Abstract
The gene-pool of wild emmer wheat, Triticum turgidum ssp. dicoccoides, harbors a rich allelic repertoire for disease resistance. In the current study, we made use of tetraploid wheat mapping populations derived from a cross between durum wheat (cv. Langdon) and wild emmer (accession G18-16) to identify and map a new powdery mildew resistance gene derived from wild emmer wheat. Initially, the two parental lines were screened with a collection of 42 isolates of Blumeria graminis f. sp. tritici (Bgt) from Israel and 5 isolates from Switzerland. While G18-16 was resistant to 34 isolates, Langdon was resistant only to 5 isolates and susceptible to 42 isolates. Isolate Bgt#15 was selected to differentiate between the disease reactions of the two genotypes. Segregation ratio of F2-3 and recombinant inbreed line (F7) populations to inoculation with isolate Bgt#15 indicated the role of a single dominant gene in conferring resistance to Bgt#15. This gene, temporarily designated PmG16, was located on the distal region of chromosome arm 7AL. Genetic map of PmG16 region was assembled with 32 simple sequence repeat (SSR), sequence tag site (STS), Diversity array technology (DArT) and cleaved amplified polymorphic sequence (CAPS) markers and assigned to the 7AL physical bin map (7AL-16). Using four DNA markers we established colinearity between the genomic region spanning the PmG16 locus within the distal region of chromosome arm 7AL and the genomic regions on rice chromosome 6 and Brachypodium Bd1. A comparative analysis was carried out between PmG16 and other known Pm genes located on chromosome arm 7AL. The identified PmG16 may facilitate the use of wild alleles for improvement of powdery mildew resistance in elite wheat cultivars via marker-assisted selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum spp.) is a major staple food crop in many parts of the world in terms of cultivated area and food source. To date, with ~620 million tons produced annually worldwide, wheat provides about one-fifth of the calories consumed by humans (FAO stat 2008). Powdery mildew, caused by the biotrophic pathogen Blumeria graminis (DC.) EO Speer f. sp. tritici Em. Marchal (syn. Erysiphe graminis f. sp. tritici), is a foliar disease of wheat, which causes severe yield reduction in areas with cool or maritime climates (e.g., Smith and Smith 1974; Bennett 1984; Hsam and Zeller 2002). In recent years, agro-technical improvements in irrigation and fertilization techniques have increased the risk of disease spreading. Yield losses ranged from 5 to 34% and in severe cases to 45% or more (Griffey et al. 1993; Hsam and Zeller 2002; Yao et al. 2007). Therefore, developing new cultivars with improved powdery mildew resistance is economically and environmentally sound approach to reduce yield losses. This approach, however, requires comprehensive exploration of potential genetic resources and an in-depth understanding of their resistance mechanisms.
It is well established that the genetic diversity of crop plants has been eroded relative to their wild relatives as a result of the genetic bottleneck associated with the domestication process (i.e., founder effect; Ladizinsky 1985) and subsequent modern breeding processes (Tanksley and McCouch 1997; Ladizinsky 1998). This genetic erosion had far-reaching agronomic consequences limiting our ability to protect crop plants from biotic and abiotic stress factors and to meet future global challenges (e.g., Harlan 1972; Zamir 2001). Using crosses between domesticated and wild species of inbreeding plants, alleles that were “left behind” during domestication may be reintroduced into the domesticated gene-pool (McCouch 2004).
Wild emmer wheat [T. turgidum ssp. dicoccoides (Körn.) Thell., also known as T. dicoccoides], is the allo-tetraploid (2n = 4x = 28; BBAA) progenitor of both domesticated tetraploid durum wheat [T. turgidum ssp. durum (Desf.) MacKey] and hexaploid (2n = 6x = 42; BBAADD) bread wheat (T. aestivum L.) (Feldman 2001). Wild emmer wheat germplasm harbors a rich allelic repertoire for improving agronomically important traits (e.g., Feldman and Sears 1981; Nevo et al. 2002; Peleg et al. 2007) including disease resistance (Fahima et al. 1998; Hsam and Zeller 2002). The earliest finding of wild emmer showing symptoms of powdery mildew was reported by Reichert (1940), decades after wild emmer had been discovered by A. Aaronsohn in 1906 (Aaronsohn and Schweinfurh 1906). Later on, the wild emmer gene-pool was shown to contain a particularly promising allelic reservoir for powdery mildew resistance (e.g., Gerechter-Amitai and van Silfhout 1984; Moseman et al. 1984). Sixty-six powdery mildew resistance genes/alleles have been reported so far in 43 loci (Pm1–Pm43) and located onto 18 different chromosomes of hexaploid wheat. Eight of these genes originated from wild emmer germplasm: Pm16 (Reader and Miller 1991), Pm26 (Rong et al. 2000), Pm30 (Liu et al. 2002), Pm36 (Blanco et al. 2008), MlZec1 (Mohler et al. 2005), MlIW72 (Ji et al. 2008), Pm41 (Li et al. 2009) and Pm42 (Hua et al. 2009).
The overall objective of this study was to explore the genetic bases of powdery mildew resistance in wild emmer wheat as a source for improving domesticated wheat. In the current study we report on (1) the identification and genetic mapping of a novel powdery mildew resistance gene, designated PmG16, derived from wild emmer wheat; (2) comparative genetic mapping of PmG16 and other known Pm genes located on the same chromosome; and (3) the establishment of colinearity between the genomic region spanning the PmG16 locus within the distal region of chromosome arm 7AL and the genomic regions on rice chromosome 6 and Brachypodium chromosome Bd1.
Materials and methods
Plant material and powdery mildew isolates
Two mapping populations previously obtained from a cross between T. durum wheat cultivar (cv. Langdon; LDN hereafter) and wild emmer wheat (T. turgidum ssp. dicoccoides accession #G18-16) (Peleg et al. 2008) were used in the current study. The first population consisted of 93 F2-derived F3 families (20 plants for each F3 family). Additionally, a population of 152 F7 RILs was developed from the same original cross by single-seed descent procedure. The two parental lines, LDN and G18-16, were tested for resistance to 47 isolates of powdery mildew (Blumeria graminis f. sp. tritici; Bgt hereafter). Forty-two Bgt isolates were collected from various wheat species (wild and domesticated) or cultivars in various locations across Israel by Dr. N. Eshed and A. Dinoor. In addition, five Bgt isolates were provided by the Federal Research Station for Agriculture, Forschungsanstalt Agroscope Reckenholz-Tänikon, Zürich, Switzerland (Table 1).
Isolate Bgt#15 (collected from durum wheat cultivar Inbar at Yavor, Western Galilee, Israel, E35o35′ N32o47′) clearly differentiated between the reactions of the two parental lines. This isolate was therefore selected for further characterization using a set of differential wheat accessions carrying known Pm resistance genes that were obtained from the National Small Grain Collection (Aberdeen, Idaho, USA) and from Prof. F.J. Zeller (Technische Universität München, Institut für Pflanzenbau und Pflanzenzüchtung, Germany). Phenotypic performance of Bgt#15 was tested on two independent progeny sets, a segregating population of 93 F2- derived F3 families and 152 F7 RILs.
Reactions to powdery mildew
Host seedlings were grown at 21°C, 12 h photoperiod. The tests for mildew resistance were conducted on 10 to 14-day-old primary leaf segments maintained on 6 g/l of agar supplemented with 50 mg/l of benzimidazole in polistyren boxes. Conditions of incubation, inoculation and disease assessment were according to Hsam and Zeller (1997). Inoculum was first produced on leaf segments of the susceptible wheat cv. Chinese Spring and dispersed in a settling tower over the plant material at densities of ~500 spores cm−2. The boxes were incubated at 15°C with 12 h photoperiod of white fluorescent light (photosynthetic photon flux density of 55–65 μmol m−2 s−1). Results were scored about 12 days after inoculation and once again 2–5 days later. Each box included segregating genotypes along with three replicates of a susceptible genotype (cv. Chinese spring) and the two parental lines as controls. The infection types (IT) of powdery mildew were recorded for symptoms on a scale of 0–4, with 0 representing no visible symptoms and with values of 1, 2, 3 and 4 representing highly resistant, resistant, susceptible and highly susceptible reactions, respectively (Mains and Dietz 1930). Score of 0–2 was recorded as resistant and 3–4 as susceptible.
Analysis of SSR and STS markers
PCR reactions of simple sequence repeat (SSR) and sequence tag site (STS) markers were carried out in a 20 μl reaction volume under the following conditions: one denaturation cycle at 94°C for 5 min, followed by 35 cycles of 94°C for 60 s, 50–65°C (depending on the primer) for 60 s, and 72°C for 90 s, followed by an elongation step of 72°C for 7 min. Fragment analysis was carried out on an automated laser fluorescence (ALF) sequencer using the computer program Fragment Analyzer ver. 1.02 (Amersham Biosciences, USA) comparing with internal size standards (Röder et al. 1995). The bread wheat cultivar Chinese Spring was used as a reference in each run to ensure size accuracy and avoid run-to-run and gel-to-gel variations. Genotyping of the SSR and STS markers was also preformed on 1–3% MetaPhor® Agarose (Cambrex, Rockland, ME, USA) stained with GelRedTM (Biotium, Hayward, CA, USA).
Developing CAPS marker XBE442572GCTAGC
Wheat expressed sequence tags (ESTs) and genome-specific primers (GSPs) to chromosome arm 7AL distal region were obtained from the wheat single nucleotide polymorphism (SNP) database (http://wheat.pw.usda.gov/SNP/new/index.shtml). Cleavage amplified polymorphic sequence (CAPS) marker XBE442572 GCTAGC was developed as described by Distelfeld et al. (2006). Comparison of 980 bp BE442572 PCR product of LDN with G18-16 sequence revealed the presence of six SNPs, one of which was used to develop a CAPS marker. Digestion of the amplification product with restriction enzyme NheI yielded fragments of 500 and 480 bp in LDN while leaving the 980-bp G18-16 product uncut (Supplementary material: Fig S1, Table S1). This 7AL locus was designated XBE442572 GCTAGC. The GenBank accession number of this EST provides a unique locus identifier together with the subscript indicating the base pair in the EST that carries the SNP.
Genetic mapping
A genetic linkage map of 2,317 cM was previously developed using the same 152 RIL mapping population based on 197 SSR and 493 DArT markers (Peleg et al. 2008). Chromosome 7A of the above genetic map included 38 markers. In the current study, we added 16 markers to this map in order to increase the marker saturation in the target region. For each segregating marker, a χ 2 analysis was performed to test for deviation from the 1:1 expected segregation ratio in the RIL population. Linkage analysis and map construction were performed based on the evolutionary strategy algorithm included in the MultiPoint package (Mester et al. 2003), as described in Peleg et al. (2008). The wheat EST sequences of STS and CAPS markers were aligned by BLASTN to the rice (Oryza sativa L.) genome sequence (using both http://blast.jcvi.org/euk-blast/index.cgi?project=osa1 and http://rice.plantbiology.msu.edu) and to the Brachypodium distachyon L. genome sequence (JGI 8X Brachy sequence, http://blast.brachybase.org/). In addition, ten of the DArT marker sequences, kindly provided by Diversity Arrays Technology Pty Ltd (Yarralumla, Australia), were compared in silico to search for colinearity with the Brachypodium and rice genomes.
Results
Reactions of the two parental lines to powdery mildew isolates
The reactions of the two parental lines (LDN and G18-16) to a set of 42 Israeli and 5 Swiss powdery mildew isolates are listed in Table 1. The cultivated durum line (LDN) was susceptible to most (37) Israeli Bgt isolates and to the 5 Swiss isolates. The wild emmer accession (G18-16) was resistant to most (29) Israeli Bgt isolates and to the Swiss isolates and susceptible to 13 Israeli isolates (Table 1). The Israeli isolate Bgt#15 was selected for further genetic mapping studies and was used to test a set of Pm differential wheat accessions (Table 2). Bgt#15 was virulent on lines carrying Pm1a, Pm1e, Pm2, Pm3a, Pm3c, Pm3f, Pm3g, Pm4b, Pm5a, Pm5b, Pm6 and Pm7 and avirulent on Pm1b, Pm3b, Pm3d, Pm4a, Pm17 and Pm9 (this last avirulence is based on the susceptibility of the line Normandie, which includes the Pm combination of Pm1a + Pm2 + Pm9). The inoculation of differential lines Pm8 and Pm3e resulted in an intermediate reaction, IT = 2–3 (Table 2).
Inheritance of powdery mildew resistance derived from wild emmer G18-16
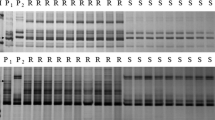
The wild parent (G18-16) was highly resistant to the powdery mildew isolate Bgt#15, whereas LDN was highly susceptible to this isolate (IT scores 0 and 4, respectively; Fig. 1). The phenotypic test of the F2-3 population (93 F2-3 families) showed the following segregation: 17 resistant, 53 segregating and 23 susceptible families. This segregation pattern fits the theoretical expected ratio of 1:2:1 for F2-3 families (Table 3). In the second stage, 152 F7 RILs developed from the original F2 population were inoculated with Bgt#15. The results showed a segregation ratio of 68 resistant and 75 susceptible RILs (9 RILs were excluded due to missing phenotypic data). This segregation pattern fits the expected theoretical ratio of 1:1 for an F7 RIL population (Table 3), thus confirming the involvement of a single dominant gene in conferring resistance to Bgt. The newly identified gene was temporarily designated PmG16.
Genetic and physical mapping of PmG16
To map the PmG16 gene, we used the genetic linkage map of 2,317 cM, previously developed using the 152 RIL population (LDN × G18-16) based on 197 SSR and 493 DArT markers (Peleg et al. 2008). PmG16 was located to the distal end of chromosome arm 7AL. To saturate the map in the PmG16 region, 16 additional markers, all but XBE442572 GCTAGC linked to known Pm genes on 7AL, were analyzed. These included the SSR markers Xwmc809 (Somers et al. 2004), Xcfa2019, Xcfa2040, Xcfa2257, Xcfa2240 (Sourdille et al. 2004), Xbarc275 (Song et al. 2005) and Xgwm1066 (Ganal and Röder 2007), and the STS markers Xsts638 (Hu et al. 1997), XstsBE406627, XstsBE445653 (Perugini et al. 2008), Xmag1705, Xmag1759, Xmag1986, Xmag2185 (Yao et al. 2007) and YP7A (He et al. 2008). A new genetic linkage map was then constructed for chromosome arm 7AL using 46 markers. Markers interfering with map stability were removed and the 7A linkage group was re-analyzed to construct a stabilized map, as recommended by Mester et al. (2003). As a result, a map consisting of 32 SSR, DArT, STS and CAPS markers and the PmG16 gene provided a basic map for calculating recombination frequencies and centiMorgan (cM) distances. Five SSR (Xwmc809, Xcfa2019, Xcfa2040, Xcfa2257 and Xgwm1066), two STS (Xmag2185 and XstsBE445653) and one EST-based markers (XBE442572 GCTAGC ; see Supplementary Material) were added to the final version of the genetic map presented in the current study. The PmG16 gene was genetically mapped to a 3.6 cM interval between the markers Xgwm344 and wPt-9217 (Fig. 2). The two DArT markers, wPt-1424 and wPt-6019, were located on the same map interval.
Genetic map of chromosome arm 7AL of wheat containing powdery mildew resistance gene PmG16 and anchored markers for the physical map of the chromosome arm 7AL and sequence of Brachypodium chromosome Bd1 and rice chromosome 6. a Genetic map of the PmG16 gene region on wheat chromosome arm 7AL. Markers are shown on the left with map distances on the right. Molecular markers that were previously assigned to the 7A wheat deletion bin map (b) are connected to the physical map with dashed lines. The PmG16 locus is framed with a black line. The four markers which served as anchors, establishing colinearity between the PmG16 genetic map and the sequences of Brachypodium and rice, are underlined. c The homologous regions on Brachypodium Bd1 are marked with gray bars. Approximate locations (bp) along Bd1 sequence are shown on the left. The round dot line linking the Xmag2185 anchor marker to the Brachypodium Bd1 indicates intermediate homology values (E value = 2.00E-12) to the Brachypodium gene Bradi1g29670.1. The dashed dot line connects this gene to the rice chromosome 6 BAC AP003621 sequence through homology to the rice locus LOC_Os06g49390. d The homologous regions on rice chromosome 6 BACs are marked with white bars (BAC No. included). AP00575, AP006616 and AP006854 are BACs representing the telomeric region of rice chromosome arm 6L. In both c and d the colinear genomic regions spanning the PmG16 locus between markers Xmag2185 and XstsBE4456531, 114 kb in Brachypodium and 1.7 Mb in rice, are marked with brace. In both c and d the length of the bars is not drawn to scale
Several markers that mapped in the PmG16 region were previously linked to the physical deletion bin map of chromosome arm 7AL (Fig. 2). Markers Xgwm282, Xgwm332, Xcfa2019 and Xcfa2040, located proximally to PmG16, were previously assigned to chromosome deletion bin 7AL-16 0.86-0.90 (Sourdille et al. 2004). The marker Xcfa2257, mapped distally to PmG16, is the only marker which was physically mapped to a proximal bin (7AL-1 0.39-0.71) (Sourdille et al. 2004). The EST-based marker XBE442572 GCTAGC served as an additional anchor to the physical deletion bin map of wheat and was assigned to chromosome bin 7AL-21 074-0.86 (http://wheat.pw.usda.gov/SNP/new/index.shtml; Fig. 2).
Analysis of DArT marker sequences revealed two incidences of high intra similarity between markers mapped by Peleg et al. (2008) to the same interval. High sequence identity (>92%) was found between wPt-1424, wPt-1023 and wPt-1259 and also between wPt-1976 and wPt-3403. Therefore, wPt-1023, wPt-1259 and wPt-3403, which were included in the genetic map presented by Peleg et al. (2008), were omitted from the current genetic map.
Comparison of PmG16 with other Pm genes on 7AL
The PmG16 gene was compared to previously published Pm genes assigned to 7AL (Fig. 3). Five dominant alleles of Pm1 were identified for this gene in T. aestivum (Pm1a and Pm1e), T. monococcum (Pm1b and Pm1c) and T. aestivum var. spelta (Pm1d) (Hsam et al. 1998; Singrün et al. 2003). Phenotypic test of a differential line carrying the Pm1a allele with Bgt#15 showed a susceptible reaction, thus differentiating PmG16 from Pm1a (Table 2). The SSR marker Xgwm344, previously reported to be closely linked to Pm1c and Pm1e alleles (0.5 and 0.9 cM, respectively; Singrün et al. 2003; Stepien et al. 2004) was found here to be 1.2 cM proximal to PmG16 (Figs. 2, 3). The STS marker Xsts638 co-segregated with the Pm1 locus (Hu et al. 1997) in addition to XstsBE406627 and XstsBE445653, which were found to be closely linked to Pm1 (Perugini et al. 2008). While the former two showed no polymorphism between the parental lines used in the current study (LDN and G18-16) the latter was mapped 21.8 cM distally from the PmG16 on chromosome arm 7AL (Figs. 2, 3).
Comparative view of the PmG16 linkage map with other previously published Pm loci on chromosome arm 7AL. Map position of Pm1a is according to Neu et al. (2002), map position of Pm1e is according to Singrün et al. (2003), map position of MlIW72 is according to Ji et al. (2008), map position of Pm37 is according to Perugini et al. (2008), map position of MIm2033 and MIm80 is according to Yao et al. (2007), map position of MIAG12 is according to Maxwell et al. (2009), map position of NCAG11 is according to Srnić et al. (2005) and map position of NCA6 is according to Miranda et al. (2007). Markers are shown on the right with map distances on the left. Common markers are connected with dotted lines
Four other Pm genes, Mlm2033, Mlm80 (Yao et al. 2007), MlIW72 (Ji et al. 2008) and MlAG12 (Maxwell et al. 2009), were located in the Pm1 genomic region. The two genes, Mlm2033 and Mlm80, derived from T. monococcum, were mapped to the Xcfa2040–Xmag2185 genetic interval (Figs. 2, 3). In addition, MlIW72 gene derived from T. dicoccoides was mapped to the same genomic interval (Ji et al. 2008) as Mlm2033 and Mlm80. In the current study, PmG16 was located in a more distal interval defined by markers Xgwm344–Xcfa2257 (Figs. 2, 3). Three other EST-based STS markers (Xmag1705, Xmag1759 and Xmag1986) located in the same region (Yao et al. 2007) could not be mapped onto the current LDN X G18-16 map due to lack of polymorphism between the parental lines.
Several other Pm genes were also reported in the 7AL region, including two recessive Pm genes (Pm9, mlRD30), three genes derived from T. monococcum (NCAG11 and NCA4, Srnić et al. 2005; and NCA6, Miranda et al. 2007) and one form T. timopheevii (Pm37, Perugini et al. 2008). These four dominant genes were mapped to a proximal segment relative to the PmG16 gene (Fig. 3).
Colinearity between PmG16 region and Brachypodium and rice genomes
CAPS marker XBE442572 GCTAGC and STS marker XstsBE445653 were mapped proximally and distally to the PmG16 gene, respectively. XBE442572 GCTAGC showed homology to Brachypodium Bd1 at physical position 28,892,870 bp (E value = 3.00E-40) and rice BAC AP003935 (E value = 1.30E-26) on chromosome 6. XstsBE445653 showed homology to Brachypodium Bd1 at physical position 25,086,137 bp (E value = 1.00E-43) and to rice BAC AP005750 (E value = 2.9E-22) (Fig. 2). Two markers located in the PmG16–XBE442572 GCTAGC interval, the STS marker Xmag2185 and the DArT marker wPt-1976, served as additional anchors for comparative genomics. The sequence of STS marker Xmag2185, located 6.6 cM from PmG16, showed homology to Brachypodium Bd1 gene Bradi1g29670.1 (genomic location 25,200,121 bp, E value = 2.00E-12). The Bradi1g29670.1 sequence showed high homology to rice BAC AP003621 (E value = 2.80E-113) on chromosome 6 and therefore established a third anchor, hence confirming the wheat–Brachypodium–rice colinearity in this genomic region (Fig. 2). The Bradi1g29670.1 sequence (5.6 kb long) showed also high homology to sequences located on other rice chromosomes, such as chromosomes 2, 5 and 1 (E value < 2.80E-135).
wPt-1976 showed homology to Brachypodium Bd1 at position 24,922,411 bp (E value = 7.00E-28), breaking the synteny along Brachypodium Bd1 and wheat chromosome arm 7AL. Furthermore, wPt-1976 also showed homology to rice chromosome 11 (E value = 1.7E-25) and moderate homology to rice chromosome 4 (E value = 3.4E-15) and to the proximal region of chromosome 6 (E value = 1.2E-13), thereby disrupting the colinearity between wheat chromosome arm 7AL and rice chromosome arm 6L (Fig. 2). The marker wPt-0494 showed only low homology to Brachypodium Bd1 and Bd4 (E value > 3.00E-09) and no homology to rice BAC sequences.
Discussion
Characterization and chromosomal location of PmG16
In the current study, a single dominant powdery mildew resistance gene derived from wild emmer was identified using the progeny of a cross between domesticated durum wheat and its wild progenitor. Isolate Bgt#15 was found virulent on a wide range of Pm genes (Table 2) and was previously reported as highly virulent on a large collection of domesticated and wild wheat germplasm (71% of the accessions were susceptible, Ben-David et al. 2008). The phenotypic reaction of the RIL population to this isolate was mapped to genomic region on wheat chromosome arm 7AL and was temporarily designated PmG16. Phenotypic response to Bgt#15 isolate at the adult plant stage of the two parental lines showed similar pattern of resistance in the wild parent G18-16 (Fig. 1) as found at the seedling stage.
Genome mapping of all 14 chromosomes of tetraploid wheat located the PmG16 gene to the long arm of chromosome 7A. Further molecular zooming on this genomic region indicated that PmG16 is located in a 3.6 cM interval between Xgwm344 and wPt-0494 (Fig. 2). Additionally, two DArT markers (wPt-1424 and wPt-6019) co-segregated with PmG16 on the same map interval. Previously mapped markers enabled us to assign PmG16 to wheat chromosome deletion bin 7AL-16 0.86-0.90. Eight SSR and CAPS markers that physically mapped to chromosome arm 7AL were found to be linked to PmG16 and served as anchors to the 7AL deletion bin map (Fig. 2). Notably, based on the markers Xcfa2040 and Xcfa2019, the more distal marker Xcfa2257, assigned to chromosome bin 7AL-1 by Sourdille et al. (2004), was genetically mapped in the current study to a more distal chromosome interval, which corresponds to chromosome bin 7AL-16 (Fig. 2). Such discrepancies between genetic and physical maps have been previously reported (Sourdille et al. 2004). Nevertheless, the map order found in the current study is highly similar to previously published wheat maps (e.g. Somers et al. 2004; Ganal and Röder 2007) and linkage mapping of chromosome arm 7AL (e.g. Yao et al. 2007; Ji et al. 2008; Perugini et al. 2008).
Remarkable clustering of DArT markers was reported in gene-rich telomeric regions (e.g., Peleg et al. 2008; Akbari et al. 2006). In the current study, we noted high similarity between some of the DArT sequences that were mapped to the same cluster of markers on chromosome arm 7AL. These results may suggest that the observed clusters of DArT markers found by Peleg et al. (2008) are actually an outcome of a duplication of these DArT sequences during the process of marker development.
Colinearity of PmG16 gene region to rice and Brachypodium
Four markers, XBE442572 GCTAGC , wPt-1976, Xmag2185 and XstsBE445653, served as anchors between the PmG16 region on chromosome arm 7AL and Brachypodium Bd1 chromosome. In addition, three out of these four markers (excluding wPt-1976) established colinearity to rice chromosome arm 6L. The two markers, Xmag2185 and XstsBE445653, spanning the PmG16 region in a 28.4 cM interval established colinearity with the currently available draft (8× depth) of Brachypodium genome on chromosome Bd1 and to the distal end of rice chromosome arm 6L. The physical stretch between those two markers in Brachypodium and rice includes 114 kb and 1.7 Mb (comprised of 10 rice BAC clones), respectively.
Some discrepancies in the colinearity between wheat and Brachypodium have been previously reported (e.g., Huo et al. 2009) and our data suggests that marker wPt-1976 is creating such macro colinearity disruption (Fig. 2). Still the results obtained in the current study demonstrate the utilization of this relatively new tool of Brachypodium genome sequence for the fine mapping of wheat genes.
The firm colinearity found near the PmG16 region of wheat and rice is in accordance with other studies. Yao et al. (2007) mapped two powdery mildew resistance genes (Mlm80 and Mlm2033) on chromosome arm 7AL (interval Xmag2185–Xcfa2040) of diploid T. monococcum (genome AA) and found a distal anchor marker (Xmag1986) to BAC clone (AP006616), adjacent to the current distal anchor (XstsBE445653), on the annotated rice sequence. Quarrie et al. (2006) found high level of colinearity between wheat ESTs assigned to chromosome deletion bin 7AL-16 0.86-0.90 and rice chromosome arm 6L, which was also in agreement with the results of Hossain et al. (2004).
The accumulated data on rice genome suggest that it has a limited benefit for map-based cloning of R-genes in cereals because of nonsyntenic map locations of R-genes between cereal species (Leister et al. 1998; Quraishi et al. 2009). Similar situation is probably true also for the use of Brachypodium genome as a model for positional cloning of R-genes in other cereals. Bossolini et al. (2007) found only partial colinearity between genes in the Lr34 genomic region and the Brachypodium genomic sequence. Nevertheless, it seems that Brachypodium and rice genomes will continue to serve as models for genomic studies in large genomes, such as wheat and barley, until these genomes are fully sequenced. In the case of R-genes, even if the target genes are missing from the orthologous position in rice or Brachypodium, the flanking colinear genes can serve as potential markers in map-based cloning efforts of wheat and barley disease resistance genes (Quraishi et al. 2009).
Comparison of PmG16 with other Pm genes on 7AL
Genomic regions located near telomeres are hot spots for chromosome evolution and recombination and are therefore associated with the emergence of novel genes and gene duplication (See et al. 2006; Akhunov et al. 2003). These processes may result in the formation of R-gene clusters. R loci may represent single genes with multiple alleles (Shepherd and Mayo 1972; e.g., Pm1 locus with six known alleles; Hsam et al. 1998; Singrün et al. 2003) and on the other hand different genes within a single cluster can confer resistance to different pathogens (Michelmore and Meyers 1998).
Furthermore, the reaction to another powdery mildew isolate (Bgt#70, Table 1) was mapped to the same genetic interval (Xgwm344–wPt-0494) as PmG16 gene (data not shown). This resistance may be conferred by the same locus/allele or even by a different locus of powdery mildew resistance situated in the same gene cluster. Several other R-genes, including resistance to powdery mildew (Pm1, Pm9 and mlRD30; Schneider et al. 1991; Singrün et al. 2004), leaf rust (Lr20; Sears and Briggle 1969), stem rust (Sr15; Hu et al. 1997), Fusarium head blight (Shen and Ohm 2007) and common bunt (Fofana et al. 2008) were mapped to the same chromosome region as PmG16.
The PmG16 gene was compared to previously published Pm genes on 7AL (Fig. 3). The Pm1 locus was identified in the early 1950s (Pugsley and Carter 1953) and later on was located to chromosome arm 7AL (Sears and Briggle 1969). Our temporarily designated gene PmG16 was genetically mapped to the same map interval as the Pm1 locus (Fig. 3) and was distinguished phenotypically only from Pm1a allele based on allelic tests (Table 2). Recently, MlAG12 gene derived from T. timopheevii was mapped to Xwmc809–Xmag2185 region (Maxwell et al. 2009), a congruent genetic interval relative to the PmG16 gene. Bearing this in mind, and although PmG16 was mapped to a different interval than Mlm2033, Mlm80 and MlIW72, additional studies are necessary to clearly differentiate between these four powdery mildew resistance genes and the new PmG16 gene. Such additional studies could include testing the different resistant wheat genotypes with different sources of Bgt inoculums [e.g., T. monococcum accession TA2003 showed resistant response to Bgt#15 (Table 2)]. Moreover allelic tests of the Pm gene cluster located on 7AL could resolve the situation by implementing a more precise approach and determine the number of different genes or alleles of the same gene that are involved. However, the currently available list of differential lines covers only part of the gene\allelic variation in this gene cluster and therefore enabling only partial resolution of this problem. A more complete answer could be obtained through implementation of the map-based cloning approach (Lin et al. 2007) of one or more of the genes comprising this cluster.
The isolation of the fungal disease resistance genes Lr10 (Feuillet et al. 2003), Lr21 (Li et al. 2009) and Pm3b (Yahiaoui et al. 2004) and the recent cloning of Yr36 and Lr34 has shown that map-based cloning is now within reach, even in hexaploid wheat (Feuillet and Keller 2005; Fu et al. 2009; Krattinger et al. 2009). The advances in development of new genomic tools for wheat studies, together with the construction of the physical map of chromosome 7A, that is under way (http://www.wheatgenome.org/index.php), are making such an approach even more feasible. The currently ongoing map-based cloning project of MlIW72 on the distal end of 7AL is the first step towards the exploration of such a genomics-based approach (Liu et al. 2010).
Conclusions and implementations for wheat improvement
The continuous increase in global human population poses huge challenges to world agriculture. Developing cultivars with good resistance to powdery mildew is the most economical and effective approach to reduce fungicide application and minimize grain-yield losses. The notion of using the wild emmer gene resources for wheat improvement has been repeatedly advocated since the discovery of the wild progenitor of cultivated wheats about a century ago (Aaronsohn 1910). In the current study, a cross between domesticated and wild wheat was used to map a newly discovered powdery mildew resistance gene. This single dominant gene, designated as PmG16, was derived from wild emmer wheat and mapped to chromosome arm 7AL. The colinearity between PmG16 genomic region and the rice and Brachypodium genomes will advance future fine mapping and cloning of PmG16 gene.
The resistance of the wild parent (G18-16) to a wide collection of Bgt isolates (Table 1) and the mapping of resistance to Bgt#70 to the same genetic interval as PmG16 suggest a significant potential for further wheat breeding. The dense genetic map of PmG16 region presented in the current study can promote its utilization via marker-assisted selection (MAS) in wheat breeding programs. Furthermore, simultaneous transfer of large DNA fragments containing multiple R-genes could act synergistically (Rommens and Kishore 2000). Therefore, transposing the tightly linked R-genes cluster found near the PmG16 region could enhance the durability of PmG16 resistance. Alternatively, the use of molecular markers for pyramiding of R-genes can allow the combination of several single R-genes together to increase resistance durability (Feuillet and Keller 2005).
References
Aaronsohn A (1910) Agricultural and botanical exploration in Palestine. Bulletin Plant Industry, US Department of Agriculture, Washington, 180:1–63
Aaronsohn A, Schweinfurh G (1906) Die Auffindung des wilden Emmers (Triticum dicoccum) in Nordpalästina. Altneuland 3:213–220
Akbari M, Wenzl P, Caig V, Carling J, Xia L, Yang S, Uszynski G, Mohler V, Lehmensiek A, Kuchel H, Hayden MJ, Howes N, Sharp P, Vaughan P, Rathmell B, Huttner E, Kilian A (2006) Diversity arrays technology (DArT) for high throughput profiling of hexaploid wheat genome. Theor Appl Genet 113:1409–1420
Akhunov ED, Goodyear AW, Geng S, Qi LL, Echalier B, Gill BS, Miftahudin, Gustafson JP, Lazo G, Chao S et al (2003) The organization and rate of evolution of wheat genomes are correlated with recombination rates along chromosome arms. Genome Res 13:753–763
Ben-David R, Peleg Z, Xie W, Saranga Y, Dinoor A, Korol AB, Fahima T (2008) Dissection of powdery mildew resistance uncover different resistance types in the Triticum turgidum L. gene pool. In: Appels R, Eastwood R, Lagudah E, Langridge P, Mackay M, McIntyre L, Sharp P (eds) Proceedings of the 11th international wheat genetics symposium. Sydney University Press, Sydney. Available at http://wheat.pw.usda.gov/GG2/Triticum/events/11IWGS/
Bennett FGA (1984) Resistance to powdery mildew in wheat: a review of its use in agriculture and breeding programmes. Plant Pathol 33:279–300
Blanco A, Gadaleta A, Cenci A, Carluccio AV, Abdelbacki AMM, Simeone R (2008) Molecular mapping of the novel powdery mildew resistance gene Pm36 introgressed from Triticum turgidum var. dicoccoides in durum wheat. Theor Appl Genet 117:135–142
Bossolini E, Wicker T, Knober PA, Keller B (2007) Comparison of orthologous loci from small grass genomes Brachypodium and rice: implications from wheat genomics and grass genome annotation. Plant J 49:704–717
Distelfeld A, Uauy C, Fahima T, Dubcovsky J (2006) Physical map of the wheat high-grain protein content gene Gpc-B1 and development of a high-throughput molecular marker. New Phytol 169:753–763
Fahima T, Röder M, Grama A, Nevo E (1998) Microsatellite DNA polymorphism divergence in Triticum dicoccoides accessions highly resistant to yellow rust. Theor Appl Genet 96:187–195
FAOstat (2008) Food and Agriculture Organization of the United Nations. Available at http://faostat.fao.org
Feldman M (2001) The origin of cultivated wheat. In: Benjean AP, Angus J (eds) The wheat book: a history of wheat breeding. Lavoisier Publishing, Paris, pp 3–56
Feldman M, Sears ER (1981) The wild gene resources of wheat. Sci Am 244:102–112
Feuillet C, Keller B (2005) Molecular markers for disease resistance: the example wheat. In: Nagata T, Lörz H, Widholm J, Lörz H, Wenzel G (eds) Molecular marker systems in plant breeding and crop improvement, vol 55. Genomics Applications in crops. Springer, Berlin, pp 353–370
Feuillet C, Travella S, Stein N, Albar L, Nublat A, Keller B (2003) Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploid wheat (Triticum aestivum L.) genome. Proc Natl Acad Sci USA 100:15253–15258
Fofana B, Humphreys DG, Cloutier S, McCartney CA, Somers DJ (2008) Mapping quantitative trait loci controlling common bunt resistance in a doubled haploid population derived from the spring wheat cross RL4452 × AC Domain. Mol Breed 21:317–325
Fu D, Uauy C, Distelfeld A, Blechl A, Epstein L, Chen X, Sela H, Fahima T, Dubcovsky J (2009) A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 323:1357–1360
Ganal MW, Röder MS (2007) Microsatellite and SNP markers in wheat breeding. In: Varshney RK, Tuberosa R (eds) Genomics-assisted crop improvement, vol 2. Genomics applications in crops. Springer, Berlin, pp 1–24
Gerechter-Amitai Z, van Silfhout C (1984) Resistance to powdery mildew in wild emmer (Triticum dicoccoides Körn.). Euphytica 33:273–280
Griffey C, Das M, Stromberg E (1993) Effectiveness of adult-plant resistance in reducing grain yield loss to powdery mildew in winter wheat. Plant Dis 77:618–622
Harlan JR (1972) Genetics of disaster. J Environ Qual 1:212–215
He XY, Zhang YL, He ZH, Wu YP, Xiao YG, Ma CX, Xia XC (2008) Characterization of phytoene synthase 1 gene (Psy1) located on common wheat chromosome 7A and development of a functional marker. Theor Appl Genet 116:213–221
Hossain K, Kalavacharla V, Lazo G, Hegstad J, Wentz M, Kianian P, Simons K, Gehlhar S, Rust J, Syamala R et al (2004) A chromosome bin map of 2148 expressed sequence tag loci of wheat homoeologous group 7. Genetics 168:687–699
Hsam SLK, Zeller FJ (1997) Evidence of allelism between genes Pm8 and Pm17 and chromosomal location of powdery mildew and leaf rust resistance genes in the common wheat cultivar ‘Amigo’. Plant Breed 116:119–122
Hsam SLK, Zeller FJ (2002) Breeding for powdery mildew resistance in common wheat (Triticum aestivum L.). In: Belanger RR, Bushnell WR, Dik AJ, Carver TLW (eds) The powdery mildews, a comprehensive treatise. St. Paul, MN, USA, pp 219–238
Hsam SLK, Huang XQ, Ernst F, Hartl L, Zeller FJ (1998) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.). 5. Alleles at the Pm1 locus. Theor Appl Genet 96:1129–1134
Hu X, Ohm H, Dweikat I (1997) Identification of RAPD markers linked to the gene Pm1 for resistance to powdery mildew in wheat. Theor Appl Genet 94:832–840
Hua W, Liu Z, Zhu J, Xie C, Yang T, Zhou Y, Duan X, Sun Q, Liu Z (2009) Identification and genetic mapping of Pm42, a new recessive wheat powdery mildew resistance gene derived from wild emmer (Triticum turgidum var. dicoccoides). Theor Appl Genet 119:223–230
Huo N, Vogel JP, Lazo GR, You FM, Ma Y, McMahon S, Dvorak J, Anderson OD, Luo MC, Gu YQ (2009) Structural characterization of Brachypodium genome and its syntenic relationship with rice and wheat. Plant Mol Biol 70(1–2):47–61
Ji X, Xie C, Ni Z, Yang T, Nevo E, Fahima T, Liu Z, Sun Q (2008) Identification and genetic mapping of a powdery mildew resistance gene in wild emmer (Triticum dicoccoides) accession IW72 from Israel. Euphytica 159:385–390
Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL, Keller B (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323:1360–1363
Ladizinsky G (1985) Founder effect in crop-plant evolution. Econ Bot 39:191–199
Ladizinsky G (1998) Plant evolution under domestication. Kluwer Academic Publishers, Dordrecht, Netherlands, pp 174–176
Leister D, Kurth J, Laurie DA, Yano M, Sasaki T, Devos K, Graner A, Schulze-Lefert P (1998) Rapid reorganization of resistance gene homologues in cereal genomes. Proc Natl Acad Sci USA 95:370–375
Li G, Fang T, Zhang H, Xie C, Li H, Yang T, Nevo E, Fahima T, Sun Q, Liu Z (2009) Molecular identification of a new powdery mildew resistance gene Pm41 on chromosome 3BL derived from wild emmer (Triticum turgidum var. dicoccoides). Theor Appl Genet 119:531–539
Lin FS, Chen Z, Que L, Wang X, Liu X, Pan Q (2007) The blast resistance gene Pi37 encodes an NBS-LRR protein and is a member of a resistance gene cluster on rice chromosome 1. Genetics 177:1871–1880
Liu Z, Sun Q, Ni Z, Nevo E, Yang T (2002) Molecular characterization of a novel powdery mildew resistance gene Pm30 in wheat originating from wild emmer. Euphytica 123:21–29
Liu Z, Zhu J, Liu Z, Cui Y, Wu H, Li S, Ouyang S, Zhao X, Xie C, Yang T, Sun Q (2010) Towards fine genetic mapping and cloning of wheat disease resistance genes by comparative genomics approach plant and animal genome XVIII conference. San Diego, California, 9–13 January, 2010, USA
Mains E, Dietz S (1930) Physiologic forms of barley mildew, Erysiphe graminis hordei Marchal. Phytopathology 20:229–239
Maxwell JJ, Lyerly JH, Cowger C, Marshall D, Brown-Guedira G, Murphy JP (2009) MlAG12: a Triticum timopheevii-derived powdery mildew resistance gene in common wheat on chromosome 7AL. Theor Appl Genet 117:103–115
McCouch SR (2004) Diversifying selection in plant breeding. PLoS Biol 2(10):e347:1507–1512
Mester D, Ronin Y, Minkov D, Nevo E, Korol AB (2003) Constructing large-scale genetic maps using an evolutionary strategy algorithm. Genetics 165:2269–2282
Michelmore RW, Meyers BC (1998) Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res 8:1113–1130
Miranda L, Perugini L, Srnic G, Brown-Guedira G, Marshall D, Leath S, Murphy J (2007) Genetic mapping of a Triticum monococcum derived powdery mildew resistance gene in common wheat. Crop Sci 47:2323
Mohler V, Zeller FJ, Wenzel G, Hsam SLK (2005) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell). 9. Gene MIZec1 from Triticum dicoccoides-derived wheat line Zecoi-1. Euphytica 142:161–167
Moseman J, Nevo E, Morshidy MAEL, Zohary D (1984) Resistance of Triticum dicoccoides to infection with Erysiphe graminis tritici. Euphytica 33:41–47
Neu C, Stein N, Keller B (2002) Genetic mapping of the Lr20-Pm1 resistance locus reveals suppressed recombination on chromosome arm 7AL in hexaploid wheat. Genome 45:737–744
Nevo E, Korol AB, Beiles A, Fahima T (2002) Evolution of wild emmer and wheat improvement: population genetics, genetic resources, and genome organization of wheats progenitor, Triticum dicoccoides. Springer, Berlin
Peleg Z, Fahima T, Saranga Y (2007) Drought resistance in wild emmer wheat: physiology, ecology and genetics. Isr J Plant Sci 55:289–297
Peleg Z, Saranga Y, Suprunova T, Ronin Y, Röder MS, Kilian A, Korol AB, Fahima T (2008) High-density genetic map of durum wheat × wild emmer wheat based on SSR and DArT markers. Theor Appl Genet 117:103–115
Perugini LD, Murphy JP, Marshall D, Brown-Guedira G (2008) Pm37, a new broadly effective powdery mildew resistance gene from Triticum timopheevii. Theor Appl Genet 116:417–425
Pugsley AT, Carter MV (1953) The resistance of twelve varieties of Triticum vulgare to Erysiphe graminis tritici. Aust J Biol Sci 6:335–346
Quarrie S, Pekic Quarrie S, Radosevic R, Rancic D, Kaminska A, Barnes J, Leverington M, Ceoloni C, Dodig D (2006) Dissecting a wheat QTL for yield present in a range of environments: from the QTL to candidate genes. J Exp Bot 57:2627–2637
Quraishi UM, Abrouk M, Bolot S, Pont C, Throude M, Guilhot N, Confolent C, Bortolini F, Praud S, Murigneux A, Charmet G, Salse J (2009) Comparative genomics in cereals: from genome-wide conserved orthologous set (COS) sequences to candidate genes for trait dissection. Funct Integr Genomics 9:473–484
Reader SA, Miller T (1991) The introduction into bread wheat of a major gene for resistance to powdery mildew from wild emmer wheat. Euphytica 53:57–60
Reichert I (1940) On the disease resistance of wild emmer. Pal J Bot 3:179–183
Röder MS, Plaschke J, Konig SU, Börner A, Sorrells ME, Tanksley SD, Ganal MW (1995) Abundance, variability and chromosomal location of microsatellites in wheat. Mol Genet Genom 246:327–333
Rommens CM, Kishore GM (2000) Exploiting the full potential of disease-resistance genes for agricultural use. Curr Opin Biotechnol 11:120–125
Rong J, Millet E, Manisterski J, Feldman M (2000) A new powdery mildew resistance gene: introgression from wild emmer into common wheat and RFLP-based mapping. Euphytica 115:121–126
Schneider DM, Heun M, Fischbeck G (1991) Inheritance of the powdery mildew resistance gene Pm9 in relation to Pm1 and Pm2 of wheat. Plant Breed 107:161–164
Sears E, Briggle L (1969) Mapping the gene Pml for resistance to Erysiphe graminis f. sp. tritici on chromosome 7A of wheat. Crop Sci 9:96–97
See DR, Brooks S, Nelson JC, Brown-Guedira G, Friebe B, Gill BS (2006) Gene evolution at the ends of wheat chromosomes. Proc Natl Acad Sci USA 103:4162–4167
Shen X, Ohm H (2007) Molecular mapping of Thinopyrum-derived Fusarium head blight resistance in common wheat. Mol Breed 20:131–140
Shepherd KW, Mayo GME (1972) Genes conferring specific plant disease resistance. Science 175:375–380
Singrün C, Hsam SLK, Hartl L, Zeller FJ, Mohler V (2003) Powdery mildew resistance gene Pm22 in cultivar Virest is a member of the complex Pm1 locus in common wheat (Triticum aestivum L. em Thell.). Theor Appl Genet 106:1420–1424
Singrün C, Hsam SLK, Zeller FJ, Wenzel G, Mohler V (2004) Localization of a novel recessive powdery mildew resistance gene from common wheat line RD30 in the terminal region of chromosome 7AL. Theor Appl Genet 109:210–214
Smith H, Smith M (1974) Surveys of powdery mildew in wheat and an estimate of national yield losses. NZ J Exp Agric 2:441–445
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Song QJ, Shi JR, Singh S, Fickus EW, Costa JM, Lewis J, Gill BS, Ward R, Cregan PB (2005) Development and mapping of microsatellite (SSR) markers in wheat. Theor Appl Genet 110:550–560
Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, Gill BS, Dufour P, Murigneux A, Bernard M (2004) Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Funct Integr Genomics 4:12–25
Srnić G, Murphy J, Lyerly J, Leath S, Marshall D (2005) Inheritance and chromosomal assignment of powdery mildew resistance genes in two winter wheat germplasm lines. Crop Sci 45:1578–1586
Stepien L, Chelkowski J, Wenzel G, Mohler V (2004) Combined use of linked markers for genotyping the Pm1 locus in common wheat. Cell Mol Biol Lett 9:819–827
Tanksley SD, McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Yahiaoui N, Srichumpa P, Dudler R, Keller B (2004) Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J 37:528–538
Yao G, Zhang J, Yang L, Xu H, Jiang Y, Xiong L, Zhang C, Zhang Z, Ma Z, Sorrells ME (2007) Genetic mapping of two powdery mildew resistance genes in einkorn (Triticum monococcum L.) accessions. Theor Appl Genet 114:351–358
Zamir D (2001) Improving plant breeding with exotic genetic libraries. Nat Rev Genet 2:983–989
Acknowledgments
This work was supported by The Israel Science Foundation grants # 608/03 and 1089/04 and equipment grants #048/99, #1478/04 and #1719/08. The authors thank A. Fahum, M. Chatzav and U. Uner for their excellent technical assistance. The authors thank B. Keller for providing five Swiss Bgt isolates. Z. Peleg is indebted to the Israel Council for Higher Education postdoctoral fellowships award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Ordon.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ben-David, R., Xie, W., Peleg, Z. et al. Identification and mapping of PmG16, a powdery mildew resistance gene derived from wild emmer wheat. Theor Appl Genet 121, 499–510 (2010). https://doi.org/10.1007/s00122-010-1326-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-010-1326-5