Abstract
Low-molecular-weight glutenin subunit (LMW-GS) Glu-B3 has a significant influence on the processing quality of the end-use products of common wheat. To characterize the LMW-GS genes at the Glu-B3 locus, gene-specific PCR primers were designed to amplify eight near-isogenic lines and Cheyenne with different Glu-B3 alleles (a, b, c, d, e, f, g, h and i) defined by protein electrophoretic mobility. The complete coding regions of four Glu-B3 genes with complete coding sequence were obtained and designated as GluB3-1, GluB3-2, GluB3-3 and GluB3-4. Ten allele-specific PCR markers designed from the SNPs present in the sequenced variants discriminated the Glu-B3 proteins of electrophoretic mobility alleles a, b, c, d, e, f, g, h and i. These markers were validated on 161 wheat varieties and advanced lines with different Glu-B3 alleles, thus confirming that the markers can be used in marker-assisted breeding for wheat grain processing quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gluten, the most important storage protein in the endosperm of common wheat (Triticum aestivum L.), comprises glutenins and gliadins (Lindsay and Skerritt 1999; Shewry and Halford 2002). Glutenins are separated into high-molecular-weight glutenin subunits (HMW-GS) and low-molecular-weight glutenin subunits (LMW-GS) according to their mobilities in sodium-dodecyl-sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (Bietz et al. 1975; Payne and Corfield 1979). These proteins are held together by disulphide bonds to form gluten macropolymers (Gras et al. 2001), contribute to fundamental aspects of dough quality such as viscoelasticity and extensibility, and consequently influence the end-use products of wheat (Payne 1987; Luo et al. 2001). The identification of specific HMW-GS and LMW-GS alleles is, therefore, an important target in improving wheat quality (Gupta et al. 1999; Eagles et al. 2001; Gale 2005).
Allelic variation of the HMW-GS and its relationship with processing quality have been studied extensively, and PCR-based DNA markers are available to discriminate the important Glu-1 alleles Ax2*, Bx7, Bx7*, Bx17, By8, By9 and Dx5 (Ma et al. 2003; Butow et al. 2004; Gale 2005; Lei et al. 2006). Compared with HMW-GS, the extensive allelic variations of LMW-GS and their overlapping mobilities with the more abundant gliadin proteins (Singh and Shepherd 1988) make it difficult to discriminate the roles of individual LMW-GS in wheat quality. Gupta and Shepherd (1990) carried out an extensive survey of LMW glutenin proteins in common wheat cultivars by SDS-PAGE and detected 20 banding patterns. Subsequently, six protein alleles were found for the Glu-A3 locus (a, b, c, d, e, f), nine for the Glu-B3 (a, b, c, d, e, f, g, h, i) and five for the Glu-D3 (a, b, c, d, e). With respect to effects on dough quality, various Glu-3 alleles were ranked for R max (maximum dough resistance, an indicator of dough strength), and the rankings of alleles were b > d > e > c at the Glu-A3 locus, i > b = a > e = f = g = h > c at the Glu-B3 and e > b > a > c > d at the Glu-D3 (Gupta et al. 1989, 1991; Metakovsky et al. 1990). For dry white Chinese noodle (DWCN) quality, Glu-A3d and Glu-B3d were considered slightly better than others (He et al. 2005). Cornish et al. (1993) found that the composition bbb for Glu-A3, Glu-B3 and Glu-D3, respectively, gave the best extensibility, and the composition bbc was almost as extensible.
Traditionally, SDS-PAGE (Jackson et al. 1996) or RP-HPLC (Margiotta et al. 1993) is used to determine allelic compositions of LMW-GS in wheat. However, difficulties in resolving the multigene families and the overlapping fractions of LMW-GS hinder their routine use, particularly for testing large populations in the early generations of wheat breeding programs. Therefore, it is important to develop functional markers to identify different LMW-GS genes (Andersen and Lübberstedt 2003). Long et al. (2005) classified 69 LMW-GS genes registered in GenBank into nine groups and established nine group-specific primer sets to discriminate the nine groups. Ikeda et al. (2006) constructed 12 group-specific markers according to the 12 groups of LMW-GS genes detected in cultivar Norin 61. Based on the allelic variation of LMW-GS gene at the Glu-A3 locus, Zhang et al. (2004) developed several PCR markers to distinguish alleles a, b, c, d, e, f and g. Zhao et al. (2007a, b) designed several gene-specific markers for discriminating haplotypes of Glu-D3 genes. Three markers were developed for different Glu-B3 haplotypes at the DNA level (Zhao et al. 2007b). However, these markers do not discriminate the Glu-B3 alleles a, b, c, d, e, f, g, h and i, and the association between Glu-B3 genes and Glu-B3 protein alleles remained unclear. Here, we report the isolation of LMW-GS genes at the Glu-B3 locus from common wheat, characterization of the relationship between the genetic haplotypes and Glu-B3 alleles defined by protein mobility and development of allele-specific STS markers for different Glu-B3 alleles. This will benefit marker-assisted breeding for wheat quality.
Materials and methods
Plant materials
Aroona (Glu-B3b) and its seven near-isogenic lines (NIL), Aroona-B3a (Glu-B3a), Aroona-B3c (Glu-B3c), Aroona-B3d (Glu-B3d), Aroona-B3f (Glu-B3f), Aroona-B3g (Glu-B3g), Aroona-B3h (Glu-B3h) and Aroona-B3i (Glu-B3i), and Cheyenne (Glu-B3e), with different Glu-B3 alleles defined by protein mobility, were used to isolate Glu-B3 genes and develop molecular markers (Table 1). Certain homologous Group 1 Chinese Spring nulli-tetrasomic lines and five Chinese varieties with the 1BL.1RS translocation were used to confirm the chromosomal locations of identified genes. Twenty varieties from our laboratory collection and 141 wheat varieties and advanced lines from the International Maize and Wheat Improvement Centre (CIMMYT) with different Glu-B3 protein alleles detected by SDS-PAGE were employed to validate the allele-specific markers.
DNA extraction and PCR amplification
Genomic DNA was extracted from seedlings or seeds using a modified CTAB procedure (Gale et al. 2001). PCR was performed using TakaRa Taq DNA polymerase (1.0 unit) in 20 μl reaction volumes containing approximately 50 ng of genomic DNA, 1× PCR buffer (1.5 mM MgCl2), 100 μM of each dNTPs and 10 pmoles of each PCR primer. PCR cycling conditions for gene-specific primers were 94°C for 5 min followed by 38 cycles at 94°C for 45 s, 56–61°C for 45 s, 72°C for 90 s and a final extension at 72°C for 8 min. PCR conditions for allele-specific markers are shown in Table 2.
Development of locus-specific primers for isolation of Glu-B3 genes
Locus-specific primers for cloning Glu-B3 genes were developed from the descriptions of Zhang et al. (2003, 2004) and Zhao et al. (2006). Eight reference genes with complete coding regions, including seven LMW-GS genes (GenBank accessions AB119006, AB164415, AB164416, AB262661, Y14104, AB062852 and AJ007746) located on the short arm of chromosome 1B, and one (AY542898) with high similarity to Glu-B3 available in GenBank, were used for primer development (http://www.ncbi.nlm.nih.gov). The genes AB062852, AB164416 and AB262661 were selected as probes for the in silico cloning of Glu-B3 genes according to He et al. (2007).
Based on the alignment of the reference genes, 63 primers were designed and 378 primer combinations were tested with the NILs and Cheyenne with different Glu-B3 alleles. Primer screening was conducted as described by Zhao et al. (2006). Finally, four Glu-B3 genes were isolated by four pairs of primers with annealing temperatures ranging from 56 to 61°C (depending on primer set) (Fig. 1). Primer sequences (5′–3′) and their relative positions in reference genes are shown in Table 1.
Electrophoresis of PCR products of four gene-specific primer sets on agarose gels. a GluB3-1, GluB3-4 and GluB3-2; 1 Chinese Spring, 2 N1AT1B, 3 N1BT1A, 4 N1BT1D, 5 N1DT1B. b GluB3-3; 1, 2, 5–7 1BL.1RS lines, 3 Aroona-B3c, 4 Aroona-B3d, 8 Aroona-B3h, 9 Aroona-B3i, 10 N1AT1B, 11 N1BT1A, 12 N1BT1D, 13 N1DT1B. M DNA Ladder 2000 (100, 250, 500, 750, 1,000 and 2,000 bp)
Sequencing of PCR products
PCR fragments with expected sizes were recovered from agarose gels and cloned into the pGEM-T Easy Vector. Recombinant clones with expected sizes were sequenced after a PCR test. To eliminate cloning pitfalls (Masci et al. 1998), the recovered fragments were also sequenced directly using the corresponding PCR primers. Each PCR and sequence procedure was repeated three to six times to avoid any technical errors. All the sequencings were performed by the Sangon Biotechnology (Shanghai, China). Sequence analysis and characterization were performed using software DNAMAN (http://www.lynnon.com).
Allele-specific PCR marker design and validation
Allele-specific PCR markers were designed based on the allelic variants of Glu-B3 following the method of Zhang et al. (2003). These markers were firstly validated with the eight Aroona NILs and Cheyenne, and then with 161 wheat varieties and advanced lines from CIMMYT, Australia and France with Glu-B3 protein mobility alleles previously identified in SDS-PAGE by other workers.
Results
Allelic variants at the Glu-B3 locus
Four Glu-B3 genes, designated GluB3-1, GluB3-2, GluB3-3 and GluB3-4, including 17 allelic variants at the DNA level were identified at the Glu-B3 locus in the eight NILs and Cheyenne.
The GluB3-1 gene had five haplotypes or allelic variants, designated as GluB3-11, GluB3-12, GluB3-13, GluB3-14 and GluB3-15 (GenBank accessions EU369699, EU369700, EU369701, EU369702 and EU369703), amplified with the primer set LB1F/LB1R from Aroona-B3a (Glu-B3a), Aroona (Glu-B3b), Cheyenne (Glu-B3e), Aroona-B3f (Glu-B3f) and Aroona-B3g (Glu-B3g), respectively. Compared with GluB3-12, four, one and two triplet-nucleotide (CAA) deletions at positions 319–330, 328–330 and 325–330 in the coding region were found in GluB3-11, GluB3-13 and GluB3-14 (Appendix Fig. A1 in the Electronic Supplementary Material), leading to four, one and two glutamine deletions in the glutamine-rich repetitive domains of the deduced peptides BP1-1, BP1-3 and BP1-4, respectively (Fig. A2). At position 292–354, GluB3-15 had a 63-bp deletion, resulting in a 21-amino acid deletion in the deduced peptide BP1-5. GluB3-11 and GluB3-15 showed an additional 3-bp (CAA) deletion at position 556–558, leading to a glutamine deletion. In addition, GluB3-11 had two SNPs, one with A–G transition at position 1,092 and the other at position 1,113 with a C–T transition; GluB3-13 had a SNP at the position 69 with a C–A substitution; GluB3-15 showed four SNPs in the coding region at positions 360, 363, 918 and 1,056, respectively; the 360th SNP of GluB3-15 resulted in a change of phenylalanine to leucine at position 105 in the N-terminal domain of the deduced peptides, whereas all the others represented synonymous changes.
The GluB3-2 gene, amplified with primer set LB2F/LB2R, had three allelic variants at the DNA level, designated as GluB3-21, GluB3-22 and GluB3-23 (GenBank accessions EU369704, EU369721 and EU369705), respectively. GluB3-21 was detected in Aroona-B3a, GluB3-22 in genotypes with protein mobility alleles b, e and f and GluB3-23 was present in Aroona-B3g. Compared with GluB3-21, both GluB3-22 and GluB3-23 had a triplet nucleotide deletion (CAA) at position 378–380 in the coding region (Fig. A3), leading to a glutamine deletion in the repetitive domain of the deduced peptides BP2-2 and BP2-3, respectively (Fig. A4). In addition, GluB3-22 contained two SNPs at positions 671 and 1,246, and the latter resulted in an amino acid change from serine to asparagine in the C-terminal conserved region.
GluB3-3 had four allelic variants, designated as GluB3-31, GluB3-32, GluB3-33 and GluB3-34 (GenBank accessions EU369715, EU369716, EU369717 and EU369718, respectively), amplified with the primer set LB3F/LB3R from Aroona-B3c, Aroona-B3d, Aroona-B3h and Aroona-B3i, respectively. Compared with GluB3-31, four SNPs were detected at positions 197, 505, 836 and 1,030 in the coding region of both GluB3-32 and GluB3-34 (Fig. A5). GluB3-34 and GluB3-32 contained an additional SNP at positions 662 and 692, respectively. GluB3-33 had SNPs at positions 186, 505, 836 and 1,180 and a double-base substitution at positions 1,030 and 1,031. In addition, GluB3-34 showed a 3-bp (CAA) deletion at position 603–605 and a triplet code (CAA) insertion between positions 651 and 652, leading to a glutamine deletion and insertion in the deduced peptide BP3-4, respectively (Fig. A6).
GluB3-4 gene was amplified with the primer set LB4F/LB4R in all the eight NILs and Cheyenne, and had five allelic variants at the DNA level, designated as GluB3-41, GluB3-42, GluB3-43, GluB3-44 and GluB3-45 (GenBank accessions EU369724, EU369719, EU369727, EU369729 and EU369720, respectively). GluB3-41 was detected in NILs with protein mobility alleles a, c and d, GluB3-42 in Aroona-B3b, GluB3-43 in Cheyenne and Aroona-B3h, GluB3-44 in Aroona-B3f and Aroona-B3g and GluB3-45 in Aroona-B3i. Compared with GluB3-41, five, four and two SNPs were present in the upstream noncoding region of GluB3-42, GluB3-43 and GluB3-45, respectively (Fig. A7). In the coding region, a SNP with a C–T base transition was found at position 669 in GluB3-45 and at position 714 in GluB3-42 and GluB3-43, respectively, leading to an amino acid substitution from serine to leucine in the deduced proteins of BP4-5, BP4-2 and BP4-3 (Fig. A8). GluB3-42, GluB3-43, GluB3-44 and GluB3-45 had a common SNP (A–G) at position 731, leading to a change from isoleucine to valine in the deduced glutamine-rich repetitive domain. At the position 770–790, a 21-bp deletion was present in GluB3-45, resulting in a 7-amino acid (QFPQQQQ) deletion at the protein level. In addition, GluB3-45 had SNPs at positions 863, 885, 890 and 894, and GluB3-42 and GluB3-43 had a SNP at position 894, respectively. At positions 1,056 and 1,135, two SNPs were detected in GluB3-44, and the former led to an amino acid mutation from methionine to threonine, whereas the latter was a synonymous mutation in the deduced C-terminal cysteine-rich domain.
Characterization of the GluB3-1, GluB3-2, GluB3-3 and GluB3-4 genes and their deduced amino acid sequences
The 17 identified allelic variants of the four Glu-B3 genes contain a complete coding sequence, including start codon, termination sequence with double-stop codons TAATAA in GluB3-2 and GluB3-3 or TGATAA in GluB3-1 and GluB3-4. GluB3-1, GluB3-2 and GluB3-4 had the AATAAA polyadenylation signals in the 3′ flanking region. GluB3-4 was longer than the other three genes at the 5′ flanking region, comprising also the endosperm boxes, CAAT box and double TATA box. Sequence alignments indicated that the homologies of the DNA sequences were 87.4–94.4% among the four gene sequences, and 99.3–99.9% among different allelic variants within each of the four gene sequences (data not shown). At the protein level, the homologies of deduced amino acid sequences of the four Glu-B3 genes (designated BP1, BP2, BP3 and BP4) were 82.4–93.0% among the four genes and 98.3–100.0% (BP4-2 was the same as BP4-3) among different allelic variants within each of the four genes (data not shown).
All the deduced amino acid sequences of the four genes contained a single open reading frame (ORF) encoding a highly conserved signal peptide of 20 amino acids and a short N-terminal conserved region with 13 amino acids, followed by a repetitive domain rich in glutamine and a C-terminal conserved domain. The C domains had three subregions typical of LMW glutenin subunit proteins (Cassidy et al. 1998). The deduced peptides BP1, BP2 and BP3, encoded by GluB3-1, GluB3-2 and GluB3-3, respectively, were characterized with the amino acids MENSHIP in the N-terminal domain, and their deduced molecular weight ranged from ~39.0 kDa (BP1-5) to ~44.6 kDa (BP3-1). BP4, corresponding to GluB3-4, started with METSHIP in the N-terminal domain with molecular weights of ~39.0 kDa (BP4-5) or ~39.8 kDa (BP4-1, BP4-2, BP4-3 and BP4-4). The deduced amino acid sequences also showed typical eight-cysteine (Cys) residues, and all can be classified into type II based on the distribution of the cysteine residues (D’Ovidio and Masci 2004).
Relationship between Glu-B3 gene haplotypes and Glu-B3 protein mobility alleles
Each of the Glu-B3 protein mobility alleles contained different haplotypes at the DNA level (Table 3). Aroona-B3a possesses the haplotypes GluB3-11, GluB3-21 and GluB3-41; Aroona (Glu-B3b) has allelic variants GluB3-12, GluB3-22 and GluB3-42; Aroona-B3c contains GluB3-31 and GluB3-41; Aroona-B3d has GluB3-32 and GluB3-41; Cheyenne (Glu-B3e) contains GluB3-13, GluB3-22 and GluB3-43; Aroona-B3f possesses GluB3-14, GluB3-22 and GluB3-44; Aroona-B3g contains GluB3-15, GluB3-23 and GluB3-44; Aroona-B3h has GluB3-33 and GluB3-43; Aroona-B3i contains GluB3-34 and GluB3-45.
Gene-specific PCR markers for Glu-B3 alleles defined by protein mobility
Based on the sequence alignment of allelic variants among each of the Glu-B3 genes, ten primer sets were developed to amplify different Glu-B3 alleles based on the detected SNPs (Table 2). In the eight NILs and Cheyenne, primer set SB1F/SB1R amplified only a 1,095-bp PCR fragment in Aroona-B3a with the Glu-B3a allele (Fig. 2a). Primer set SB2F/SB2R was designed for the Glu-B3b allele in Aroona with a 1,549-bp PCR fragment (Fig. 2b). For the Glu-B3c allele in Aroona-B3c, SB3F/SB3R generated a unique 472-bp PCR product (Fig. 2c). Primer set SB4F/SB4R was used to identify the Glu-B3d allele in Aroona-B3d, producing a 662-bp band (Fig. 2d). In Cheyenne with the Glu-B3e allele, a specific 669-bp PCR product was generated with the primer set SB5F/SB5R (Fig. 2e). Primer set SB7F/SB7R was used to detect the Glu-B3g allele in Aroona-B3g with an 853-bp PCR fragment (Fig. 2f). To discriminate the Glu-B3h allele from others, primer set SB8F/SB8R was developed; it generated a 1,022-bp band in Aroona-B3h (Fig. 2g). Primer set SB9F/SB9R uniquely amplified a 621-bp PCR fragment in Aroona-B3i with the Glu-B3i allele (Fig. 2h). Since it was difficult to design a specific primer set for Glu-B3f, primer set SB6F/SB6R was developed to amplify Glu-B3f and Glu-B3g in Aroona-B3f and Aroona-B3g, respectively (Fig. 2i). In combination with SB7F/SB7R, this primer set can be used to identify Glu-B3f. In addition, primer set SB10F/SB10R was designed to amplify Glu-B3b, e and f in Aroona, Cheyenne and Aroona-B3f, respectively (Fig. 2j), and this set can be used to verify the former primer sets.
Electrophoresis of PCR products amplified from the eight NILs and Cheyenne on agarose gels using ten allele-specific markers: a gluB3a, b gluB3b, c gluB3c, d gluB3d, e gluB3e, f gluB3g, g gluB3h, h gluB3i, i gluB3fg, j gluB3bef. Materials used as PCR templates were as follows: 1 Aroona-B3a (a), 2 Aroona-B3b (b), 3 Aroona-B3c (c), 4 Aroona-B3d (d), 5 Cheyenne (e), 6 Aroona-B3f (f), 7 Aroona-B3g (g), 8 Aroona-B3h (h), 9 Aroona-B3i (i). PCR product sizes and conditions are as listed in Table 2. M DNA Ladder 2000 (100, 250, 500, 750, 1,000 and 2,000 bp)
Validation of Glu-B3 allele-specific markers
Eight NILs and Cheyenne used in cloning the Glu-B3 genes, and additional 161 wheat varieties and advanced lines were used to validate the ten Glu-B3 allele-specific markers (Table 4). The results were in accordance with those detected by SDS-PAGE except for five genotypes (entries 28, 68, 121, 152 and 165). In particular, the entry 121, which was detected to possess Glu-B3j or Glu-B3g allele in SDS-PAGE (Fig. 3), was determined to have Glu-B3g allele by the markers gluB3fg and gluB3g. Thirty-two genotypes with the 1BL.1RS translocation showed no target PCR product in the test with molecular markers, indicating the presence of the protein allele Glu-B3j.
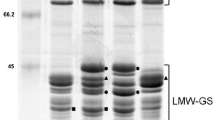
SDS-PAGE patterns of glutenin subunits of wheat varieties and advanced lines. 1 entry no. 43 in Table 4, 2 no. 48, 3 no. 52, 4 Pavón (Control), 5 no. 30, 6 no. 33, 7 no. 39, 8 no. 41, 9 no. 53, 10 no. 121, 11 no. 40, 12 no. 46, 13 no. 47, 14 Opata (Control)
Discussion
The LMW-GS proteins are critical components of the gluten complex of wheat and are encoded by a highly variable gene family. Because of their importance in wheat flour quality and difficulties in discriminating them by traditional SDS-PAGE techniques, it was necessary to develop allele-specific markers to identify different LMW-GS alleles (Gupta et al. 1999; Gale 2005; Bagge et al. 2007). Zhang et al. (2004) reported seven Glu-A3 markers to discriminate different Glu-A3 alleles. Several STS markers were also developed for different Glu-D3 gene haplotypes (Zhao et al. 2007a, b). In this study, ten allele-specific primer sets were successfully designed for the nine Glu-B3 alleles defined by protein mobility. The ten markers were validated with eight Aroona NILs and Cheyenne, and 161 wheat varieties and advanced lines from CIMMYT, Australia and France.
The GluB3-1 and GluB3-2 genes were present in varieties with protein based alleles a, b, e, f and g, and the GluB3-3 allele was present in those with c, d, h and i only. As the three genes were isolated by the gene-specific primer sets LB1F/LB1R, LB2F/LB2R and LB3F/LB3R, respectively, we also designed new primer sets from within the coding regions of these genes to perform PCR, and confirmed the distinctions between the genes. In a previous study, Zhao et al. (2007b) found that the primer set T13F4/T13R3 was null in varieties with Glu-B3c, d, h and i. The GluB3-4 genes were present in varieties with the protein alleles a, b, c, d, f, g, h, and i, and thus the haplotypes of gene variants present in different varieties share common genes as well as carrying unique variants.
Currently, more than 200 LMW-GS genes have been registered in GenBank. About 30 sequences were located on chromosome 1B and 13 of them had complete coding sequences (Van Campenhout et al. 1995; D’Ovidio et al. 1997, 1999; Masci et al. 1998; Ikeda et al. 2002; Maruyama-Funatsuki et al. 2005; Huang and Cloutier 2008). In the present study, four Glu-B3 genes were identified, including the complete coding sequences of 17 allelic variants. Sequence alignments indicated that GluB3-1 was 99.1–99.7% identical in sequence to AB262661 and EU189088. In particular, GluB3-11 had only three SNP differences from AB262661 and EU189088 in the aligned domain. The three allelic variants of GluB3-2 showed lower identities to the genes in GenBank, with the highest similarity of 93.4% between GluB3-23 and AB164415, indicating that GluB3-2 might be a new LMW-GS gene identified at the Glu-B3 locus. GluB3-3 shared 99.5–100.0% identity with AB164415, and GluB3-33 had the same sequence as AB164415. GluB3-4 shared 99.1–99.9% of identities with AB062852 and X84960, and GluB3-41 had only 1 bp difference from X84960 at position 1,303 in the coding region.
Based on the first N-terminal amino acid of the mature protein, LMW-GS were divided into three types: LMW-m, LMW-s and LMW-i, corresponding to methionine, serine and isoleucine, respectively (Lew et al. 1992). In this study, BP4 started with the amino acids METSHIP in the N-terminal domain and could therefore be classified as a LMW-m type. The LMW-m type was found to be the main type by gene sequencing, but the LMW-s type was the predominant type found by N-terminal sequencing of proteins (Lew et al. 1992; Masci et al. 2002). It was suggested that the LMW-s type might originate from post-translational cleavage by an asparaginyl peptidase (Masci et al. 1998; Dupont et al. 2004). Consequently, BP1, BP2 and BP3 starting with MENSHIP in the N-terminal domain could be classified as LMW-s types based on asparagine at the third position (Ikeda et al. 2002, 2006). Previous studies also indicated that such classification of LMW-m and LMW-s type genes might be highly tenuous, as the LMW-m and LMW-s type genes have high homologies in wheat and its relatives (Jiang et al. 2008; Huang and Cloutier 2008).
The overall results of this study showed that the protein mobility alleles determined by SDS-PAGE were consistent with the screening results obtained using the allele-specific markers in 165 of 170 genotypes. The inconsistent results for five genotypes were attributed to the low discrimination power of SDS-PAGE in distinguishing some LMW-GS and the impurity of some materials. The ten Glu-B3 allele-specific markers can be used in the marker-assisted breeding aimed at the improvement of wheat quality.
References
Andersen JR, Lübberstedt T (2003) Functional markers in plants. Trends Plant Sci 8:554–560
Bagge M, Xia XC, Lübberstedt T (2007) Functional markers in wheat. Curr Opin Plant Biol 10:211–216
Bietz JA, Shepherd KW, Wall JS (1975) Single kernel analysis of glutenin: use in wheat genetics and breeding. Cereal Chem 52:513–532
Butow BJ, Gale KR, Ikea J, Juhász A, Bedö Z, Tamás L, Gianibelli MC (2004) Dissemination of the highly expressed Bx7 glutenin subunit (Glu-B1al allele) in wheat as revealed by novel PCR markers and RP-HPLC. Theor Appl Genet 109:1525–1535
Cassidy BG, Dvorak J, Anderson OD (1998) The wheat low-molecular-weight glutenin genes: characterization of six new genes and progress in understanding gene family structure. Theor Appl Genet 96:743–750
Cornish GB, Burridge PM, Palmer GA, Wrigley CW (1993) Mapping the origins of some HMW and LMW glutenin subunit alleles in Australian germplasm. In: Proceedings of the 43rd Australian cereal chemistry conference, Sydney, 12–16 September 1993, pp 255–260
D’Ovidio R, Masci S (2004) The low-molecular-weight glutenin subunits of wheat gluten. J Cereal Sci 39:321–339
D’Ovidio R, Simeone M, Masci S, Porceddu E (1997) Molecular characterization of an LMW-GS gene located on chromosome 1B and the development of primers specific for the Glu-B3 complex locus in durum wheat. Theor Appl Genet 95:1119–1126
D’Ovidio R, Marchitelli C, Cardelli LE, Porceddu E (1999) Sequence similarity between allelic Glu-B3 genes related to quality properties of durum wheat. Theor Appl Genet 93:455–461
Dupont FM, Vensel W, Encarnacao T, Chan R, Kasarda DD (2004) Similarities of omega gliadin from Triticum urartu to those encoded on chromosome 1A of hexaploid wheat and evidence for their post-translational processing. Theor Appl Genet 108:1299–1308
Eagles HA, Bariana HS, Ogbonnaya FC, Rebetzke GJ, Hollamby GJ, Henry RJ, Henschke PH, Carter M (2001) Implementation of markers in Australian wheat breeding. Aust J Agric Res 52:1349–1356
Gale KR (2005) Diagnostic DNA markers for quality traits in wheat. J Cereal Sci 41:181–192
Gale KR, Ma W, Zhang W, Rampling L, Hill AS, Appels R, Morris P, Morrel M (2001) Simple high-throughput DNA markers for genotyping in wheat. In: Eastwood R, Hollamby G, Rathjen T, Gororo N (eds) Proceedings of 10th Australian wheat breeding assembly, Wheat Breeding Society of Australia, Mildura, VIC, 16–21 September 2001, pp 26–31
Gras PW, Anderssen RS, Keentock M, Bekes F, Appels R (2001) Gluten protein functionality in wheat flour processing: a review. Aust J Agric Res 52:1311–1323
Gupta RB, Shepherd KW (1990) Two-step one-dimensional SDS-PAGE analysis of LMW subunits of glutenin. I. Variation and genetic control of the subunits in hexaploid wheats. Theor Appl Genet 80:65–74
Gupta RB, Singh NK, Shepherd KW (1989) The cumulative effects of allelic variation in LMW and HMW glutenin subunits on physical dough properties in the progeny of two bread wheats. Theor Appl Genet 77:57–62
Gupta RB, Bekes F, Wrigley CW (1991) Prediction of physical dough properties from glutenin subunit composition in bread wheats. Cereal Chem 68:328–333
Gupta PK, Varshney RK, Sharma PC, Ramesh B (1999) Molecular markers and their application in wheat breeding. Plant Breed 118:369–390
He ZH, Liu L, Liu JJ, Xia XC, Peña RJ (2005) Composition of HMW and LMW glutenin subunits and their effects on dough properties, pan bread, and noodle quality of Chinese bread wheats. Cereal Chem 82:345–350
He XY, He ZH, Zhang LP, Sun DJ, Morris CF, Fuerest EP, Xia XC (2007) Allelic variation of polyphenol oxidase (PPO) genes located on chromosomes 2A and 2D and development of functional markers for the PPO genes in common wheat. Theor Appl Genet 115:47–58
Huang XQ, Cloutier S (2008) Molecular characterization and genomic organization of low molecular weight glutenin subunit genes at the Glu-3 loci in hexaploid wheat (Triticum aestivum L.). Theor Appl Genet 116:953–966
Ikeda TM, Nagamine T, Fukuoka H, Yano H (2002) Identification of new low-molecular-weight glutenin subunit genes in wheat. Theor Appl Genet 104:680–687
Ikeda TM, Araki E, Fujita Y, Yano H (2006) Characterization of low-molecular-weight glutenin subunit genes and their protein products in common wheats. Theor Appl Genet 112:327–334
Jackson EA, Morel MH, Sontag-Strohm T, Branlard G, Metakovsky EV, Redaelli R (1996) Proposal for combining the classification systems of alleles of Gli-1 and Glu-3 loci in bread wheat (Triticum aestivum L.). J Genet Breed 50:321–336
Jiang CX, Pei YH, Zhang YZ, Li XH, Yao DN, Yan YM, Ma WJ, Hsam SLK, Zeller FJ (2008) Molecular cloning and characterization of four novel LMW glutenin subunit genes from Aegilops longissima, Triticum dicoccoides and T. zhukovskyi. Hereditas 145:92–98
Lei ZS, Gale KR, He ZH, Gianibeli C, Larroque O, Xia XC, Butow BJ, Ma W (2006) Y-type gene specific markers for enhanced discrimination of high-molecular weight glutenin alleles at the Glu-B1 locus in hexaploid wheat. J Cereal Sci 43:94–101
Lew EJL, Kuzmicky DD, Kasarda DD (1992) Characterization of low-molecular-weight glutenin subunits by reversed-phase high-performance liquid chromatography, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and N-terminal amino acid sequencing. Cereal Chem 69:508–515
Lindsay MP, Skerritt JH (1999) The glutenin macropolymer of wheat flour doughs: structure–function perspectives. Trends Food Sci Technol 10:247–253
Long H, Wei YM, Yan ZH, Baum B, Nevo E, Zhen YL (2005) Classification of wheat low-molecular-weight glutenin subunit genes and its chromosome assignment by developing LMW-GS group-specific primers. Theor Appl Genet 111:1251–1259
Luo C, Griffin WB, Branlard G, McNeil DL (2001) Comparison of low- and high- molecular-weight wheat glutenin allele effects on flour quality. Theor Appl Genet 102:1088–1098
Ma W, Zhang W, Gale KR (2003) Multiplex-PCR typing of high molecular weight glutenin alleles in wheat. Euphytica 134:51–60
Margiotta B, Colaprico G, D’Ovidio R, Lafiandra D (1993) Characterization of high Mr subunits of glutenin by combined chromatographic (RP-HPLC) and electrophoretic separation and restriction fragment length polymorphism (RFLP) analyses of their encoding genes. J Cereal Sci 17:221–236
Maruyama-Funatsuki W, Takata K, Funatsuki H, Tabiki T, Ito M, Nishio Z, Kato A, Saito K, Yahata E, Saruyama H, Yamauchi H (2005) Identification and characterization of a novel LMW-s glutenin gene of a Canadian Western extra-strong wheat. J Cereal Sci 41:47–57
Masci S, D’Ovidio R, Lafiandra D, Kasarda DD (1998) Characterization of a low-molecular-weight glutenin subunit gene from bread wheat and the corresponding protein that represents a major subunit of the glutenin polymer. Plant Physiol 118:1147–1158
Masci S, Rovelli L, Kasarda DD, Vensel WH, Lafiandra D (2002) Characterisation and chromosomal localization of C-type low-molecular-weight glutenin subunits in the bread wheat cultivar Chinese Spring. Theor Appl Genet 104:422–428
Metakovsky EV, Wrigley CW, Bekes F, Gupta RB (1990) Gluten polypeptides as useful genetic markers of dough quality in Australian wheats. Aust J Agric Res 41:289–306
Payne PI (1987) Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu Rev Plant Physiol 38:141–153
Payne PI, Corfield KG (1979) Subunit composition of wheat glutenin proteins, isolated by gel filtration in a dissociating medium. Planta 145:83–88
Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53:947–958
Singh NK, Shepherd KW (1988) Linkage mapping of genes controlling endosperm storage proteins in wheat. 1. Genes on the short arms of group-1 chromosomes. Theor Appl Genet 75:628–641
Van Campenhout S, Stappen JV, Sagi L, Volckaert G (1995) Locus-specific primers for LMW glutenin genes on each of the group 1 chromosomes of hexaploid wheat. Theor Appl Genet 91:313–319
Zhang W, Gianibelli MC, Ma W, Rampling L, Gale KR (2003) Identification of SNPs and development of AS-PCR markers for γ-gliadin alleles in Triticum aestivum. Theor Appl Genet 107:130–138
Zhang W, Gianibelli MC, Rampling L, Gale KR (2004) Characterisation and marker development for low molecular weight glutenin genes from Glu-A3 alleles of bread wheat (Triticum aestivum L.). Theor Appl Genet 108:1409–1419
Zhao XL, Xia XC, He ZH, Gale KR, Lei ZS, Appels R, Ma WJ (2006) Characterization of three low-molecular-weight Glu-D3 subunit genes in common wheat. Theor Appl Genet 113:1247–1259
Zhao XL, Xia XC, He ZH, Lei ZS, Appels R, Yang Y, Sun QX, Ma W (2007a) Novel DNA variations to characterize low molecular weight glutenin Glu-D3 genes and develop STS markers in common wheat. Theor Appl Genet 114:451–460
Zhao XL, Ma W, Gale KR, Lei ZS, He ZH, Sun QX, Xia XC (2007b) Identification of SNPs and development functional markers for LMW-GS genes at Glu-D3 and Glu-B3 loci in bread wheat (Triticum aestivum L.). Mol Breed 20:223–231
Acknowledgments
The authors are very grateful to Prof. Robert McIntosh, University of Sydney, for reviewing this manuscript. Aroona and its NILs were kindly provided by Dr. Marie Appelbee and Prof. Ken Shepherd, SARDI Grain Quality Research Laboratory, Adelaide, South Australia, Cheyenne was provided by Dr. Baoyun Li, China Agricultural University, Beijing, and Chinese Spring and nulli-tetrasomic lines N1A-T1D, N1B-T1A, N1B-T1D and N1D-T1B were provided by Prof. Robert McIntosh. The study was supported by the National Science Foundation of China (30671296 and 30830072), National Basic Research Program (2009CB118300) and National 863 Programs (2006AA10Z1A7 and 2006AA100102).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by J. W. Snape.
L. H. Wang and X. L. Zhao contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L.H., Zhao, X.L., He, Z.H. et al. Characterization of low-molecular-weight glutenin subunit Glu-B3 genes and development of STS markers in common wheat (Triticum aestivum L.). Theor Appl Genet 118, 525–539 (2009). https://doi.org/10.1007/s00122-008-0918-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-008-0918-9