Abstract
Pearl millet is an important component of food security in the semi-arid tropics and is assuming greater importance in the context of changing climate and increasing demand for highly nutritious food and feed. Molecular tools have been developed and applied for pearl millet on a limited scale. However, the existing tool kit needs to be strengthened further for its routine use in applied breeding programs. Here, we report enrichment of the pearl millet molecular linkage map by exploiting low-cost and high-throughput Diversity Arrays Technology (DArT) markers. Genomic representation from 95 diverse genotypes was used to develop a DArT array with circa 7,000 clones following PstI/BanII complexity reduction. This array was used to genotype a set of 24 diverse pearl millet inbreds and 574 polymorphic DArT markers were identified. The genetic relationships among the inbred lines as revealed by DArT genotyping were in complete agreement with the available pedigree data. Further, a mapping population of 140 F7 Recombinant Inbred Lines (RILs) from cross H 77/833-2 × PRLT 2/89-33 was genotyped and an improved linkage map was constructed by integrating DArT and SSR marker data. This map contains 321 loci (258 DArTs and 63 SSRs) and spans 1148 cM with an average adjacent-marker interval length of 3.7 cM. The length of individual linkage groups (LGs) ranged from 78 cM (LG 3) to 370 cM (LG 2). This better-saturated map provides improved genome coverage and will be useful for genetic analyses of important quantitative traits. This DArT platform will also permit cost-effective background selection in marker-assisted backcrossing programs as well as facilitate comparative genomics and genome organization studies once DNA sequences of polymorphic DArT clones are available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pearl millet [Pennisetum glaucum (L.) R. Br.] is a monocot species belonging to the Poaceae family. It has a relatively small diploid genome (2n = 2x = 14) with a DNA content of 1C = 2.36 pg (Martel et al. 1997). It is a highly cross-pollinated crop and has a large number of wild relatives (n = 5, 8 and 9) including many with 2n = 14 with which it can be intercrossed (Jauhar 1968, 1981; Jauhar and Hanna 1998). Globally, pearl millet is the fifth most important food-grain following rice, wheat, maize and sorghum, and also has importance as a forage and stover crop. It is grown on more than 26 million ha in arid and semi-arid regions of Asia and Africa, where it is often a basic staple among the poorest people but has received relatively little attention of researchers compared with its potential contribution to humanity and is still regarded as an ‘orphan’ crop. Pearl millet hybrids are largely generated from relatively narrow gene pools and current breeding programs do not make use of wild pearl millets except as donors of specific traits such as apomixis (Ozias-Akins et al. 1998), or resistance to pests and diseases (Wilson et al. 2004) and make only limited use of landrace germplasm. Genetic diversity studies in Pennisetum germplasm offer possibilities of identifying diverse germplasm for utilization in improving pearl millet open-pollinated varieties and hybrids. These efforts require effective DNA marker-based fingerprinting strategies for rapid assessment of genetic relationships. Such DNA markers are also required for construction of genetic linkage maps for efficient QTL mapping (the first step in genetic dissection of target traits) and marker-assisted selection for trait introgression, as molecular markers play an important role in improving our understanding the genetic basis of economically important traits and are efficient tools to speed up crop improvement.
During the past decades, various molecular markers such as RFLPs (Liu et al. 1994), STSs (Gale et al. 2001), AFLPs (vom Brocke et al. 2003), single-strand conformational polymorphisms (Bertin et al. 2005), genomic SSRs (Qi et al. 2001, 2004; Allouis et al. 2001; Budak et al. 2003) and genic SSRs (Mariac et al. 2006; Senthilvel et al. 2008) have been developed for pearl millet. Most of these are gel-based marker systems and have limited capability to rapidly assay large numbers of marker loci. Although some of these limitations can be overcome by utilizing specialized hardware such as high-throughput capillary electrophoresis machines, which can improve allelic discrimination ability, reproducibility and speed, still there are limitations related to the sequential nature and high assay costs of these marker technologies as well as the requirement of DNA sequence information to expand currently available marker toolkits. Thus, the available pearl millet marker systems significantly limit the capacity of breeding programs to obtain sufficient return on investment to justify routine use of marker-assisted selection.
Marker technologies are undergoing a transition from predominantly serial assays that measure the size of DNA fragments to hybridization-based assays with high multiplexing levels. Two hybridization-based technologies have emerged as high potential marker systems viz., Single Nucleotide Polymorphisms (SNPs) and Diversity Arrays Technology (DArT) (Jaccoud et al. 2001). It has been established that SNPs are the most abundant marker type, promising nearly unlimited supplies of markers (Chee et al. 1996). Although the progress in genome sequencing and SNP identification has been impressive in humans and a limited number of model organisms, the high cost of SNP marker discovery and assay development has limited their applicability for many crops, especially for the ‘orphan’ crops and polyploid species.
It has been found that DArT performs well in polyploid species, can be rapidly developed for practically any genome in contrast to SNPs, and offers a practical solution to the problems associated with development and application of markers for orphan crops. It is a cost-effective, solid-state platform, hybridization-based marker technology that offers a high multiplexing level (being able to simultaneously genotype several thousand loci per assay), while being independent of sequence information (Jaccoud et al. 2001; Wenzl et al. 2004). The cost of DArT markers per data point has been reported to be tenfold lower than the cost of SSRs (Xia et al. 2005). This genotyping method was developed originally for rice (Jaccoud et al. 2001) and has subsequently been used in genetic mapping and fingerprinting studies in many other plants including barley (Wenzl et al. 2004, 2006; Hearnden et al. 2007), cassava (Xia et al. 2005; Hurtado et al. 2008), Arabidopsis (Wittenberg et al. 2005), pigeonpea (Yang et al. 2006), wheat (Akbari et al. 2006; Crossa et al. 2007; White et al. 2008; Neumann et al. 2010), sorghum (Mace et al. 2008), the Festuca–Lolium complex (Kopecký et al. 2009), Musa (Risterucci et al. 2009; Amorim et al. 2009), oat (Tinker et al. 2009), and rye (Bolibok-Bragoszewska et al. 2009).
Genetic linkage maps developed so far for pearl millet were mainly based on RFLPs and SSRs (Liu et al. 1994; Qi et al. 2004) and generally provided less than optimal genome coverage and marker density. Genetic maps produced for four different pearl millet crosses were integrated to develop a consensus map of 353 RFLP and 65 SSR markers (Qi et al. 2004). In this map, 85% of the markers were clustered and occupied less than one-third of the total map length. This phenomenon was independent of the cross. Extreme localization of recombination toward the chromosome ends, resulting in gaps on the genetic map of 30 cM or more in the distal regions, is typical for pearl millet. This unequal distribution of recombination has consequences for the transfer of genes controlling important agronomic traits from donor to elite pearl millet germplasm. Prior to this study, fewer than 100 PCR-compatible pearl millet markers had published map positions. The length of published linkage maps so far ranged from 280 cM (Jones et al. 2002) to 675 cM (Senthilvel et al. 2008), and the marker density of these maps is completely inadequate for their exploitation in applied plant breeding. Recently, Pedraza-Garcia et al. (2010) developed a PCR-based linkage map for pearl millet having 196 markers (66 SRAPs, 63 RAPDs, 27 ISSRs, 31 pearl millet, 6 sorghum, and 3 maize SSRs). This map consisted of nine linkage groups that spanned about 1,796 cM. Two out of expected seven linkage groups had a gap that split them into two separate linkage sub-groups. Hence, there is a need to develop more number of markers and saturate these maps to facilitate their further exploitation.
DArT has a potential to generate hundreds of high-quality markers with a cost- and time-competitive trade-off (Kilian et al. 2005). The high number of DArT markers generated in a single assay not only provides a precise estimate of genetic relationships among genotypes, but also their relatively even distribution across the genome (depending upon the endonucleases used in complexity reduction) offers real advantages for a range of plant breeding and genomics applications. In this context, we attempted to develop a low-cost, high-throughput DArT marker platform for pearl millet and assess its utility in diversity analysis and high-density linkage map construction.
Materials and methods
Plant material
A set of 95 diverse genotypes (listed as electronic supplementary information S1), representing the entire diversity of wild and cultivated pearl millet accessions held in the ICRISAT Genebank, was used to develop the genotyping array. A sub-set of 24 pearl millet inbred lines (Table 1) was used for diversity analysis. A mapping population of 140 F7 RILs from the cross H77/833-2 (female parent) × PRLT2/89-33 (male parent) was used to generate a linkage map based on DArT and SSR genotyping.
Development of DArT array
Preparation of genomic representation
Genomic representations were generated using a PstI based complexity reduction method, essentially as described by Wenzl et al. (2004). Different combinations of PstI and various frequent-cutting restriction enzymes (BanII, ApoI, AluI, BstNI, HpaII, TaqI, MseI, StyI) were tested and the PstI/BanII combination was selected to construct the library. Restriction digestions and adapter ligation were performed simultaneously to minimize fragment-to-fragment ligation. The procedure involved digestion of 50 ng of DNA with 1.4U each of rare-cutter enzyme PstI and frequent-cutter BanII, ligation of a PstI adapter (Adapter 1: 5′CACGATGGATCCAGTGCA3′ and Adapter 2: 5′CTGGATCCATCGTGCA3′) with T4 DNA ligase (NEB, UK), and amplification of small adapter-ligated fragments. For amplification, 1-μl aliquots of the ligation product were used as template in 50-μl amplification reactions with 2U of Taq DNA polymerase (Sibenzyme Ltd., Russia) and PstI + 0 primer (5′GATGGATCCAGTGCAG 3′). The PCRs were conducted on a thermal cycler (PTC-200, MJ Research) using the following conditions: 94°C for 4 min, followed by 30 cycles of 94°C for 20 s, 58°C for 40 s, 72°C for 1 min, and final extension at 72°C for 7 min.
Cloning and amplification of the fragments from representations
A library was prepared by ligating the pooled genomic representations into the PCR2.1-TOPO vector using the TOPO cloning kit (Invitrogen, USA) and transforming these into an electrocompetent E. coli strain (TOP10) according to the manufacturer’s instructions. Transformants were selected on a medium containing ampicillin and X-gal. Individual white colonies (containing recombinant plasmids) were picked by toothpicks and grown in 384-well plates containing LB medium supplemented with 100 mg/ml ampicillin and a freezing mix. The inserts were amplified in a 15-μl reaction containing 0.1 μM each of forward and reverse M13 primers (F: 5′ GTTTTCCCAGTCACGACGTTG 3′ and R: 5′ TGAGCGGATAACAATTTCACACAG 3′) and 0.3 U of Taq polymerase (Sibenzyme Ltd., Russia) under the cycling conditions: 95°C for 3 min, 57°C for 35 s, 72°C for 1 min, followed by 40 cycles of 94°C for 35 s, 52°C for 35 s, 72°C for 1 min. The approximate size of amplified inserts ranged from 200 to 1,000 bp. After amplification, the PCR products were precipitated with one volume of isopropanol at room temperature and washed once with 100 μl of 77% ethanol. The ethanol was then removed and the products dried per Jaccoud et al. (2001). The DNA was pellet was re-suspended in spotting buffer and the resulting solutions of clean insert DNA fragments from 7,680 clones were used in printing a replicated array.
Printing and processing of array
The 7,680 fragments were spotted onto poly-l-lysine-coated slides (Erie Scientific Co., USA) using a MicroGridII arrayer (Biorobotics, UK) in replication with approximately 10% missing spots. After printing, slides were left at room temperature for at least one day and then processed by heating them in hot water at 92°C for 2 min and drying by centrifugation.
Genotyping of individual DNA samples using DArT array
Fluorescent labeling of representations
Genomic representations were generated individually from the 24 pearl millet inbred lines and 140 F7 RILs of the mapping population using the same complexity reduction method used for library construction (PstI/BanII). Representations were precipitated with one volume of isopropanol, denatured at 95°C for 3 min, and labeled with Cy3/Cy5-dUTP, random decamers using the exo− Klenow fragment of E. coli DNA polymerase I (NEB, UK).
Hybridization and washing
Labeled representations (targets) were mixed with 50 μl of DArT hybridizer (50:5:1 mixture of ExpressHyb buffer (Clontech, USA), 10 g/l herring sperm DNA (Sigma), and the 6-FAM-labeled poly-linker fragment of the plasmid that was used for library preparation and denatured at 95°C for 3 min). The poly-linker fragment was used as a reference to determine the amount of DNA spotted on the array for each clone (Jaccoud et al. 2001). The denatured target was pipetted directly onto the microarray surface and covered with a glass coverslip (24 × 60 mm, Menzel-Glazer, Germany). Slides were quickly placed into a 65°C water bath for overnight hybridization. After overnight hybridization at 65°C, the coverslips were removed, slides were placed into slide-racks, and washed in 4 steps; Step 1: 1× SSC + 0.1% SDS for 5 min: Step 2: in 1× SSC for 5 min, Step 3: in 0.2× SSC for 2 min and Step 4: in 0.02× SSC for 30 s. Slides were centrifuged and dried in vacuum desiccators as described by Jaccoud et al. (2001).
Slide scanning and polymorphism scoring
Slides were scanned using a confocal laser scanner (Tecan LS300) and images were generated for each fluorescent dye using the appropriate laser/filter combination at appropriate wavelengths (Cy3: 543 nm, Cy5: 633 nm, 6-FAM: 488 nm). A software package developed by DArT P/L, (Australia), DArTsoft, was used to automatically analyze each batch of TIF image pairs generated in the experiment. The software localized spots, rejected those with a weak reference signal, computed and normalized the relative hybridization intensities [= log (Cy3target/FAMreference)] of all spots, calculated the median value for replicate spots, identified polymorphic clones by using a combination of ANOVA and fuzzy K-means clustering at a fuzziness level of 1.5 and finally, the relative hybridization intensities of polymorphic clones in the representation hybridized to a slide were converted into present (“1”) or absent (“0”) scores based on the membership probability estimates computed by the clustering algorithm. The clustering algorithm also provided a probability estimate for each individual genotype call. Markers that showed conflicting scores between the replicates or could not be scored in either of the replicates were noted as ‘unknown’.
Diversity analysis
The DArTsoft-generated ‘0’ and ‘1’ scores for the polymorphic DArT markers were used to assess the genetic relationship among a set of 24 inbred lines of pearl millet. DARwin 5.0 software (Perrier and Jacquemoud-Collet 2006) was used to calculate a pair-wise dissimilarity index (Jaccard) and a dendrogram was constructed based on the unweighted neighbor-joining algorithm implemented in DARwin.
Linkage map construction
A set of 80 SSR marker data generated in a different study was used along with DArT marker data to construct an integrated linkage map. Among the SSR markers used, the PSMP series genomic SSR markers were obtained from Qi et al. (2001, 2004) and Allouis et al. (2001). The CTM series genomic SSR markers were published by Budak et al. (2003). The ICMP series EST-SSR markers were published by Senthilvel et al. (2008). The remaining ISEP series EST-SSRs are the subject of a manuscript that is being prepared for submission to an appropriate journal (Rajaram et al. under preparation). The scores of all polymorphic DArT and SSR markers for the RIL population progenies were converted into genotype codes (‘A’ = female parent homozygote and ‘B’ = male parent homozygote) according to their parental allele constitutions. The linkage groups were obtained using GMendel version 0.8b (Holloway and Knapps 1993) at a LOD threshold value of 4.0. The order of markers in each linkage group was finalized by RECORD software (van Os et al. 2005), and distances between marker loci calculated using the Haldane mapping function. A graphical representation of the map was drawn using MapChart software (Voorrips 2002). The DArT markers were named with the prefix “PgPb” where ‘Pg’ stands for Pennisetum glaucum, ‘P’ for PstI (primary restriction enzyme used) and ‘b’ for BanII (secondary restriction enzyme used) followed by numbers corresponding to their unique clone ID.
Results
Genetic relationship among the inbred lines
A total of 574 DArT marker clones (7.5%) were polymorphic in a panel of 24 pearl millet inbreds including 11 mapping parental lines and two elite products of marker-assisted backcrossing. The call rate, which reflects the total percentage of non-missing scores for a specific clone across all samples in the experiment ranged from 80.5 to 100% with an average of 91.6% and the scoring reproducibility was 100%. Polymorphism information content (PIC) values of individual polymorphic DArT markers ranged from 0.04 to 0.50 with an average of 0.30.
Cluster analysis discriminated the 24 pearl millet inbreds into three main clusters (Fig. 1). Cluster I consisted of 12 inbred lines, including elite restorers, downy mildew resistance sources of West African origin, and Iniadi landrace-derived lines. Cluster II consisted of 11 inbreds, which were mainly seed parents and genetic stocks, whereas inbred ICMB 90111-P6, derived from the ICRISAT Early Composite, was entirely separated from these first two clusters. Of 12 inbreds grouped in Cluster I, ICMP 85410-P7 is semi-dwarf due to recessive alleles at the d2 locus, while all other lines in this cluster are genetically tall, having the dominant D2 alleles at this locus. At least four lines (IPC 804-P4, ICMP 85410-P7, PRLT 2/89-33, and 863B-P2) are derived from the Iniadi landrace. H 77/833-2, H 77/833-2-P5(NT), ICMP 451-P6, ICMP 451-P8, ICMP 85410-P7, ICMR 01004, ICMR 01007 and IPC 804-P4 are strong male-fertility restorers for the A1 cytoplasmic-genetic male-sterility system, whereas 863B-P2 is a strong maintainer. ICMP 451-P6, ICMP 451-P8, ICMP 85410-P7, PRLT 2/89-33, and 863B-P2 are downy mildew resistant hybrid parents and parents of pearl millet mapping populations. The two inbreds P1449-2-P1 and P310-17-Bk, which are tall and downy mildew resistant mapping population parents of West African origin, were grouped together. Six fertility restorer lines (H 77/833-2, H 77/833-2-P5(NT), ICMR 01004, ICMR 01007, ICMP 451-P8, and ICMP 451-P6) in this cluster were grouped together more tightly. Among these, H 77/833-2 is derived from a Rajasthani landrace population and H 77/833-2-P5(NT) is a sub-selection (probably derived from an outcross) of H 77/833-2. Both are susceptible to downy mildew and rust. ICMP 451-P8 and ICMP 451-P6 grouped together as both of these are derived from the same inbred line (LCSN 72-1-2-1-1) and are moderately resistant to downy mildew and resistant to rust. ICMR 01004 is moderately susceptible to rust, whereas ICMR 01007 is resistant to rust, but both are resistant to downy mildew and were bred by marker-assisted backcrossing of disease resistance from donor parent ICMP 451-P6 into the genetic background of recurrent parent H 77/833-2. The known genetic relationships of these six elite pollinator lines are well-reflected by the cluster analysis.
Unweighted neighbour-joining dendrogram of 24 pearl millet inbred lines constructed using a Jaccard dissimilarity matrix based on presence/absence of 574 DArT markers. Numbers at branching points indicate per cent bootstrap support of individual nodes; only values (>50% are reported (bootstrap no. = 1,000). DMR downy mildew resistance, dms downy mildew susceptible, RR rust resistance, rs rust susceptible, hl recessive hairy foliage at Hl/hl locus on LG6, Hl dominant non-hairy foliage at Hl/hl locus on LG6, D2 dominant tall allele at D2/d2 semi-dwarf locus on LG4, d2 recessive dwarf allele at D2/d2 semi-dwarf locus on LG4, d1 recessive dwarf allele at D1/d1 semi-dwarf locus on LG1
In cluster II, five inbreds (ICMB 841-P3, Tift 23D2B1-P5, 81B-P6 and 81B-P8, IP 18293-P152) were grouped together, four of which are d 2 dwarf lines. Out of these five inbreds, genetically tall ICMB 841-P3 and d 2 dwarf Tift 23D2B1-P5 are expected to cluster together as they share genetically tall Tift 23B1 as a common ancestor and the two sub-selections of 81B, namely 81B-P6 and 81B-P8, were also clustered with these as 81B is a product of an induced mutation breeding program based on Tift 23D2B1. Among these inbreds, ICMB 841-P3, Tift 23D2B1-P5, 81B-P6, and 81B-P8 are maintainers for the A1 cytoplasmic-genetic male-sterility (CMS) system, have strongly pubescent (hl allele), green (p allele) foliage, whereas IP 18293-P152 has non-pubescent (Hl allele) purple (P allele) foliage. ICMB 841-P3, 81B-P6, 81B-P8, and IP 18293-P152 are all at least moderately resistant to downy mildew, while Tift 23D2B1-P5 is highly susceptible to downy mildew, but all five are highly susceptible to rust. The other four inbreds (ICMB 89111-P6, WSIL-P8, PT 732B-P2, and LGD 1-B-10) of cluster II were grouped together and all are d 2 dwarf lines with non-hairy (Hl allele) green (p allele) foliage. Out of these, ICMB 89111-P6 and LGD 1-B-10 are downy mildew susceptible while WSIL-P8 is downy mildew resistant, and all three of these lines are rust susceptible. PT 732-P2 is a maintainer for the Aβ CMS system and has moderate resistance to both rust and downy mildew; whereas ICMB 89111-P6 is a maintainer of the A1 CMS system. W504-1-P1 and Tift 238D1-P158 were the other two inbreds in cluster II, and both are highly susceptible to a diverse range of downy mildew isolates of Indian origin (Hash et al. 2006). W504-1-P1 is genetically tall and Tift 238D1-P158 is a d 1 dwarf fertility restorer line for the A1 CMS system.
Linkage mapping
Segregation analysis indicated that distortion was found at 136 (34.9%) out of 389 marker loci analyzed for the 137 RILs studied. A set of 37 marker loci (9.5%) showed distortion in favor of the H 77/833-2 alleles, whereas 99 (25.4%) showed distortion in favor of PRLT 2/89-33 alleles. Out of 321 mapped markers, 112 (34.9%) showed significant segregation distortion from the expected 1:1 Mendelian ratio. The number of mapped marker loci showing segregation distortion in favor of PRLT 2/89-33 alleles was more (82; 25.5%) than that for H 77/833-2 alleles (30; 9.3%). The marker loci that showed distortion in favor of H 77/833-2 alleles were distributed on LG 2 (29 of 80 mapped loci) and LG 4 (1 out of 47 mapped loci). The 82 markers that showed distortion in favor of PRLT2/89-33 alleles were distributed across all seven linkage groups, but were concentrated on LG 1 (32 of 61 mapped loci) and LG 6 (25 of 39 mapped loci). LG 2 and LG 4 showed regions with distortion favoring alleles of either parent.
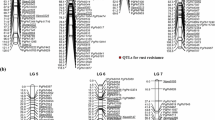
A total of 389 markers (309 DArT and 80 SSR markers) were used for assembling the linkage map using data from 137 RILs. From these, 321 loci (258 DArTs and 63 SSRs) were mapped across 7 linkage groups using GMendel at a LOD threshold value of 4.0 and a recombination frequency threshold of 0.4 (Fig. 2). The map had a total length of 1148 cM (Haldane), with an average density of 0.28 markers/cM, and an average adjacent-marker interval length of 3.66 cM. The length of individual linkage groups ranged from 77 (LG 3) to 370 cM (LG 2) with the average length of 164 cM. The total number of mapped loci per linkage group ranged from 28 (LG 5) to 80 (LG 2), with an average of 45.8 loci. The average adjacent-marker interval lengths ranged from 2.15 (LG 6) to 4.69 cM (LG 2), with corresponding map densities ranging from 0.22 to 0.48 markers/cM for LG 2 and LG 6, respectively. Map distance between adjacent markers varied from 0 to 35.3 cM and 91.7% of the intervals (288 out of 314 intervals) were <10 cM. There were 26 map regions (8.3%) with adjacent-marker distances >10 cM and the largest distance between adjacent markers was observed on LG 2 (35.3 cM). Of the 321 markers placed on the genetic linkage map, 61 were distributed on LG 1, 80 on LG 2, 30 on LG 3, 47 on LG 4, 28 on LG 5, 39 on LG 6, and 36 on LG 7. The details of each linkage group are described in Table 2.
Genetic linkage map for LG 1 thru LG 7 of the (H 77/833-2 × PRLT 2/89-33)-based pearl millet RIL population. Cumulative map distances in cM (Haldane) and marker names are shown on the left and right side of each linkage group, respectively. SSR marker names are underlined and DArT marker names begin with the prefix PgPb. Markers that showed distorted segregation are shown in italics followed by an asterisk
Discussion
In the present study, the usefulness of DArT markers in pearl millet was demonstrated by their ability to effectively describe the genetic relationships among a set of pearl millet inbred lines. Such genetic differences in pearl millet have been studied previously by morphological and isozyme analysis (Tostain et al. 1987; Tostain and Marchais 1989; Tostain 1992). Subsequently, RAPDs (Chowdari et al. 1998), RFLPs (Bhattacharjee et al. 2002), and SSRs (Budak et al. 2003; Kapila et al. 2008) and combinations of SSRs with other PCR-compatible markers (Thudi et al. 2010) have been used to estimate pearl millet genetic diversity. The power of DArT fingerprinting method lies in its ability to compare different genomes at a large number of loci in a single assay, at an average cost per marker locus that is very low compared with PCR-compatible markers. The large number of markers that are simultaneously assayed by DArT provide a high level of resolution in genetic diversity studies. Genetic distance estimates derived using DArT are more likely to be accurate because the ‘random’ nature of DArT markers should reduce the ascertainment bias when compared with technologies relying on targeted marker development.
The DArT-based cluster analysis discriminated well between the 24 pearl millet inbreds assessed in this study. Clusters generated using DArT were in complete agreement with the available pedigree data. Most inbreds derived from the Iniadi landrace formed a perfect sub-cluster within Cluster I (the exception being d 2 dwarf lines ICMB 89111-P6 and LGD 1-B-10, which contributed to a loose sub-cluster within cluster II). In Cluster II, five inbreds (ICMB 841-P3, Tift 23D2B1-P5, 81B-P6 and 81B-P8, and IP 18293-P152) were grouped together, four of which are d 2 dwarf lines. Out of these five inbreds, genetically tall ICMB 841-P3 and d 2 dwarf Tift 23D2B1-P5, are expected to cluster together as they share genetically tall Tift 23B1 as a common ancestor and the two sub-selections of 81B, namely 81B-P6 and 81B-P8, were also clustered with these as 81B is a product of an induced mutation breeding program based on Tift 23D2B1. The known pedigree relationships of these five lines are much better captured by this DArT-based diversity analysis than by the analysis based on SSR and SSCP-SNP marker data recently reported by Thudi et al. (2010).
Marker-assisted breeding is generally more efficient when molecular maps are well saturated, as this provides an increased chance of detecting polymorphic markers in any genetic background in any genomic region of interest. This is the first report in which DArT markers were mapped in pearl millet. In this study, segregation distortion was observed for 35% of the total marker loci analyzed. The phenomenon of segregation distortion can be an important limitation in map construction as it may affect both the establishment of linkage groups and estimation of recombination frequencies. Calculations of linkage distance usually assume no segregation distortion, which could cause overestimation of recombination frequency between linked markers (Paran et al. 1995). Numerous examples of segregation distortion have been reported in many crop species including barley (Graner et al. 1991; Devaux et al. 1995), rice (Causse et al. 1994; Xu et al. 1997), maize (Wendel et al. 1987; Lu et al. 2002), and wheat (Blanco et al. 2004; Peng et al. 2000; Quarrie et al. 2005). Segregation distortion is most commonly observed in interspecific crosses; however, previous studies showed that distortion phenomenon also occurs in intraspecific pearl millet crosses (Liu et al. 1994; Busso et al. 1995). While segregation distortion is a common phenomenon in different types of mapping populations, be it F2, RILs or double haploid (DH), RIL populations have the highest potential for such distortions due to repeated generations of selection forces (Singh et al. 2007), which can be accentuated by loss of vigor with enforced inbreeding in outcrossing species such as pearl millet.
In most previous studies, segregation distortion in favor of the female parent alleles was observed (Singh et al. 2007). In contrast, the present data showed distortion in favor of the male parent alleles in some genomic regions and female parent alleles in others. This result should not be considered as a surprise if we take into account the variety of mechanisms that could contribute to the observed distortions such as meiotic drive, preferential abortion of gametes, effects of unusual gametophyte factors, non-random fertilization, and viability selection at post-syngamic stages (Lyttle 1991). Clearly, these factors may work simultaneously and in opposite directions, favoring the alleles of the two parents in different genomic regions. Segregation distortion favoring alleles from a male parent has previously been reported in pearl millet by Liu et al. (1994), Azhaguvel (2001) and Kolesnikova (2001). It has been suggested that such segregation distortion is highly likely in pearl millet because of its protogynous nature (Liu et al. 1994) and sensitivity to inbreeding depression.
The genetic map spans 1,148 cM corresponding to an average of 3.6 cM per marker. The linkage map constructed in this study is more highly saturated, includes more markers, and has smaller marker intervals than any previously constructed pearl millet genetic maps constructed with RFLPs and/or SSRs. The genome coverage achieved makes the present map particularly useful to select markers for use in whole-genome breeding strategies and to saturate genomic regions of interest in other mapping populations. The map showed a high level of genome coverage and distribution of markers was reasonably uniform. This resulted largely from the inclusion of DArT and EST-SSRs. These markers typically show improved genome coverage compared with anonymous (non-coding) SSRs or AFLPs, which are characteristically clustered proximal to centromeric regions (Ramsay et al. 2000) in regions with relatively low recombination frequencies. In contrast, markers from gene-rich regions of the genome (such as those targeted by the PstI rare-cutting endonuclease used in the complexity-reduction protocol for the pearl millet DArT platform described here) end up covering a larger portion of the linkage map (if not the physical map) because of the relatively higher recombination rates in there. The difference in genome coverage is thought to reflect the processes used to develop each type of marker. Anonymous SSRs are usually developed from random genomic libraries, in which microsatellites located in the heterochromatic regions are overrepresented (Röder et al. 1998) and the development of EST-SSRs from genic regions reduces the representation of regions that are rich in repetitive DNA (Parida et al. 2006; Chabane et al. 2005). It appeared that DArT markers may have a stronger tendency than genomic SSR and AFLP markers in particular, to map to gene-rich regions (Vuylsteke et al. 1999) provided that the right combinations of endonucleases are used in complexity reduction. The occurrence of PstI-based DArT marker clusters in distal regions of chromosome arms was observed in previous mapping studies on barley (Wenzl et al. 2004) and wheat (Akbari et al. 2006; Semagn et al. 2006). Similar clustering in distal regions was also found in tetraploid wheat using PstI-based AFLP markers (Peng et al. 2000). The higher density of such clusters in distal regions could well be related to the trend of PstI-based markers to map in gene-rich, hypomethylated regions of the genome (Langridge and Chalmers 1998; Moore 2000).
SSR marker orders from the present study were compared with those from maps based on SSRs only (recently developed unpublished maps of H 77/833-2 × PRLT 2/89-33 and 81B-P6 × ICMP 451-P8 provided by Mr. Rajaram) and were almost identical except for swapping of marker orders within small blocks on a few linkage groups (data not shown). Such differences in marker order among genetic maps based on populations of moderate size are not unexpected, as genetic mapping only gives an indication of the relative positions and genetic distances of the markers to each other (Sourdille et al. 2004). Moreover, inconsistency in map position of these few SSRs could be explained by the presence of closely linked DArT loci. The order of loci was also compared with an integrated DArT-SSR pearl millet map based on cross (81B-P6 × ICMP451-P8) (Supriya et al., unpublished data) and the order was very similar with limited marker order swapping. A total of 78 markers representing all 7 linkage groups of pearl millet were mapped in both populations, which will permit the development of a well-saturated pearl millet consensus linkage map combining DArT and SSR markers.
The results obtained from the present study indicate that DArT provides high-quality markers that can be used to construct medium-density genetic linkage maps for plants even when no sequence information is available. An additional advantage is that DArT clones can readily be sequenced and thus provide information for their conversion into PCR-based markers, and for linkage group alignment with genomes of other species for which aligned DNA sequence information is available. This can be advantageous in cases when there are not yet any inexpensively assayed markers closely flanking a potential target QTL that could be used in foreground selection for the favorable allele.
References
Akbari M, Wenzl P, Caig V, Carling J, Xia L, Yang S, Uszynski G, Mohler V, Lehmensiek A, Kuchel H, Hayden MJ, Howes N, Sharp P, Vaughan P, Rathmell B, Huttner E, Kilian A (2006) Diversity arrays technology (DArT) for high throughput profiling of hexaploid wheat genome. Theor Appl Genet 113:1409–1420
Allouis S, Qi X, Lindup S, Gale MD, Devos KM (2001) Construction of a BAC library of pearl millet, Pennisetum glaucum. Theor Appl Genet 102:1200–1205
Amorim EP, Vilarinhos AD, Cohen KO, Amorim VBO, Santos-Serejo JA, Silva SO, Pestana KN, Santos VJ, Paes NS, Monte DC, Rei RV (2009) Genetic diversity of carotenoid-rich bananas evaluated by diversity arrays technology (DArT). Genet Mol Biol 32:96–103
Azhaguvel P (2001) Linkage map construction and identification of QTLs for downy mildew (Sclerospora graminicola) resistance in pearl millet (Pennisetum glaucum (L.) R. Br.). PhD thesis, Department of Agricultural Botany, Agricultural College and Research Institute, Tamil Nadu Agricultural University, Madurai, Tamil Nadu, India, p 204
Bertin I, Zhu JH, Gale MD (2005) SSCP-SNP in pearl millet-a new marker system for comparative genetics. Theor Appl Genet 110:1467–1472
Bhattacharjee R, Bramel PJ, Hash CT, Kolesnikova-Allen MA, Khairwal IS (2002) Assessment of genetic diversity within and between pearl millet landraces. Theor Appl Genet 105:666–673
Blanco A, Simeone R, Cenci A, Gadaleta A, Tanzarella OA, Porceddu E, Salvi S, Tuberosa R, Figliuolo G, Spagnoletti P, Röder MS, Korzun V (2004) Extension of the “Messapia x dicoccoides” linkage map of Triticum turgidum (L.) Thell. Cell Mol Biol Lett 9:529–541
Bolibok-Brągoszewska H, Heller-Uszyńska K, Wenzl P, Uszyński G, Kilian A, Rakoczy-Trojanowska M (2009) DArT markers for the rye genome-genetic diversity and mapping. BMC Genomics 10:578
Budak H, Pedraza F, Cregan PB, Baenzinger PS, Dweikat I (2003) Development and utilization of SSRs to estimate the degree of genetic relationships in a collection of pearl millet germplasm. Crop Sci 43:2284–2290
Busso CS, Liu CJ, Hash CT, Witcombe JR, Devos KM, de Wet JMJ, Gale MD (1995) Analysis of recombination rate in female and male gametogenesis in pearl millet (Pennisetum glaucum) using RFLP markers. Theor Appl Genet 90:242–246
Causse MA, Fulton TM, Cho YG, Ahn SN, Chunwongse J, Wu KS, Xiao JH, Yu ZH, Ronald PC, Harrington SE, Second G, McCouch SR, Tanksley SD (1994) Saturated molecular map of the rice genome based on an interspecific backcross population. Genetics 138:1251–1274
Chabane K, Ablett GA, Cordeiro GM, Valkoun J, Henry RJ (2005) EST versus genomic derived microsatellite markers for genotyping wild and cultivated barley. Genet Resour Crop Evol 52:903–909
Chee M, Yang R, Hubbell E, Berno A, Huang XC, Stern D, Winkler J, Lockhart DJ, Morris MS, Fodor SPA (1996) Accessing genetic information with high-density DNA arrays. Science 274:610–614
Chowdari KV, Davierwala AP, Gupta VS, Ranjekar PK, Govila OP (1998) Genotype identification and the assessment of genetic relationships in pearl millet (Pennisetum glaucum L.) using the (GATA) 4 microsatellite and RAPDs. Theor Appl Genet 97:154–162
Crossa J, Burgueno J, Dreisigacker S, Vargas M, Herrera-Foessel SA, Lillemo M, Singh RP, Trethowan R, Warburton M, Franco J, Reynolds M, Crouch JH, Ortiz R (2007) Association analysis of historical bread wheat germplasm using additive genetic covariance of relatives and population structure. Genetics 177:1889–1913
Devaux P, Kilian A, Kleinhofs A (1995) Comparative mapping of the barley genome with male and female recombination-derived, doubled haploid populations. Mol Gen Genet 249:600–608
Gale MD, Devos KM, Zhu JH, Allouis S, Couchman MS, Liu H, Pittaway TS, Qi XQ, Kolesnikova-Allen M, Hash CT (2001) New molecular marker technologies for pearl millet improvement. Int Sorghum Millets Newsl 42:16–22
Graner A, Jahoor A, Schondelmaier J, Siedler H, Pillen K, Fischbeck G, Wenzel G, Herrmann RG (1991) Construction of an RFLP map of barley. Theor Appl Genet 83:250–256
Hash CT, Thakur RP, Rao VP, Bhasker Raj AG (2006) Evidence for enhanced resistance to diverse isolates of pearl millet downy mildew through gene pyramiding. Int Sorghum Millets Newslett 47:134–138
Hearnden PR, Eckermann PJ, McMichael GL, Hayden MJ, Eglinton JK, Chalmers KJ (2007) A genetic map of 1,000 SSR and DArT markers in a wide barley cross. Theor Appl Genet 115:383–391
Holloway JL, Knapp SJ (1993) GMendel 3.0 Users guide. Department of Crop and Soil Science, Oregon State University. Corvallis, OR
Hurtado P, Olsen KM, Buitrago C, Ospina C, Marin J, Duque M, de Vicente C, Wongtiem P, Wenzel P, Kilian A, Adeleke M, Fregene M (2008) Comparison of simple sequence repeat (SSR) and diversity array technology (DArT) markers for assessing genetic diversity in cassava (Manihot esculenta Crantz). Plant Genetic Resour Char Utili 6(3):208–214
Jaccoud D, Peng K, Feinstein D, Kilian A (2001) Diversity arrays: a solid state technology for sequence information independent genotyping. Nucleic Acids Res 29(4):e25
Jauhar PP (1968) Inter- and intra-genomal chromosome pairing in an interspecific hybrid and its bearing on basic chromosome number in Pennisetum. Genetica 39:360–370
Jauhar PP (1981) Cytogenetics and breeding of pearl millet and related species. Progress and topics in cytogenetics, vol 1. Alan R Liss, New York, pp 1–289
Jauhar PP, Hanna WW (1998) Cytogenetics and genetics of pearlmillet. Adv Agron 64:1–26
Jones ES, Breese WA, Liu CJ, Singh SD, Shaw DS, Witcombe JR (2002) Mapping quantitative trait loci for resistance to downy mildew in pearl millet: field and glasshouse screens detect the same QTL. Crop Sci 42:1316–1323
Kapila RK, Yadav RS, Plaha P, Rai KN, Yadav OP, Hash CT, Howarth CJ (2008) Analysis of genetic diversity in pearl millet inbreds using microsatellite markers. Plant Breed 127:33–37
Kilian A, Huttner E, Wenzl P, Jaccoud D, Carling J, Caig V, Evers M, Heller-Uszynska, Cayla C, Patarapuwadol S, Xia L, Yang S, Thomson B (2005) The fast and the cheap: SNP and DArT-based whole genome profiling for crop improvement. In: Tuberosa R, Phillips RL, Gale M (eds) Proceedings of the international congress “In the wake of the double helix: from the green revolution to the gene revolution”, 27–31 May 2003, Avenue Media, Bologna, Italy, pp 443–461
Kolesnikova MA (2001) Mapping new quantitative trait loci (QTL) for downy mildew resistance in pearl millet. PhD thesis, Russian National Academy of Sciences, Moscow, Russia. (English and Russian) p 266
Kopecký D, Bartoš J, Lukaszewski AJ, James H, Baird JH, Černoch V, Kölliker R, Rognli OA, Blois H, Caig V, Lübberstedt T, Studer B, Shaw P, Doležel J, Kilian A (2009) Development and mapping of DArT markers within the Festuca–Lolium complex. BMC Genomics 10:473
Langridge P, Chalmers K (1998) Techniques for marker development. In: Proceedings of the 9th international wheat genet symposium, vol 1. Saskatchewan, Canada, pp 107–117
Liu CJ, Witcombe JR, Pittaway TS, Nash M, Hash CT, Busso CS, Gale MD (1994) An RFLP-based genetic map in pearl millet (Pennisetum glaucum). Theor Appl Genet 89:481–487
Lu H, Romero-Severson J, Bernardo R (2002) Chromosomal regions associated with segregation distortion in maize. Theor Appl Genet 105:622–628
Lyttle TW (1991) Segregation distorters. Annu Rev Genet 25:511–557
Mace ES, Xia L, Jordan DR, Halloran K, Parh DK, Huttner E, Wenzl P, Kilian A (2008) DArT markers: diversity analyses and mapping in Sorghum bicolor. BMC Genomics 9:26
Mariac C, Luong V, Kapran I, Mamadou A, Sagnard M, Deu M, Chantereau J, Gerard B, Ndjeunga J, Bezançon G, Pham J, Vigouroux Y (2006) Diversity of wild and cultivated pearl millet accessions (Pennisetum glaucum [L.] R. Br.) in Niger assessed by microsatellite markers. Theor Appl Genet 114:49–58
Martel E, De Nay D, Siljak-Yakovlev S, Brown S, Sarr A (1997) Genome size variation and basic chromosome number in pearl millet and fourteen related Pennisetum species. J Hered 88:139–143
Moore G (2000) Cereal chromosome structure, evolution and pairing. Annu Rev Plant Physiol Plant Mol Biol 51:195–222
Neumann K, Kobiljski B, Dencic S, Varshney RK Borner A (2010) Genome-wide association mapping: a case study in bread wheat (Triticum aestivum L.). Mol. Breeding, DOI 10.1007/s11032-010-9411-7
Ozias-Akins P, Roche D, Hanna WW (1998) Tight clustering and hemizygosity of apomixis-linked molecular markers in Pennisetum squamulatum implies genetic control of apospory by a divergent locus that may have no allelic form in sexual genotypes. Proc Nat Acad Sci (USA) 95:5127–5132
Paran I, Goldman I, Tanksley SD, Zamir D (1995) Recombinant inbred lines for genetic mapping in tomato. Theor Appl Genet 90:542–548
Parida SK, Kumar KAR, Dalal V, Singh NK, Mohapatra T (2006) Unigene derived microsatellite markers for the cereal genomes. Theor Appl Genet 112:808–817
Pedraza-Garcia F, Specht JE, Dweikat I (2010) A new PCR-based linkage map in pearl millet. Crop Sci 50:1754–1756
Peng JH, Fahima T, Röder MS, Li YC, Grama A, Ronin YI, Korol AB, Nevo E (2000) Molecular genetic maps in wild emmer wheat, Triticum dicoccoides: genome-wide coverage, massive negative interference, and putative quasi-linkage. Genome Res 10:1059–1061
Perrier X, Jacquemoud-Collet JP (2006) DARwin software (http://darwin cirad fr/darwin)
Qi X, Lindup S, Pittaway TS, Allouis S, Gale MD, Devos KM (2001) Development of simple sequence repeat markers from bacterial artificial chromosome without sub-cloning. Biotechniques 31:355–362
Qi X, Pittaway TS, Lindup S, Liu H, Wateran E, Padi FK, Hash CT, Zhu J, Gale MD, Devos KM (2004) An integrated genetic map and new set of simple sequence repeat markers for pearl millet, Pennisetum glaucum. Theor Appl Genet 109:1485–1493
Quarrie SA, Steed A, Calestani C, Semikhodskii A, Lebreton C, Chinoy C, Steele N, PljevljakusiT D, Waterman E, Weyen EJ, Schondelmaier J, Habash DZ, Farmer P, Saker L, Clarkson DT, Abugalieva A, Yessimbekova M, Turuspekov Y, Abugalieva S, Tuberosa R, Sanguineti M-C, Hollington PA, Aragués R, Royo A, Dodig D (2005) A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross Chinese Spring × SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor Appl Genet 110:865–880
Ramsay L, Macaulay M, degli Ivanissevich S, MacLean K, Cardle L, Fuller J, Edwards KJ, Tuvesson S, Morgante M, Massari A, Maestri E, Marmiroli N, Sjakste T, Ganal M, Owell W, Waugh R (2000) A simple sequence repeat-based linkage map of barley. Genetics 156:1997–2005
Risterucci AM, Hippolyte I, Perrier X, Xia L, Caig V, Evers M, Huttner E, Kilian A, Jean-Christophe Glaszmann JC (2009) Development and assessment of diversity arrays technology for high-throughput DNA analyses in Musa. Theor Appl Genet 119:1093–1103
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Semagn K, Bjornstad A, Skinnes H, Maroy AG, Tarkegne Y, William M (2006) Distribution of DArT, AFLP, and SSR markers in a genetic linkage map of a doubled-haploid hexaploid wheat population. Genome 49:545–555
Senthilvel S, Jayashree B, Mahalakshmi V, Sathish Kumar P, Nakka S, Nepolean T, Hash CT (2008) Development and mapping of simple sequence repeat markers for pearl millet from data mining of expressed sequence tags. BMC Plant Biol 8:119–127
Singh K, Ghai M, Garg M, Chhuneja P, Kaur P, Schnurbusch T, Keller B, Dhaliwal HS (2007) An integrated molecular linkage map of diploid wheat based on a Triticum boeoticum × T. monococcum RIL population. Theor Appl Genet 115:301–312
Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, Gill BS, Dufour P, Murigneux A, Bernard M (2004) Microsatellite based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomics 4:12–25
Thudi M, Senthilvel S, Bottley A, Hash CT, Reddy AR, Feltus AF, Paterson AH, Hoisington DA, Varshney RK (2010) A comparative assessment of the utility of PCR-based marker systems in pearl millet. Euphytica 174:253–260
Tinker NA, Kilian A, Wight CP, Heller-Uszynska K, Wenzl P, Rines HW, Bjørnstad A, Howarth CJ, Jannink JL, Anderson JM, Rossnage BG, Stuthman DD, Sorrells ME, Jackson EW, Tuvesson S, Kolb FL, Olsson O, Federizzi LC, Carson ML, Ohm HW, Molnar SJ, Scoles GJ, Eckstein PE, Bonman JM, Ceplitis A, Langdon T (2009) New DArT markers for oat provide enhanced map coverage and global germplasm characterization. BMC Genomics 10:39
Tostain S (1992) Enzyme diversity in pearl millet (Pennisetum glaucum L.) 3. Wild millet. Theor Appl Genet 83:733–742
Tostain S, Marchais L (1989) Enzyme diversity in pearl millet (Pennisetum glaucum). 2. Africa and India. Theor Appl Genet 77:634–640
Tostain S, Riandey MF, Marchais L (1987) Enzyme diversity in pearl millet (Pennisetum glaucum). 1. West Africa. Theor Appl Genet 74:188–193
van Os H, Stam P, Visser RGF, van Eck HJ (2005) RECORD: a novel method for ordering loci on a genetic linkage map. Theor Appl Genet 112:30–40
vom Brocke K, Christinck A, Weltzien ER, Presterl T, Geiger HH (2003) Farmers’ seed systems and management practices determine pearl millet genetic diversity patterns in semi-arid regions of India. Crop Sci 43:1680–1689
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Vuylsteke M, Mank R, Antonise R, Bastiaans E, Senior ML, Stuber CW, Melchinger AE, Lübberstedt T, Xia XC, Stam P, Zabeau M, Kuiper M (1999) Two high-density AFLP linkage maps of Zea mays L.: analysis of distribution of AFLP markers. Theor Appl Genet 99:921–935
Wendel JF, Edwards MD, Stuber CW (1987) Evidence for multilocus genetic control of preferential fertilization in maize. Heredity 58:297–302
Wenzl P, Kudrna D, Jaccoud D, Huttner E, Kleinhofs A, Kilian A (2004) Diversity arrays technology (DArT) for whole genome profiling of barley. Proc Natl Acad Sci USA 101(26):9915–9920
Wenzl P, Li H, Carling J, Zhou M, Raman H, Paul E, Hearnden P, Maier C, Xia L, Caig V, Ovesna J, Cakir M, Poulsen D, Wang J, Raman R, Smith K, Muehlbauer GJ, Chalmers KJ, Kleinhofs A, Huttner E, Killian A (2006) A high-density consensus map of barley linking DArT markers to SSR, RFLP and STS loci and phenotypic traits. BMC Genomics 7:206
White J, Law JR, Mackay I, Chalmers KJ, Smith JSC, Kilian A, Powell W (2008) The genetic diversity of UK, US and Australian cultivars of Triticum aestivum measured by DArT markers and considered by genome. Theo Appl Genet 116:439–453
Wilson WP, Hess DE, Hanna WW, Kumar KA, Gupta SC (2004) Pennisetum glaucum subsp. monodii accessions with Striga resistance in West Africa. Crop Protect 23:865–870
Wittenberg AH, Van der Lee T, Cayla C, Kilian A, Visser RG, Schouten HJ (2005) Validation of the high-throughput marker technology DArT using the model plant Arabidopsis thaliana. Mol Gen Genomics 274:30–39
Xia L, Peng K, Yang S, Wenzl P, Carmen de Vicente M, Fregene M, Kilian A (2005) DArT for high-throughput genotyping of cassava (Manihot esculenta) and its wild relatives. Theor Appl Genet 110:1092–1098
Xu Y, Zhu L, Xiao J, Huang N, McCouch SR (1997) Chromosomal regions associated with segregation distortion of molecular markers in F2, backcross, doubled haploid and recombinant inbred populations of rice (Oryza sativa L.). Mol Gen Genet 253:535–545
Yang S, Pang W, Harper J, Carling J, Wenzl P, Huttner E, Zong X, Kilian A (2006) Low level of genetic diversity in cultivated pigeonpea compared to its wild relatives is revealed by diversity arrays technology. Theor Appl Genet 113:585–595
Acknowledgments
This study was supported by the ‘Generation Challenge Programme’ of the Consultative Group on International Agricultural Research (CGIAR) and by the Department of Biotechnology, Government of India. The authors thank DArT P/L, Australia for providing technical know-how, software, and helpful discussions and the Council of Scientific and Industrial Research (CSIR), India for providing a Junior Research Fellowship to Mr V. Rajaram.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schulman.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Supriya, A., Senthilvel, S., Nepolean, T. et al. Development of a molecular linkage map of pearl millet integrating DArT and SSR markers. Theor Appl Genet 123, 239–250 (2011). https://doi.org/10.1007/s00122-011-1580-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-011-1580-1