi LL, Gulya TJ, Seiler GJ, Hulke BS, Vick BA (2011) Identification of resistance to new virulent races of rust in sunflowers and validation of DNA markers in the gene pool. Phytopathology 101:241–249
Quresh Z, Jan CC, Gulya TJ (1993) Resistance to sunflower rust and its inheritance in wild sunflower species. Plant Breed 110:297–306
Radwan O, Bouzidi MF, Vear F, Philippon J, Tourvieille de Labrouhe D, Nicolas P, Mouzeyar S (2003) Identification of non-TIR-NBS-LRR markers linked to the Pl5/Pl8 locus for resistance to downy mildew in sunflower. Theor Appl Genet 106:1438–1446
Radwan O, Gandhi S, Heesacker A, Whitaker B, Taylor C, Plocik A, Kesseli R, Kozik A, Michelmore R, Knapp SJ (2008) Genetic diversity and genomic distribution of homologs encoding NBS-LRR disease resistance proteins in sunflower. Mol Genet Genomics 280:111–125
Rashid KY (2006) Sunflower rust races in Manitoba. http://www.umanitoba.ca/afs/agronomists_conf/proceedings/2006/Rashid_sunflower_rust_races.pdf (verified 03 August 2010)
Richter TE, Ronald PC (2000) The evolution of disease resistance genes. Plant Mol Biol 42:195–204
Sackston WE (1962) Studies on sunflower rust. III. Occurrence, distribution, and significance of race Puccinia helianthi Schw. Can J Bot 40:1449–1458
Salmeron JM, Oldroyd GE, Rommens CM, Scofield SR, Kim HS et al (1996) Tomato Prf is a member of the leucine-rich repeat class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster. Cell 86:123–133
Sendall BC, Kong GA, Goulter KC, Aitken EAB, Thompson SM, Mitchell JHM, Kochman JK, Lawson W, Shatte T, Gulya TJ (2006) Diversity in the sunflower: Puccinia helianthi pathosystem in Australia. Australas Plant Path 35:657–670
Slabaugh MB, Yu JK, Tang SX, Heesacker A, Hu X, Lu GH, Bidney D, Han F, Knapp SJ (2003) Haplotyping and mapping a large cluster of downy mildew resistance gene candidates in sunflower using multilocus intron fragment length polymorphisms. Plant Biotechnol J 1:167–185
Song WY, Pi LY, Wang GL, Gardner J, Holsten T et al (1997) Evolution of the rice Xa21 disease resistance gene family. Plant Cell 9:1279–1287
Stakman EC, Stewart DM, Loegering WQ (1962) Identification of physiological races of Puccinia graminis var. tritici. USDA-ARS Bulletin E-617
Tang S, Yu JK, Slabaugh MB, Shintani DK, Knapp SJ (2002) Simple sequence repeat map of the sunflower genome. Theor Appl Genet 105:1124–1136
Tang S, Kishore VK, Knapp SJ (2003) PCR-multiplexes for a genome-wide framework of simple sequence repeat marker loci in cultivated sunflower. Theor Appl Genet 107:6–19
Trevet IW, Rawson AJ, Cherry E, Saxon RB (1951) A method for the collection of microscopic particles. Phytopathology 41:282–285
Wei F, Wing RA, Wise RP (2002) Genome dynamics and evolution of the Mla (powdery mildew) resistance locus in barley. Plant Cell 14:1903–1917
Yahiaoui N, Arichumpa P, Dudler R, Keller B (2004) Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J 37:528–538
Yang SM, Antonelli EF, Luciano A, Luciani ND (1986) Reactions of Argentine and Australian sunflower rust differentials to four North American cultures of Puccinia helianthi from North Dakota. Plant Dis 70:883–886
Yang SM, Dowler WM, Luciano A (1989) Gene Pu 6 : a new gene in sunflower for resistance to Puccinia helianthi. Phytopathology 79:474–477
Yang S, Jiang K, Araki H, Ding J, Yang YH, Tian D (2007) A molecular isolation mechanism associated with high intra-specific diversity in rice. Gene 394:87–95
Yu JK, Tang S, Slabaugh MB, Heesacker A, Cole G, Herring M et al (2003) Towards a saturated molecular genetic linkage map for cultivated sunflower. Crop Sci 43:367–387
Zhou T, Wang Y, Chen JQ, Araki H, Jing Z, Jiang K, Shen J, Tian D (2004) Genome-wide identification of NBS genes in rice reveals significant expansion of divergent non-TIR NBS Genes. Mol Genet Genomics 271:402–415
Acknowledgments
We thank Drs. Justin Faris and Chao-Chien Jan for critical review of the manuscript, and Angelia Hogness and Megan Ramsett for excellent technical assistance both in the lab and greenhouse. This project was supported by the USDA-ARS CRIS Project No. 5442-21000-034-00D.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Bervillé.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Rights and permissions
About this article
Cite this article
Qi, L.L., Hulke, B.S., Vick, B.A. et al. Molecular mapping of the rust resistance gene R 4 to a large NBS-LRR cluster on linkage group 13 of sunflower. Theor Appl Genet 123, 351–358 (2011). https://doi.org/10.1007/s00122-011-1588-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-011-1588-6