Abstract
Nine single segment substitution lines (SSSLs) in rice, which contain quantitative trait loci (QTLs) for tiller number on substituted segments detected in previous studies, were selected as materials to analyse dynamic expression of the QTLs in this study. These SSSLs and their recipient parent, Hua-jing-xian 74 (HJX74), were grown in four different environments and were measured for tiller number at nine different growth stages. An indirect methodology was applied in QTL mapping through analyzing multi-environment phenotypic data. Dynamics of three types of effects (including total effect, main effect, and QE interaction effect) of QTLs was released. It was shown that nine QTLs exhibited statistically significant effects only at certain stages. Effects of a QTL, although insignificant at certain stages, displayed dynamic change with the growth of rice plants. Two common features of nine QTLs were detected, one is no expression within 7 days after transplanting, and the other is opposite expression existed during the whole growth period. Nine QTLs largely focused on expression in certain stages, and accordingly were suggested to partition into three types, expression in prophase, both in prophase and in anaphase, and evenly during the whole stage. It may be reasonable explanation that dynamics of main effects of QTLs are likely due to gene expression selectly at certain times, while dynamics of QE interaction effects of QTLs might attribute to the subrogation of environmental factors. Examination of the association between QE interaction effect and specified environmental factors across stages may provide useful information on how an environmental factor regulates QTL expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice tiller is a specialized grain-bearing branch that is formed on the un-elongated basal internodes and grows independently of the mother stem (culm) by means of its own adventitious roots (Li 1979). The development of tillering is affected by various environmental factors including manuring, planting density, and climatic circumstances such as light, temperature, water supply, and so on. Tillering in rice is one of the most important agronomic traits for grain production because tiller number per plant determines panicle number, a key component of grain yield (Yan et al. 1998a). On other hand, tillering is a trait for which the expression changes over time and can easily be measured (Wu et al. 1999). Hence, tiller number usually serves as a suitable model trait for the study of developmental behaviors (Xu and Shen 1991). For these reasons, the elucidation of genetic bases influencing tiller number per plant in rice is important for the development of high-yielding cultivars. By traditional statistical analysis, there have been lots of reports about genetics of rice tillers (Murai and Kinoshita 1986; Perera et al. 1986; Xu and Shen 1991). Tiller number in rice is generally considered to be controlled by a polygenic system with a relatively low heritability of 29.8–49.6% (Xiong 1992). Using molecular marker maps and QTL mapping technology, mapping of QTLs for tiller number has been reported. There are mainly two common methods used for QTL mapping on rice tillers, one by analyzing the mutant materials (Tang et al. 2001; Li et al. 2003a; Ishikawa et al. 2005; Zou et al. 2005; Jiang et al. 2006); and the other by using the conventionally separating populations such as recombinant inbred lines (RILs) or doubled haploid lines (DHLs) (Yan et al. 1998a;Wu et al. 1999;Hittalmani et al. 2002, 2003; Liu et al. 2006). At least 17 Mendelian genes for rice tillers have been identified by mutant analyzing (http://www.gramene.org/db/mutant/search.core), and numerous QTLs for rice tillers have been located on ten rice chromosomes (Xiao et al.1995a, b; Lin et al.1996; Wu et al. 1996; Jiang et al. 2004).
According to the theory of developmental genetics, genes are expressed selectively at different growth stages (Zhu 1995). This means that different QTLs may have different expression dynamics during stages of trait development, even though they may have the same final effects. The conventional statistical results have revealed that the development of morphological traits occurs through the actions and interactions of many genes that might behave differentially during growth periods, and that gene expression is modified by interactions with the environment (Atchley and Zhu 1997). To understand the genetic functions of QTLs thoroughly, therefore, we should know not only their effects at a given time or stage, but also their expression dynamics through the whole growth stage in different environments. In recent years, there have been numerous studies to map QTLs and to estimate their effects for rice developmental traits at different stages (Bradshaw and Settler 1995; Plomion et al. 1996; Price and Tomos 1997; Yan et al. 1998a; Wu et al. 1999; Dong et al. 2004). Although these studies have provided some evidence of differential activities of QTLs during ontogenesis, they can not obtain the unbiased estimation of QTLs since they were conducted on populations such as F2, RILs, or DHLs, and only in one single environment (Eshed and Zamir 1995; McCouch and Doerge 1995; Yano et al. 1997; Zhu 1999; Wang et al. 1999). To achieve high resolution mapping of QTLs, Eshed and Zamir (1995) proposed the application of a novel introgression line (IL) population for the mapping of quantitative traits associated with yield in tomato. In rice, similar populations such as chromosome segment substitution lines (CSSLs), backcross inbred lines (BILs), and single segment substitution lines (SSSLs) were developed and applied widely in the genetic study of quantitative traits (Yano et al. 1997, 2000, 2001; Wan et al. 2003; Zhang et al. 2004; He et al. 2005a, b, c; Xi et al. 2006; Tian et al. 2006). To track the performance of individual QTL across environments, Zhu (1998) proposed an indirect methodology to map QTLs with QE interaction effects through analyzing experimental data derived from multi-environments and since then this approach was extensively used on QTL mapping in a DHL population of rice (Yan et al. 1998b, 1999; Hittalmani et al. 2003; Li et al. 2003b).
Recently, we have made two basic researches on QTL mapping for rice tiller with SSSLs. One was to genetically explore the developmental behavior of rice tiller numbers grown in individual environment, in which 14 out of 26 SSSLs were detected with QTLs and dynamic expression of them was revealed (Zhao et al. 2008). Another was to elucidate QE interactions of QTLs for rice tiller number at the final stage in six different environments, in which 18 out of 35 SSSLs were detected with QTLs and QTL components of them were analyzed by the indirect method (Liu et al. 2008). In this paper, nine SSSLs, which were identified with QTLs on tiller number in previous two papers, were selected as materials, and their tiller numbers were investigated with time-dependent measures. The indirect method (Zhu 1998) was applied to analyze data derived from four environments at each of nine stages. The aims of the study were to detect dynamics of main effects and QE interaction effects of nine QTLs through investigating successively the behavior of QTLs at nine stages. Then the temporal expression and the QE interaction effects of nine QTLs for the development of tillers in rice were discussed.
Materials and methods
Plant materials
Hua-jing-xian 74 (HJX74), an elite indica variety from South China, was selected as the recipient, and 24 varieties including 14 indica and 10 japonica varieties collected worldwide were used as donors (Zhang et al. 2004). The development scheme of the SSSLs, through backcrossing and SSR marker selection, was described previously by Xi et al. (2006). Each SSSL contains only one substituted segment from a donor in HJX74 genetic background (Zhang et al. 2004). Nine SSSLs identified with QTLs on rice tiller number in previous studies (Zhao et al. 2008; Liu et al. 2008), were selected as materials in this research (Table 1).
Field trials and tiller number evaluated
Phenotype experiments were similar to previous studies described by Zhao et al. (2008) and Liu et al. (2008). Experiments were conducted at the experimental farm in South China Agricultural University, Guangzhou (at ~113° east longitude and ~23° north latitude), China in two cropping environments, spring season (from March to July) and fall season (from July to November) during 2004 and 2006. HJX74 and 6 SSSLs were grown in four experimental environments, and 3 SSSLs in three experimental environments (Table 1). In all experiments, germinated seeds were sown in a seedling bed and seedlings were transplanted to a paddy field 20 days later, with two plants per hill spaced at 16.7 cm × 20.0 cm. Each plot consisted of thirteen 6.2 m long rows with 32 plants and all plots were arranged in a completely randomized block design with three replications. The management of the field experiments was in accordance with local standard practices. From 7 days after transplanting onwards, all tillers per hill were measured every 7 days in 20 central plants (fixed through all measuring stages) from each plot until all lines had headed. At a total of nine different stages, tiller numbers were continuously recorded during the whole rice growth period. The average tiller numbers of 20 plants in each plot at various measuring stages were used as raw data in the analysis.
Estimate of genetic effects
The genetic model of agronomic trait (Zhu 1992, 1994, 1996; Zhu and Weir 1996) was employed to study the inheritance of tiller number in rice. Genetic effects were estimated based on phenotypic value at time t,
-
(1)
For the genetic experiment conducted only in one environment, the phenotypic performance of the jth genetic entry in the kth block can be expressed by,
where μ (t) = population mean at time t, fixed; G j(t) = genetic main effect of jth genotype at time t, \( G_{j(t)} \sim N\left( {0,\sigma_{G(t)}^{2} } \right); \) B k(t) = block effects of kth block at time t, \( B_{k(t)} \sim N\left( {0,\sigma_{B(t)}^{2} } \right); \) and ε jk(t) = residual effect at time t, \( \varepsilon_{jk(t)} \sim N\left( {0,\sigma_{\varepsilon (t)}^{2} } \right). \)
-
(2)
For the genetic experiments conducted within multi environments, the phenotypic performance of the jth genetic entry in the kth block within hth environment can be expressed by,
where μ (t) = population mean at time t, fixed; E h(t) = environment effect of hth environment at time t, \( E_{h(t)} \sim N\left( {0,\sigma_{E(t)}^{2} } \right); \) G j(t) = genetic main effect of jth genotype at time t, \( G_{j(t)} \sim N\left( {0,\sigma_{G(t)}^{2} } \right); \) GE hj(t) = genotype × environment interaction effect between jth genotype and hth environment at time t, \( {\text{GE}}_{hj(t)} \sim N\left( {0,\sigma_{{{\text{GE}}(t)}}^{2} } \right); \) B hk(t) = block effects of kth block within hth environment at time t, \( B_{hk(t)} \sim N\left( {0,\sigma_{B(t)}^{2} } \right); \) and ε hjk(t) = residual effect at time t, \( \varepsilon_{hjk(t)} \sim N\left( {0,\sigma_{\varepsilon (t)}^{2} } \right). \)
The restricted maximum likelihood (REML) method, the generalized least square (GLS) method and the best linear unbiased prediction (BLUP) method were used to estimate variance components, to predict fixed and random genetic effects in models (Zhu 1994), respectively. All genetic effects for tiller number at different measuring stages in rice were estimated by using the QTModel0.70Beta software package (http://ibi.zju.edu.cn/software/qtmodel/index.html).
QTL mapping
QTL could be indirectly searched for complicated quantitative traits such as those with GE interaction effects or with time-dependent measures of developmental behavior (Zhu 1998). First, the G effects and the GE interaction effects of HJX74 and all individual SSSLs for tiller number were estimated according to the models in one environment and within multi environments mentioned above. Next, QTLs were mapped using these estimated values as input data separately. Obviously, QTL identified according to the G effect in model (1) was expected to contain mixed effect of QTLs. While QTLs obtained on the G effect and the GE interaction effect in model (2) were with the main effect and the QE interaction effect, respectively. Genetic effects estimated for each SSSL were compared to those for HJX74 with an alpha level less than 0.05 or 0.01 by t test of one tail. The t values can be calculated by the following equation:
where \( \hat{G}_{j} {\text{ and }}\hat{G}_{0} , \widehat{\text{GE}}_{j} {\text{ and }}\widehat{\text{GE}}_{0} \) are the estimations of genetic effect or components of genetic effects for SSSL and HJX74, respectively. \( \widehat{\text{SE}}_{(G)} {\text{ and }}\widehat{\text{SE}}_{{({\text{GE}})}} \) are the estimations of mean standard errors of them, respectively. df e is degrees of freedom of residual errors. QTL effect values were calculated by the differences of genetic effects between each SSSL and HJX74.
Results
Ontogenetic changes and environmental effects on tiller number
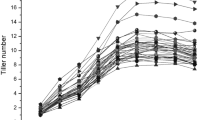
Averages of tiller number over nine SSSLs and HJX74 at nine measuring stages in four environments are presented in Fig. 1. Tiller numbers in four environments show S-shaped curves. Generally, tiller number continues to increase until the highest tillering stage, then to descend to the final observation. The fast increase in tiller number appears before the highest tillering stage. There is a time interval to keep the highest tiller number. After then tiller number begins to descend, and the speed of decline steps down until the productive tiller number is determined. The curves of tiller number exhibit apparent differences among four environments, indicating that environmental factors have an important role on tiller appearance. The effects of four environments show huge difference, and change also at various stages (Table 2). In spring seasons (including E1 and E4), environmental effects generally tend to reduce tiller appearances during early stages, and turn to enhance tillers during late stages. Reversely, environmental effects during early stages in fall seasons (including E2 and E3) rise tillers (Table 2). So tiller numbers in spring seasons were often observed to be lower during stages t1–t3 but higher since stage t6 than those in fall seasons (Fig. 1).
Dynamics of genetic variance components
There is a dynamic change in the two genetic variance components during whole ontogeny (Fig. 2). Their curves also show approximately S-shaped change. The variance due to genetic main effect rises rapidly until stage t5, then decreases slightly between stages t5 and t6, and since then declines successively. The GE interaction variance reaches the utmost value at stage t3, then descends successively with a slight increase between stages t4 and t6. Narrow sense heritability, defined as \( H_{(G)}^{2} = V_{(G)} /V_{P} \) (where V (G) and V P are genetic main effect variance and phenotypic variance, respectively), is initially zero, then increases successively to 39.1% at stage t6 and then begins to descend to 15.7% at stage t8. At the final observation, the narrow sense heritability ends with 24.5%. GE interaction heritability, defined as \( H_{{({\text{GE}})}}^{2} = V_{{({\text{GE}})}} /V_{P} \) (where V (GE) is GE interaction variance), is reasonably constant during the whole ontogeny varying from 2.5% at stage t1 to 11.0% at stage t6. These results indicate that variations of tiller numbers at various stages are partially influenced by genetic factors of both genetic main effect and GE interaction effect. Broad sense heritability, defined as \( H_{{(G + {\text{GE}})}}^{2} = {{\left( {V_{(G)} + V_{{({\text{GE}})}} } \right)} \mathord{\left/ {\vphantom {{\left( {V_{(G)} + V_{{({\text{GE}})}} } \right)} {V_{P} }}} \right. \kern-\nulldelimiterspace} {V_{P} }}, \) varies from 2.5 to 50.1% through the whole period of plant growth. In addition, it is also detected that genetic variations on tiller numbers occur largely during mid-stages from t5 to t6.
Estimations and proportions of genetic variance components for tiller numbers at various stages. V(G) and V(GE) are the genetic main effect variance and the GE interaction effect variance for tiller number, respectively. H 2(G) and H 2(GE) are their proportions occupying phenotypic variance. t1–t9 indicate measuring stages of tiller number, setting 7 days between stages
Developmental behavior of nine QTLs for tillers
QTL mapping based on data at various stages in individual environment leads the identification of developmental behavior of nine QTLs for tillers in rice (Table 3). These QTLs distribute in corresponding marker intervals on chromosomes 1, 2, 3, 6 and 8 (Liu et al. 2008). All QTLs express effects with dynamic patterns during the whole stage of rice growth in any experimental environment (Table 3). First, the effects of QTLs estimated are statistically significant only at certain stages in certain environments. In environment E2, five QTLs (Tn1-1, Tn2, Tn3-1, Tn6-2 and Tn8) were detectable from stages t2 to t9 since they are always with relatively large effects during these stages. The remainder QTLs were not always detected at all stages investigated. Except for stage t1 when no significant QTLs were detected, the average number of detectable QTLs per stage in four environments was \( 10.5 \pm 2.1\left( {\bar{x} \pm {\text{SD}}} \right), \) ranging from 8 to 14 (Table 3). Many QTLs could not be detected at a given stage since the accumulated effects of them were too small before that stage. For example, QTL Tn1-1, with the accumulated effect less than 0.88, was undetectable at any stage in E1 (Table 3). Secondly, the estimated effects of each QTL differed across developmental stages. The effects of QTL Tn1-1 in E1, for instance, descended in succession from 0.01 at stage t1 to the peak point of −0.88 at stage t6, and then rose to −0.14 at stage t9. The remainder QTLs exhibited the developmental schedules of themselves (Table 3). Generally, QTLs expressed effects in S-shaped curves during the whole growth period, but the dynamic procedures differed across both QTLs and environments. In addition, QTL effects estimated in different environments exhibited obvious deviations. QTL Tn1-1 at stage t2, for instance, possessed the estimated effects of −0.01, −2.62, −0.43, and 0.23 in E1, E2, E3, and E4, respectively (Table 3). We also noticed that the numbers and the effect directions of QTLs varied across environments. There were five (out of seven) and three (out of eight) QTLs to be detected with significant effects in E1 and E4, respectively. All of nine QTLs investigated were significant in E2, but only two of them were detected in E3. These QTLs were with negative values in E2, and positive in E3 and E4. In E1, three positive and two negative QTLs were significant (Table 3). Many QTLs could not be detected in a given environment, which is largely due to the absence of statistically significant effects or without experimental data.
Main effects of nine QTLs at nine stages
In a specific environment, the total effect of a QTL includes two components, the main effect and QTL × environment (QE) interaction effect. QTL mapping based on data derived from multi-environments can identify the two components (Zhu 1998; Yan et al. 1998b, 1999). Main effects of nine QTLs estimated according to genetic main effects in model (2) are shown in Table 4. Although all QTLs had their dynamic curves of main effects during the whole stage, only six of them including QTLs Tn1-1, Tn1-2, Tn2, Tn3-1, Tn6-2 and Tn6-3 were statistically significant at certain stages. It means that these QTLs could express effects stably with the constant magnitudes across all of measurement environments. For the remaindering three QTLs (Tn3-2, 6-1 and Tn8), their main effects were weak, indicating that they had little common effect over different environments. The significance between the main effect and the total effect of a QTL expressed in a specific environment was inconsistent sometimes. For instance, QTL Tn1-1, which was detected with significant main effect from stages t2 to t9 (Table 4), was insignificant on its total effect in all experimental environments except for E2 (Table 3). On other hand, QTL Tn3-2, which was detected with significant total effects from stages t2 to t7 in E2 (Table 3), was weak on its main effect at these stages (Table 4). These differences perhaps attribute to the cause that the total genetic effect of QTL mixed QE interaction effect of the QTL and part of residual effect. Clearly, main effects of individual QTL exhibit also dynamic change with the growth of rice plant. For QTL Tn1-1, for instance, the main effect was initially zero, then rose to the peak point of 1.02 at the stage t5, after then descended and finally reached to 0.61 (Table 4).
QE interaction effects of nine QTLs at nine stages by four environments
QE interaction effects of nine QTLs by four environments estimated according to GE interaction effects in model (2) are shown in Table 5. Only some of QE interaction effects are statistically significant. For instance, QE interaction effect at Tn1-1 was significant with the estimated values of −0.65 at stage t6 in E3. Whereas the remainder QE interaction effects for this QTL were statistically insignificance (Table 5). QE interaction effects vary also with the development of rice plant. For instance, QE interaction effect at Tn3-2 in E2 was initially zero, ascended in succession to the peak of 1.33, then descended to 0.51 at stage t5, and close behind rose again to 0.65 at stage t6, after then declined again to reach finally 0.11 (Table 5). Each of QTLs had the schedule of itself in a specific environment. QE interaction effects depended on both QTL and environment, namely that QE interaction effects differed both across QTLs and across environments. For example, QTL Tn1-1 and QTL Tn1-2 in environment E3 exhibited 0.25 and 0.68 of QE interaction effects at stage t3, respectively, while QE interaction effects of QTL Tn1-1 by environments E1, E2, E3 and E4 were 0.03, 0.33, −0.65 and 0.02, respectively (Table 5).
Discussions
It has been long time to recognize that complex traits develop through the actions of many genes that might behave differentially during growth periods and across environments (Atchley and Zhu 1997). Although molecular marker techniques have provided powerful tools to dissect these complex traits, it still is difficult to directly handle different developmental stages in various environments for QTL mapping (Yan et al. 1998a; Wu et al. 1999). An indirect method to analyze the developmental behavior and its interaction with environments for QTL mapping was proposed and applied (Zhu 1998; Yan et al. 1999), which could result in more information about dynamic gene expression and QE interaction for developmental trait. In this article, the suggested indirect approach was used in searching QTLs on rice tiller number for developmental behavior and QE interaction. First, it detected more number of significant QTLs than those detected at one specific stage in any individual environment. Many QTLs, which were undetectable at specific stages in certain environments, were identified with time-dependent measures in multi environments (Tables 3, 4, 5). Secondly, it revealed dynamic changes of QTLs through the whole developmental stages. The results indicated that ontogenetic curves of QTLs differed across environments (Tables 3, 4, 5). Finally, it provided information on the magnitude and the nature of the identified main effects and QE interactions for each putative QTL through jointly analyzing multi-environment data (Tables 4, 5). This study has two apparent advantages. One advantage is that a single QTL was tailed after through a continuance observation of tiller number to a single segment substitution line. Because a SSSL contains only one substituted segment from a donor in HJX74 genetic background, all the genetic variation between it and HJX74 can be thought to associate with the substituted segment (Eshed and Zamir 1995). Thus the dynamics tailed after and QE interaction effects estimated for a QTL in this paper should be more real and more dependable than those in previous studies that used conventional mapping populations as experimental materials. It is obvious that SSSLs are particular useful for investigating functional genomics of quantitative trait and providing direct materials for fine QTL mapping and plant breeding further. This paper is the first report on detection of developmental behavior and QE interaction effect of QTLs with SSSLs so far. Another advantage in this paper is that a putative QTL detected in one specific environment was decomposed into two components, main effect and QE interaction effect of QTL, so that their dynamics could be tailed after. Main effect of QTL is independent of changes in environmental conditions, while QE interaction effect is significantly affected by changes in environmental conditions (Yan et al. 1999; Cao et al. 2001). They reflect the stable and unstable parts of QTL, respectively.
Dynamics for tiller number
The theory of developmental genetics considers that QTL may have expression dynamics during the trait development, even though they may have the same final effects (Zhu 1995; Atchley and Zhu 1997). Therefore, it is necessary to reveal dynamic expression of QTL at serial stages. The developmental behavior of rice tiller number and plant height in a DH population were analyzed, and dynamic expression of QTLs for the two traits was revealed (Yan et al. 1998a, b; Cao et al. 2001). We also analyzed data derived from a single environment, and elucidated the developmental behavior of tiller number in rice with a set of single segment substitution lines (Zhao et al. 2008). In this paper, data derived from nine stages in four environments was further analyzed on tiller numbers. Dynamics of phenotype on tiller number is obvious (Fig. 1), which depends on environmental factors, genetic factors, and residual effects. At stages in a specific experiment, the environmental effects exhibited also dynamic change. Relative to the fall experiments (E2 and E3), environmental effects in spring season (averaged effects over E1 and E4) induced generally tiller numbers to descend in early period and to ascend in late stage (Table 2). In our experiments, spring season (from March to July) might be with relative low photothermal quotient (PTQ, radiation/temperature) in early period, therein the environmental factors would reduce tillering. Reason is that radiation drives growth (biomass accumulation), but temperature drives development (leaf appearance). Leaf appearance determines the window a tiller has for emergence. A low PTQ means little growth during the window for tiller appearance and hence little excess assimilates to produce tillers. While relative high PTQ or other favored environmental factors in late stage of spring season would increase tiller numbers. Reversely, early period and late stage during fall season (from July to November) had relative high and low PTQ or other adverse environmental factors, and hence their environmental factors would increase and decrease tillers, respectively (Table 2). The observation supports the fundamental that PTQ may play a role on crop growth and development (Leon et al. 2001). Genetic factors influencing tillers include genotype and genotype × environment interaction. Analysis of variance indicated that two genetic factors played an important role on tiller variations (Fig. 2). At an assigned stage, phenotypic variations of tiller number were always partially due to the two genetic factors. Although the proportion of phenotypic variance due to genotype × environment interaction effect (namely GE interaction heritability) kept approximately consistent, the narrow sense heritability changed across stages. At molecule level, genetic variations of tiller number derive from variations of QTL effects. Genetic main effect largely roots in main effect of QTLs, and GE interaction effect dose QE interaction effect. Both main effects and QE interaction effects of nine QTLs on tiller number at nine stages varied across stages (Tables 4, 5). Most main effects of QTLs approximately exhibited S-shaped curves, and came forth the utmost values during stage t5 or t6 (Table 4), which is consistent with the stage having the highest narrow sense heritability (Fig. 2). In conclusion, it is the dynamics of various influencing factors that resulted in the developmental behavior of tillering at different stages. The variations at three levels of phenotypic value, genetic effect and QTL effect were basically consistent.
QE interaction effects of QTLs
QE interaction is one of important genetic components affecting trait development, especially quantitative traits. QTL detected in one environment might confound the main effect and the QE interaction effect of QTL (Yan et al. 1998b; 1999). Zhu (1998) proposed a method to obtain predicted genetic main effects and GE interaction effects for exploring two components of QTL effects, main effects, and QE interaction effects. QE interaction effects of QTLs for many plant type traits and heading date in a rice DH population were estimated by the indirect method (Yan et al. 1998b, 1999; Li et al. 2003b). We also predicted components of QTLs on rice panicle number in a set of single segment substitution lines by using the method, in which the QE interaction effects were analyzed (Liu et al. 2008). In this paper, we determined three types of QTL effects, total effect, genetic main effect, and QE interaction effect of QTL. Their differences can be explained as follows. According to model (1) \( y_{jk(t)} = \mu_{(t)} + G_{j(t)} + B_{k(t)} + \varepsilon_{jk(t)} , \) the total effect of a QTL (Table 3) was estimated using the genetic effect value. Genetic main effect and QE interaction effect of a QTL originated from genetic main effect and GE interaction effect in model (2) \( y_{{{{hjk}}(t)}} = \mu_{(t)} + E_{h(t)} + G_{j(t)} + {\text{GE}}_{hj(t)} + B_{hk(t)} + \varepsilon_{hjk(t)} . \) Comparing two models, G j(t) in model (1) should include \( G_{j(t)} + {\text{GE}}_{{{{hj}}(t)}} \) and part of \( B_{{{{hk}}(t)}} + \varepsilon_{{{{hjk}}(t)}} \) in model (2) since μ (t) in model (1) equals to μ (t) + E h(t) in model (2). Thereby, the total effect of a QTL may be the mixture of main effect, QE interaction effect of the QTL and other effects, rather than the pure genetic effects. QTL effects estimated on a single environment in previous studies are biased, and comparing QTLs and their effects among multiple environments can not infer QE interaction effects. Total effects of nine QTLs estimated in this paper, being just as comparisons, are apparently different from sums of their main effects and QE interaction effects (Tables 3, 4, 5). Main effect and QE interaction effect of a QTL reflect two aspects of the existence of a QTL, environmental independency and environmental sensitivity. At any specific situation, a QTL includes always the two components. The suggestion of three types of QTLs (Liu et al. 2008) still takes effect in this paper. Those QTLs with only main effects are stable across environments, and marker-assisted selection for them can take place in any experimental environments. Whereas those QTLs with both main effects and QE interaction effects or with only QE interaction effects are unstable, which are usable only in specific environment. Furthermore, we found an interesting finding that QE interaction effects of nine QTLs fall into two groups with some exceptions regarding the seasonal patterns of QTL expression: spring (including E1 and E4) versus fall (including E2 and E3) (Table 5). This implied that environmental factors in spring season and in fall season would induce different expression of QTL. Perhaps the differences attribute to distinct PTQ between two crop seasons. Examination of the change of PTQ among different environments may provide useful information on how PTQ regulates QTL expression (Leon et al. 2001).
Temporal expression of QTLs
At the molecular level, ontogenetic changes in traits like tiller number of rice are manifested by different temporal patterns of gene expression (Zhu 1995; Atchley and Zhu 1997). In this paper, we examined successively the effects of nine QTLs at nine developmental stages in rice, and found that they exhibited dynamic change across stages (Tables 4, 5). Since QTL effect estimated at stage t is the accumulative value from initial time to stage t, QTL effect between stages t-1 and t can be inferred by their difference (Wu et al. 1999). Two common features of expression on nine QTLs are detected: (1) within 7 days after transplanting all QTLs didn’t express effects; (2) before a certain stage QTLs express effects largely in one direction but since then in opposite direction. Accordingly, there are three types of QTLs to be summed up. The first type is those expressed mainly in prophase. The second is those expressed twice, one in prophase and one in anaphase. QTL Tn6-3 belongs to the third type, which evenly expressed effects time after time (Table 4). It is the temporal expression of QTLs that result in genetic variations on tillers across developmental stages. Since, main effect of QTL is independent of changes in environmental conditions, and mean environmental factors over four experiments could be assumed to be consistent across stages, it might be the reasonable interpretation that gene expresses effects selectly at certain times (Yan et al. 1998a, b; Wu et al. 1999). Similarly, the same features with main effects of QTLs were also gained from QE interaction effects of QTLs (Table 5). However, QE interaction effect of QTL depends on environment conditions, and environmental factors in an experiment were distinct across stages, thus the temporal expression of QE interaction effects of QTLs might be induced by environmental factors on the spot. The expression difference likely attributes to the subrogation of environmental factors. Understanding the stages of QTL expression would help breeders in deciding when to use QTLs in their breeding program.
References
Atchley WR, Zhu J (1997) Developmental quantitative genetics, conditional epigenetical variability and growth in mice. Genetics 147:765–776
Bradshaw HD, Settler RF (1995) Molecular genetics of growth and development in populus. IV. Mapping QTLs with large effects on growth, form, and phenology traits in a forest tree. Genetics 139:963–973
Cao GQ, Zhu J, He CX, Gao YM, Yan JQ (2001) Impacts of epistasis and QTL × environment interaction for developmental behavior of plant height in rice (Oryza sativa L.). Theor Appl Genet 103:153–160
Dong YJ, Kamiunten HS, Ogawa TF, Tsuzuki EJ, Terao HY, Lin DZ, Matsuo MH (2004) Mapping of QTLs for leaf developmental behavior in rice (Oryza sativa L.). Euphytica 138:169–175
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141:1147–1162
He FH, Xi ZY, Zeng RZ, Zhang GQ (2005a) Developing single segment substitution lines (SSSLs) in rice (Oryza sativa L.) using advanced backcrosses and MAS. Acta Genet Sinica 32(8):825–831 (in Chinese)
He FH, Xi ZY, Zeng RZ, Zhang GQ (2005b) Mapping heading date QTL in rice (Oryza sativa L.) using single segment substitution lines (SSSLs). Acta Agric Sinica 38(8):1505–1513 (in Chinese)
He FH, Zeng RZ, Xi ZY, Talukdar A, Zhang GQ (2005c) Identification of QTLs for plant height and its components by using single segment substitution lines in rice (Oryza sativa L.). Rice Science 12(3):151–156
Hittalmani S, Shashidhar HE, Bagali PG, Huang N, Sidhu JS, Singh VP, Khush GS (2002) Molecular mapping of quantitative trait loci for plant growth, yield and yield related traits across three diverse locations in a doubled haploid rice population. Euphytica 125:207–214
Hittalmani S, Huang N, Courtois B, Venuprasad R, Shashidhar HE, Zhuang JY, Zheng KL, Liu GF, Wang GC, Sidhu JS, Srivantaneeyakul S, Singh VP, Bagali PG, Prasanna HC, McLaren G, Khush GS (2003) Identification of QTL for growth- and grain yield-related traits in rice across nine locations of Asia. Theor Appl Genet 107:679–690
Ishikawa S, Maekawa M, Arite T, Onishi K, Takemura I, Kyozuka J (2005) Supression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46:79–86
Jiang GH, Xu CG, Li XH, He YQ (2004) Characterization of the genetic basis for yield and its component traits of rice revealed by doubled haploid population. Acta genetica sinica 31(1):63–72
Jiang GH, Guo LB, Xue DW, Zeng DL, Zhang GH (2006) Genetic analysis and gene-mapping of two reduced-culm- number mutants in rice. J Integr Plant Biol 48(3):341–347
Leon AJ, Lee M, Andrade FH (2001) Quantitative trait loci for growing degree days to flowering and photoperiod response in Sunflower (Helianthus annuus L.). Theor Appl Genet 102:497–503
Li YH (1979) Morphology and Anatomy of Grass Family Crops. Shanghai Science and Technology Press, Shanghai, pp 138–142 (in Chinese)
Li X, Qian Q, Fu Z, Wang Y, Xiong G, Zeng D, Wang X, Liu X, Teng S, Fujimoto H, Yuan M, Luo D, Han B, Li J (2003a) Control of tillering in rice. Nature 422:618–621
Li ZK, Yu SB, Lafitte HR, Huang N, Courtois B, Hittalmani S, Vijayakumar CHM, Liu GF, Wang GC, Shashidhar HE, Zhuang JY, Zheng KL, Singh VP, Sidhu JS, Srivantaneeyakul S, Khush GS (2003b) QTL × environment interactions in rice. I. Heading date and plant height. Theor Appl Genet 108:141–153
Lin HX, Qian HR, Zhuang JY, Lu J, Min SK, Xiong ZM, Huang N, Zheng KL (1996) RFLP mapping of QTLs for yield and related characters in rice (Oryza sativa L.). Theor Appl Genet 92:920–927
Liu GF, Yang J, Xu HM, Zhu J (2006) Genetic analysis on tiller number and plant height per plant in rice (Oryza sativa L.). J Zhejiang Univ (Agriculture and life science) 32(5):527–534
Liu GF, Zhang ZM, Zhu HT, Zhao FM, Ding XH, Zeng RZ, Li WT, Zhang GQ (2008) Detection of QTLs with additive effects and additive-by-environment interaction effects on panicle number in rice (Oryza sativa L.) by using single segment substituted lines. Theor Appl Genet 116:923–931
McCouch SR, Doerge R (1995) QTL mapping in rice. Trends Genet 11:482–487
Murai M, Kinoshita T (1986) Diallel analysis of traits concerning yield in rice. Jpn J Breed 36:7–15
Perera ALT, Senadhira D, Lawrence MJ (1986) Genetic architecture of economically important characters and prediction of performance of recombinant inbred lines in rice. In: Rice Genetics. IRRI, Manila, pp 564–578
Plomion C, Durel CE, O’Malley DM (1996) Genetic dissection of height in maritime pine seedlings raised under accelerated growth conditions. Theor Appl Genet 93:849–858
Price AH, Tomos AD (1997) Genetic dissection of root growth in rice (Oryza sativa L.): II. Mapping quantitative trait loci using molecular markers. Theor Appl Genet 95:143–152
Tang JB, Zeng WY, Wang WM, Ma BT, Liu Y (2001) The genetic analysis and gene mapping of a few-tillering mutant in rice. Sci China (Ser C) 3:208–212
Tian F, Li DJ, Fu Q, Zhu ZF, Fu YC, Wang XK, Sun CQ (2006) Construction of introgression lines carrying wild rice (Oryza rufipogon Griff.) segments in cultivated rice (Oryza sativa L.) background and characterization of introgressed segments associated with yield-related traits. Theor Appl Genet 112:570–580
Wan JL, Zhai HQ, Wan JM, Hideshi Y, Atsushi Y (2003) Mapping QTL for traits associated with resistance to ferrous iron toxicity in rice (Oryza sativa L.), using japonica chromosome segment substitution lines. Acta Genetica Sinica 30(10):893–898
Wang DL, Zhu J, Li ZK, Paterson AH (1999) Mapping QTLs with epistatic effects and QTLs by environment interaction by mixed linear model approaches. Theor Appl Genet 99:1255–1264
Wu P, Zhang GQ, Huang N (1996) Identification of QTLs controlling quantitative characters in rice using RFLP markers. Euphytica 89:349–354
Wu WR, Li WM, Tang DZ, Lu HR, Worland AJ (1999) Time-related mapping of quantitative trait loci underlying tiller number in rice. Genetics 151:297–303
Xi ZY, He FH, Zeng RZ, Zhang ZM, Ding XH, Li WT, Zhang GQ (2006) Development of a wide population of chromosome single segment substitution lines in the genetic background of an elite cultivar of rice (Oryza sativa L.). Genome 49:476–484
Xiao J, Li J, Yuan L, Tanksley L (1995a) Dominance is the major genetic basis of heterosis in rice as revealed by QTL analysis using molecular markers. Genetics 140:745–754
Xiao J, Li J, Yuan L, Tanksley L (1995b) Identification of QTLs affecting traits of agronomic importance in a recombinant inbred population derived from a subspecific rice cross. Theor Appl Genet 92:230–244
Xiong ZM (1992) Research outline on rice genetics in China. In: Xiong ZM, Cai HF (eds) Rice in China. Chinese Agricultural Science Press, Beijing, p 4057
Xu YB, Shen ZT (1991) Diallel analysis of tiller number at different growth stages in rice (Oryza Sativa L.). Theor Appl Genet 83:243–249
Yan JQ, Zhu J, He C, Benmoussa M, Wu P (1998a) Quantitative trait loci analysis for the developmental behavior of tiller number in rice (Oryza sativa L.). Theor Appl Genet 97:267–274
Yan JQ, Zhu J, He C, Benmoussa M, Wu P (1998b) Molecular dissection of developmental behavior of plant height in rice (Oryza sativa L.). Genetics 150:1257–1265
Yan JQ, Zhu J, He C, Benmoussa M, Wu P (1999) Molecular marker-assisted dissection of genotype × environment interaction for plant type traits in rice (Oryza sativa L.). Crop Sci 39:538–544
Yano M, Harushima Y, Nagamura Y, Kurata N, Minobe Y, Sasaki T (1997) Identification of quantitative trait loci controlling heading date in rice using a high–density linkage map. Theor Appl Genet 95:1025–1032
Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait loci in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. The Plant Cell 12:2473–2483
Yano M, Kojima S, Takahashi Y, Lin HX, Sasaki T (2001) Genetic control of flowering time in rice, a short-day plant. Plant Physiology 127:1425–1429
Zhang GQ, Zeng RZ, Zhang ZM, Ding XH, Li WT, Liu GM, He FH, Tulukdar A, Huang CF, Xi ZY, Qin LJ, Shi JQ, Zhao FM, Feng MJ, Shan ZL, Chen L, Guo XQ, Zhu HT, Lu YG (2004) The construction of a library of single segment substitution lines in rice (Oryza sativa L.). Rice Genet Newsl 21:85–87
Zhao FM, Liu GF, Zhu HT, Ding XH, Zeng RZ, Zhang ZM, Li WT, Zhang GQ (2008) Unconditional and conditional QTL mapping for tiller numbers at various stages with single segment substitution lines in rice (Oryza sativa L.). Agric Sci China 7:257–265
Zhu J (1992) Mixed model approaches for estimating variances and covariances. J Biomath 7(1):1–11
Zhu J (1994) General genetic models and new analysis methods for quantitative traits. J Zhejiang Agric Univ 20(6):551–559 (in Chinese)
Zhu J (1995) Analysis of conditional genetic effects and variance components in developmental genetics. Genetics 141:1633–1639
Zhu J (1996) Analysis methods for seed models with genotype × environment interactions. Acta Genetica Sinica 23(1):56–68 (in Chinese)
Zhu J (1998) Mixed model approaches of mapping genes for complex quantitative traits. In: Wang LZ, Dai JR (eds) Proceedings of genetics and crop breeding of China. Chinese Agricultural Science and Technology Publication House, Beijing, pp 19–20
Zhu J (1999) Mixed model approaches of mapping genes for complex quantitative traits. J Zhejiang Univ (Natural Science) 33:327–335
Zhu J, Weir BS (1996) Diallel analysis for sex-linked and maternal effects. Theor Appl Genet 92(1):1–9
Zou JH, Chen ZX, Zhang SY, Zhang WP, Jiang GH et al (2005) Characterizations and fine mapping of a mutant gene for high tillering and dwarf in rice (Oryza sativa L.). Planta 222:604–612
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30330370 and 30830074).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Cooper.
G. Liu and R. Zeng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, G., Zeng, R., Zhu, H. et al. Dynamic expression of nine QTLs for tiller number detected with single segment substitution lines in rice. Theor Appl Genet 118, 443–453 (2009). https://doi.org/10.1007/s00122-008-0911-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-008-0911-3