Abstract
A novel cytoplasmic male sterility (CMS) was identified in Brassica juncea, named as hau CMS (00-6-102A). Subsequently, the male sterility was transferred to B. napus by interspecific hybridization. The hau CMS has stable male sterility. Flowers on the A line are absolutely male sterile, and seeds harvested from the line following pollinations with the maintainer gave rise to 100% sterile progeny. The anthers in CMS plants are replaced by thickened petal-like structures and pollen grains were not detected. In contrast, in other CMS systems viz. pol, nap, tour, and ogu, anthers are formed but do not produce viable pollen. The sterility of hau CMS initiates at the stage of stamen primordium polarization, which is much earlier compared with the other four CMS systems. We have successfully transferred hau CMS from B. juncea to B. napus. Restorer lines for pol, ogu, nap, and tour CMS systems were found to be ineffective to restore fertility in hau CMS. Sixteen out of 40 combinations of mitochondrial probe/enzyme used for RFLP analysis distinguished the hau CMS system from the other four systems. Among these sixteen combinations, five ones alone could distinguish the five CMS systems from each other. The evidence from genetic, morphological, cytological and molecular studies confirmed that the hau CMS system is a novel CMS system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapeseed (Brassica napus L.) is one of the major oilseed crops grown widely in European countries, Canada, China and Australia. Cytoplasmic male sterility (CMS) is an important approach to exploit heterosis in this crop (Fu 1995). CMS is a common trait in angiosperms (Kaul 1988) and is maternally inherited resulting in failure to produce functional pollen. CMS occurs in a variety of plant species and is often associated with novel mitochondrial open reading frames, which interfere with the proper functioning of mitochondria and pollen development (Hanson 1991; Hanson and Folkerts 1992; Bonen and Brown 1993). Several CMS systems have been reported in rapeseed which include Raphanus/ogu (Ogura 1968), tour (Mathias 1985; Stiewe and Röbbelen 1994), polima or pol (Fu 1981), and nap (Shiga and Baba 1971, 1973; Thompson 1972). Pol CMS was discovered in 1972 (Fu 1981), and became the first CMS system to be extensively utilized for hybrid seed production (Fan et al. 1986; Röbbelen 1991). The pol CMS is sensitive to environment in certain nuclear backgrounds leading to breakdown of sterility, which reduces hybridity levels in F1 hybrids.
A number of studies have been carried out for the characterization and identification of CMS systems in many crops including rice, wheat, sugar beet, sunflower, common bean, onion, Arabidopsis, pearl millet and radish based on mitochondrial DNA analyses (Delorme et al. 1997; Schnable and Wise 1998; Horn and Friedt 1999; Nahm et al. 2005). According to restorer and maintainer relationships, rice cytoplasm in sterile lines was classified into WA type, HL type and Dian-type (Li 1980). Analyses of mitochondria DNA sequence can also distinguish the cytoplasm types in CMS rice and maize (Kadowaki et al. 1988; Wei et al. 1997). In maize, mtDNA of the T, S and C group had unique electrophoresis bands, which could be used to classify the CMS types (Kemble et al. 1980). So far, nine CMS lines in B. napus have been characterized into four types as listed above (pol, ogu, nap, tour) by analysis of restorer and maintainer relationships and mtDNA RFLPs (Yang et al. 1998). The recent reports of CMS in Brassica include CMS 681A of spontaneous origin in a mutant line of a B. napus cv. Xiangyu 13. It has the same restorer and maintainer relationship as pol CMS but possessed different mitochondrial DNA sequences (Liu et al. 2005). Another CMS system named “126-1” was identified in a population of doubled haploids of synthetic B. napus ISN 706 and subsequently transferred to B. juncea (Sodhi et al. 2006). Several CMS systems of alloplasmic origin have also been reported in B. juncea containing cytoplasm of several wild species viz. B. oxyrrhina (Prakash and Chopra 1990), B. tournefortii (Pradhan et al. 1991; Arumugam et al. 1996), Diplotaxis siifolia (Rao et al. 1994), Moricandia arvensis (Prakash et al. 1998), Erucastrum canariense (Prakash et al. 2001), Diplotaxis erucoides (Bhat et al. 2006 ) and D. berthautii (Bhat et al. 2007).
We have identified a new cytoplasmic male sterility system in mustard (B. juncea), and we report here its characterizations based on genetic, morphological, cytological and molecular studies, and its transfer to B. napus by interspecific hybridization.
Materials and methods
Plant materials
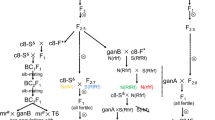
All materials used in this study belong to Huazhong Agricultural University (HAU) and are listed in Table 1. The hau CMS (00-6-102A) was originally found as a spontaneous male sterile mutant in B. juncea in the experimental field of HAU in 1999 (T.D. Fu, unpublished). The hau CMS (00-6-102A) lines were examined for 10 consecutive generations during a 5-year period in Lanzhou in summer (altitude 2,200 m, latitude 37°N, average temperature 20.4°C) and in Wuhan in spring (altitude 50 m, latitude 30°N, average temperature 17°C) in China. The maintainer was another B. juncea line 00-6-102B. Lines 6w-301 and 6w-307 of B. napus were used as recurrent parents in crosses with hau CMS for ten generations (BC10) to establish this CMS system in B. napus.
The assessment of sterility
To assess “sterility degree” in the 11th generation plants of backcross, 3,000 flowers from 300 plants (ten flowers per inflorescence per plant) were covered with pollination bags to prevent pollinations. The number of flowers inside the pollination bags resulting in pods were recorded at maturity. To assess “sterility rate”, 1,000 plants of B. juncea hau CMS were planted, and the fertility of individual plants (the number of plants with stamens in flowers) was recorded at flowering.
Cytological studies
Immature buds (about 1.8 mm in length) of 00-6-102A and 00-6-102B were randomly collected for cytological study. They were fixed in carnoy I solution for 24 h and subsequently transferred to 70% ethanol for long-term storage. The buds were embedded in wax, sectioned and observed under Leica DMLB microscope (wetzlar, Gemany) equipped with CCD (LEICA DL73300F) (Yu and Fu 1988).
Analysis of restorer and maintainer relationship
Fourteen breeding lines (restorers and maintainers of different CMS systems) were tested for their ability to act as restorers and maintainers across several CMS lines (Table 1). Pollination was carried out by hand and F1 seeds were harvested and F1 plants were assessed for the degree of male sterility. The following categories could be recognized according to the method of Pahwa et al. (2004): (1) Male fertile: flowers with normal sized petals and anthers containing fertile pollen, and seed set on selfing to a level similar to that in a fertile euplasmic check. (2) Male sterile: flowers with narrow petals and rudimentary anthers with little or no fertile pollen and no seed set on selfing. (3) Partially fertile: flowers having medium sized petals and small mal-formed anthers positioned significantly below the stigma with reduced pollen load and poor seed set on selfing.
DNA extraction and mitochondrial probes
Buds collected from five different CMS and maintainer lines in the field were used for extraction of total genomic DNA. Total DNA from six lines, one maintainer line and five different CMS, were extracted using a modified CTAB method (Doyle and Doyle 1990). Young and healthy buds were ground into a fine powder with liquid nitrogen. About 10 g of frozen powder was transferred into pre-labelled 50 ml polypropylene tubes, which were chilled with liquid nitrogen. A preheated (65°C) 20 ml extraction buffer (0.5 M NaCl, 100 mM Tris–HCl (pH 8.0), 50 mM EDTA (pH 8.0), 2% CTAB) was adjusted to pH 7.5 and 0.5 ml β-mercaptoethanol was then added to each tube. Each tube was mixed thoroughly with gentle agitation and incubated for 60 min at 65°C. Chloroform: isoamyl alcohol (24:1) was then added to the tubes and mixed thoroughly with gentle agitation. The tubes were then centrifuged for 15 min at 6,500 rpm. Supernatant DNA was precipitated by the addition of an equal volume of ice-cold isopropanol. The precipitated DNA was rinsed twice with 70% ethanol, and transferred to a sterile 1.5 ml Eppendorf tube. The precipitated DNA was then dissolved in sterile water and quantified using agarose gel electrophoresis. DNA concentration and purity were measured by a Beckman spectrophotometer at a wavelength of 260 and 280 nm.
Mitochondrial probes were either cloned and PCR amplified based on published information of Arabidopsis thaliana (NC_001284.2, GI:26556996), wheat, maize or B. napus (Table 2).
RFLP analysis of mitochondrial DNA
RFLP analysis was performed as previously described (Kang et al. 2001), with some minor modifications. Total DNA was digested with five different restriction enzymes—EcoRI, EcoRV, HindIII, BamHI, PstI. Restriction digestion was carried out with 2 U restriction enzymes per microgram of DNA. Approximately 20 μg DNA was digested for 12 h with each enzyme, with the total volume of 50 μl. Genome DNA was separated on 0.8% agar in 1× TAE buffer, and then transferred to nylon membrane, after which the membrane was conserved in 20× SSC. Mitochondrial specific probes were used for RFLP analysis (Landgren et al. 1996; Delorme et al 1997; Nahm et al. 2005). The probes were labelled with α-[32P]dCTP. The labelled probes were denatured by alkaline treatment with a final concentration of 0.4 N NaOH, and were added to filters in 30 ml of hybridization buffer (5× SSC, 0.4% SDS, 5× Denhardt’s reagent). Hybridization was conducted at 65°C for 16 h. Filters were washed twice (cold 5 min and hot 15 min at 65°C) with 1× SSC, 0.1% SDS, and then again with 0.5× SSC, 0.1% SDS (hot 15 min at 65°C). The filters were then placed under Agfa CP-BU film for several days, depending on the strength of the signal.
Results
Development of hau CMS in B. juncea and in B. napus
The male sterility found in the spontaneous mutant in B. juncea could be maintained in this species After ten generations of backcrossings with B. juncea 00-6-102B, a stable sterile B. juncea line (hau CMS) was established. The “sterility degree” of 3,000 flowers was 100% and the “sterility rate” of 1,000 plants was also 100% in Lanzhou and Wuhan, indicating the stability of hau CMS in two different environments. This CMS was transferred to B. napus through interspecific hybridization. The resultant B. napus CMS was also absolute and stable when 6w-301 and 6w-307 were used as maintainers.
Cytological observation of hau CMS anther development
Anthers of hau CMS line 00-6-102A transformed into thickened petal-like structures, which had no anther and filament (Fig. 1). These structures were similar in shape and colour to normal petals. However, the transverse section showed that they were thicker with increased cellular layers than normal petals and had no pollen sacs (Fig. 2). The stamen primordium deviated from the normal polarization and formed petal primordium, which developed into petal-type structures at the stamen location. These characteristics differed from other CMS systems reported so far. The pollen abortion stage was earlier than other CMS types (Yu and Fu 1988; Grant et al. 1986). The anther development of maintainer 00-6-102B was normal, and each anther had four normal pollen sacs in papilionaceous shape (Fig. 2). The A-lines of pol, nap, tour, and ogu CMS systems formed anthers, but were devoid of functional pollen.
Micrographs (×40) of microstructure of anther development. a B. juncea hau CMS line 00-6-102A: stamens completely degenerate into small and thick petals. Petals have many layers of cell, no polarization of archesporial cell. b B. juncea hau maintainer line 00-6-102B (normal cytoplasm): the outermost layer is calyx, which co-exists with petals, and there are six normal stamens with normal anther development
The restorer and maintainer relationship of hau CMS
When pol CMS restorer 6-300R was crossed with five different CMS systems, progenies of pol CMS were fertile but progenies of the other four CMS lines were completely male sterile. When pol CMS restorers such as 3706, 3721, 5148, 5200, 5900, 71-1, Hui10 were crossed with five CMS systems, progenies of pol CMS and nap CMS were all fertile but progenies of the other three CMS lines were completely male sterile (Table 3). Similarly, tour CMS restorer 6-260R restored the fertility of tour CMS but not the other four CMS lines. Ogu CMS restorer 6-301R was also specific only to ogu CMS, and nap CMS restorer 5021C was specific to nap CMS. When crossed to restorers of other CMS systems, the progenies of hau CMS, were all sterile. Therefore, none of the restorers of other CMS systems was effective in restoring hau CMS (Table 3). To identify restorers and maintainers of hau CMS in B. napus, more than 140 different lines of wide origin were used as pollinators in Lanzhou and Wuhan during a 5-year period (data not shown), but restorer lines could not be found. On being crossed to a synthetic B. napus 05A-409 (the progeny of hybrids between B. oleracea and B. rapa), the progenies of hau CMS were partially fertile but no seed set was obtained on selfing.
RFLP analysis of mitochondrial DNA
The polymorphism of mitochondrial DNA was detected with mitochondrial probes atp1, orf222, atp6, atp9, cob, coxII, Orf222-nad5-orf139, coxI, atpα and Orf263-atp6. Out of 40 mitochondrial probe/enzyme combinations, 16 combinations exhibited polymorphism in Table S1 (Electronic supplementary material), such as atp6/HindIII, atp6/EcoRI, atp6/EcoRV, atp9/BamHI, atp9/EcoRI and atp1/BamHI (Fig. 3). Hau CMS 00-6-102A showed different banding patterns from the other four CMS lines and its maintainer line (00-6-102B). Out of 16 combinations, five probe/enzyme combinations alone distinguished the five CMS systems from each other (Table S1 of ESM; Fig. 3f). The atp6 probe in combinations with three restriction endonucleases (HindIII, EcoRI and EcoRV) distinguished the hau cytoplasm from pol, nap, ogu and tour (Fig. 3a–c). In combination of atp6/HindIII, the main band (3.9 kb) of pol, nap, ogu and tour cytoplasms was the same as that of the maintainer line of hau (00-6-102B), but different from the main band (3.0 kb) of hau cytoplasms (Fig. 3a). In two other combinations (atp6/EcoRI and atp6/EcoRV), only one band was observed for each material, and the polymorphism of the bands could distinguish the hau from pol, nap, ogu and tour cytoplasms (Fig. 3b, c). The atp9 probe in combinations with two restriction endonucleases (EcoRI and BamHI) also allowed us to distinguish the hau from the other cytoplasms (Fig. 3d, e). The combination of atp1/BamHI alone could distinguish the five CMS from each other (Fig. 3f). These results indicated that the mitochondrial DNA of hau CMS 00-6-102A was different from those of pol, nap, ogu and tour.
Southern analysis between five CMS and one maintainer lines. Total DNAs were digested with EcoRV, EcoRI, HindIII and BamHI, blotted onto a nylon membrane and hybridized with a gene-specific atp9, atp1 and atp6 mitochondrial probe. a atp6/HindIII, b atp6/EcoRI, c atp6/EcoRV, d atp9/BamHI, e atp9/EcoRI, f atp1/BamHI. Lanes 1 pol CMS, 2 hau CMS 00-6-102A, 3 hau maintainer 00-6-102B, 4 tour CMS, 5 nap CMS, 6 ogu CMS, M markers
Discussion
Investigations on the hau CMS in B. juncea along with pol, nap, ogu, and tour CMS on cytology, general genetics and molecular genetics show that hau CMS is a novel type of cytoplasmic male sterility. Its sterility is stable and complete with the sterility degree and sterility rate both 100%. When the sterile cytoplasm of hau CMS was introduced into B. napus following interspecific hybridization, the sterility was very stable and absolute. Compared with other CMS systems, stable and complete sterility of hau system makes it very suitable for Cruciferous vegetable breeding where the potential of heterosis has already been reported (Ke et al. 1992). This sterility is probably due to its complete absence of anthers and their transformation to petals.
Several approaches have been used to characterize and classify various CMS systems by researchers, based on the original cytoplasm classification, restorer and maintainer relationships classification (Beckett 1971), anther abortion stage classification (Yu and Fu 1988), molecular genetic classification, and sporophytic and gametophytic sterility classification. In the present investigation, we adopted an integrated approach to classify the five types of CMS systems at morphological, cellular, genetic and molecular levels and confirmed that the hau CMS is different from others. The integrated approach to classify CMS systems, outlined in this paper, helps to avoid unnecessary duplicated researches and to correctly select CMS systems for different breeding purposes.
The main disadvantage of the pol CMS system is that male sterility is sensitive to environment in different nuclear backgrounds, leading to its breakdown and selfing during the process of hybrid seed production (Fu 1995). Nap CMS is very sensitive to temperature, thus limiting its use in hybrid production. Tour CMS has poor “sterility degree” with pollen production and is not used in hybrid production (Fu 1995). At present, pol and ogu CMS are widely used over the world. The discovery of hau CMS helps to increase the genetic diversity of CMS. The hau CMS system is not affected by environment and also the sterility degree and rate were both 100%. This CMS was introgressed to B. napus and Cruciferous vegetables (such as B. juncea var. capitata Hort.ex Li, B. juncea var. multiceps Tsen et Lee, B. juncea Czern. et Coss. var. tsatsai Mao, B. rapa Linn. var. purpurea Bailey and B. rapa var. pekinensis ) by hybridization. The sterility of obtained CMS lines was stable and complete, in contrast to other four CMS types.
The hau CMS system appears to result from a disruption of anther development at a very early stage of stamen primordium differentiation. In contrast, the anther development of pol CMS was inhibited at the polarization stage of the archespore, therefore no pollen sacs were formed (Yu and Fu 1988). The anther sterility of nap CMS results from delayed pollen development and indehiscent anthers (Grant et al. 1986). The anther development of ogu CMS was inhibited at the tetrad to single nucleus pollen formation stage. The development of sporule was similar to fertile lines, but tetrad release was very difficult and the duration was also long (Yu and Fu 1988). The results indicate that the hau CMS was unique and different from other CMS systems in rapeseed. Development disruption of the pol, tour, ogu, and nap CMS systems must occur at a later stage than the hau CMS system, as these systems form normal-looking anthers, but fail to develop normal archesporial or microspore cells.
The identification of the restorer and maintainer relationship is one of the most classical methods to differentiate the types of sterile cytoplasm. The hau CMS line 6W-301A has the same nucleus genetic background as its B. napus maintainer line 6W-301 after ten backcross generations (BC10). The results of test crossing with restorer lines indicated that fertility in the hau CMS was not restored by restorer lines from pol, ogu, nap and tour CMS systems. Therefore we conclude that hau CMS is a distinct type of cytoplasmic male sterility. A maintainer line for hau CMS has been developed but a restorer line has not been found so far. The absence of a restorer means that the hau CMS is not currently useful for rapeseed breeding or production. However, we observed that some progenies of hau CMS were partially fertile when crossed to a few testing materials, which indicates that the test materials carry some restorer genes for hau CMS.
RFLP technology was effective in identifying several polymorphisms in mitochondrial DNA, which clearly distinguished the five different CMS systems. The restoration and maintenance of kos CMS were the same as that of ogu CMS in radish. But RFLP and sequence analysis reveal that their mechanisms are different (Sakai and Imamura 1992). Similarly, 681A and pol CMS have the same restoring–maintaining relationship and belong to the same type of CMS revealed on the basis of restoring–maintaining relationship. However, their ORFs of sterility genes are probably different as well (Liu et al 2005).
In the present hau CMS, we need to study the fertility transition mechanism of hau CMS, to search for the restoring lines and to isolate and clone the Rf gene(s). It will be very interesting to study the expression of Rf gene and the interaction between Rf gene and sterility associated mtDNA location. We aim at discovering the mechanism of CMS fertility, maintenance and restoration. The molecular classification conducted in this research can be used as a foundation for the future cloning of the CMS genes, the study of transcription process and the expression of the genes at enzyme level.
References
Arumugam N, Mukhopadhyay A, Gupta V, Pental D, Pradhan AK (1996) Synthesis of hexaploid (AABBCC) somatic hybrids:a bridging material for transfer of ‘tour’ cytoplasmic male sterility to different Brassica species. Theor Appl Genet 92:762–768
Beckett JB (1971) Classification of male-sterile cytoplasms in maize (Zea mays). Crop Sci 11:724–727
Bhat SR, Vijayan P, Ashutosh, Dwivedi KK, Prakash S (2006) Diplotaxis erucoides-induced cytoplasmic male sterility in Brassica juncea is rescued by the Moricandia arvensis restorer: genetic and molecular analyses. Plant Breed 125:150–155
Bhat SR, Kumar P, Prakash S (2007) An improved cytoplasmic male sterile (Diplotaxis berthautii) Brassica juncea: identification of restorer and molecular characterization. Euphytica. doi:10.1007/s10681-007-9467-6
Bonen L, Boer PH, Mclntosh JE, Gray MW (1987) Nucleotide sequence of the wheat mitochondrial gene for subunit I of cytochrome oxidase. Nucleic Acids Res 15:6734
Bonen L, Brown GG (1993) Genetic plasticity and its consequences: perspectives on gene organization and expression in plant mitochondria. Can J Bot 71:645–660
Braun CJ, Levings CS III (1985) Nucleotide sequence of the F1-ATPase a subunit gene from maize mitochondria. Plant Physiol 79:571–577
Delorme V, Keen CL, Rai KN, Leaver CJ (1997) Cytoplasmic-nuclear male sterility in pearl millet:comparative RFLP and transcript analyses of isonuclear male-sterile lines. Theor Appl Genet 95:961–968
Dewey RE, Levings CS III, Timothy DH (1985a) Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol 79:914–919
Dewey RE, Schuster AM, Levings CS III, Timothy DH (1985b) Nucleotide sequence of F0-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci USA 82:1015–1019
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fan Z, Stefansson BR, Sernyk JL (1986) Maintainers and restorers for three male-sterility—inducing cytoplasms in rape (Brassica napus L. ). Can J Plant Sci 66:229–234
Fox TD, Leaver CJ (1981) The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell 26:315–323
Fu TD (1981) Production and research of rapeseed in the People’s Republic of China. Eucarpia Cruciferae Newsl 6:6–7
Fu TD (1995) Breeding and utilization of rapeseed hybrid. Hubei Science and Technology Press, Wuhan, pp 42–135
Grant I, Beversdorf WD, Peterson RL (1986) A comparative light and electron microscopic study of microspore and tapetal development in male fertile and cytoplasmic male sterile oilseed rape (Brassica napus). Can J Bot 64:1055–1086
Hanson MR (1991) Plant mitochondrial mutations and male sterility. Annu Rev Genet 25:461–486
Hanson MR, Folkerts O (1992) Structure and function of the higher plant mitochondrial genomes. Int Rev Cytol 141:129–172
Horn R, Friedt W (1999) CMS Sources in sunflower: different origin but same mechanism? Theor Appl Genet 98:195–201
Kadowaki K, Osumi T, Nemoto H, Harada K, Shinjyo C (1988) Mitochondrial DNA polymorphism in male-sterile cytoplasm of rice. Theor Appl Genet 75:234–236
Kang BC, Nahm SH, Huh JH, Yoo HS, Yu JW, Lee MH, Kim BD (2001) An interspecific (Capsicum annuum x C. chinese) F2 linkage map in pepper using RFLP and AFLP markers. Theor Appl Genet 102:531–539
Kaul MLH (1988) Male sterility in higher plants. Springer, Berlin
Ke GL, Zhao ZY, Song YZ (1992) Breeding of alloplasmic male sterile line CMS3411-7 in Chinese cabbage (Brassica campestris L. ssp. pekinensis (Lour.) Olsson) and its application. Acta Hort Sin 19:333–340
Kemble RJ, Gunn RE, Flavell RB (1980) Classification of normal and male sterile cytoplasms in maize. II. Electrophoretic analysis of DNA species in mitochondria. Genetics 95:451–458
Landgren M, Zetterstrand M, Sundberg E, Glimelius K (1996) Alloplasmic male-sterile Brassica lines containing B. tournefortii mitochondria express an ORF 3′ of the atp6 gene and a 32 kDa protein. Plant Mol Biol 32:879–890
L’Homme Y, Stahl RJ, Li XQ, Hameed A, Brown GG (1997) Brassica nap cytoplasmic male sterility is associated with expression of a mtDNA region containing a chimeric gene similar to the pol CMS-associated orf224 gene. Curr Genet 31:325–335
Li ZB (1980) A preliminary discussion about the classification of male sterile lines of rice in China. Acta Agron Sin 6:17–28
Liu Z, Guan C, Zhao F, Chen S (2005) Inheritance and mapping of a restorer gene for the rapeseed cytoplasmic male sterile line 681A. Plant Breed 124:5–8
Mathias R (1985) Transfer of cytoplasmic male sterility from brown mustard (Brassica juncea (L.) Coss.) into rapeseed (Brassica napus L.). Z Pflanzenzüchtg 95:371–374
Nahm SH, Lee HJ, Lee SW, Joo GY, Harn CH, Yang SG, Min BW (2005) Development of a molecular marker specific to a novel CMS line in radish (Raphanus sativus L.). Theor Appl Genet 111:1191–1200
Ogura H (1968) Studies on the new male-sterility in Japanese radish with special reference to the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima Univ 6:39–78
Pahwa RS, Banga SK, Gogna KPS, Banga SS (2004) Tournefortii male sterility in Brassica napus. Identification, expression and genetic characterization of male fertility restorers. Plant breed 123:444–448
Pradhan AK, Mukhopadhyay A, Pental D (1991) Identification of the putative cytoplasmic donor of a CMS system in Brassica juncea. Plant Breed 106:204–208
Prakash S, Chopra VL (1990) Male sterility caused by cytoplasm of Brassica oxyrrhina in B. campestris and B. juncea. Theor Appl Genet 79:285–287
Prakash S, Kirti PB, Bhat SR, Gaikwad K, Dinesh Kumar V, Chopra VL (1998) A Moricandia arvensis-based cytoplasmic male sterility and fertility restoration system in Brassica juncea. Theor Appl Genet 97:488–492
Prakash S, Ahuja I, Upreti HC, Dinesh Kumar V, Bhat SR, Kirti PB, Chopra VL (2001) Expression of male sterility in alloplasmic Brassica juncea with Erucastrum canariense cytoplasm and the development of a fertility restoration system. Plant Breed 120:479–482
Rao GV, Batra-Sarup V, Prakash S, Shivanna KR (1994) Development of a new cytoplasmic male-sterile system in Brassica juncea through wide hybridization. Plant Breed 112:171–174
Röbbelen G (1991) Citation at the occasion of presenting the GCIRC Superior Scientist Award to FU Tingdong. In: Proceedings of eighth international rapeseed congress, vol 1. Sasktoon, Canada, pp 2–5
Sakai T, Imamura J (1992) Alteration of mitochondrial genomes containing atpA genes in the sexual progeny of cybrids between Raphanus sativus CMS line and Brassica napus cv. Westar. Theor Appl Genet 84: 923–929
Schnable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180
Shiga T, Baba S (1971) Cytoplasmic male sterility in rape plants (Brassica napus L.). Jpn J Breed 21:16–17
Shiga T, Baba S (1973) Cytoplasmic male sterility in oilseed rape, Brassica napus L., and its utilization to breedling. Jpn J Breed 23:187–197
Sodhi YS, Chandra A, Verma JK, Arumugam N, Mukhopadhyay A, Gupta V, Pental D, Pradhan AK (2006) A new cytoplasmic male sterility system for hybrid seed production in Indian oilseed mustard Brassica juncea. Theor Appl Genet 114:93–99
Stiewe G, Röbbelen G (1994) Establishing cytoplasmic male sterility in Brassica napus by mitochondrial recombination with B. tournefortii. Plant Breed 113:294–304
Thompson KF (1972) Cytoplasmic male sterility in oil-seed rape plants. Heredity 29:253–257
Yang GS, Fu TD, Brown GG (1998) The genetic classification of cytoplasmic male sterility systems in Brassica napus L. Sci Agric Sin 31:27–31
Yu FQ, Fu TD (1988) Cytological study on anther development of several varieties of Brassica napus L. Chinese J Oil Crop Sci 4:23–25
Wei GW, Zheng YL, Zhang DH, Liu JL (1997) MtDNA heterogeneity of cytoplasmic male sterility in maize. Acta Genet Sin 24:66–77
Acknowledgments
This research was supported by The National Programs on the Development of Basic Research Special Foundation of China (2001CB1088), special “948” program (2003-Q04) of China and The Program for Changjiang Scholars and Innovative Research Team for Universities (IRT0442). The authors are grateful to Professor Shyam Prakash from Indian Agricultural Research Institute, Professor Qifa Zhang in Huazhong Agricultural University, Dr. Wallace Cowling, Dr. Sheng Chen and Dr. Guijun Yan in the University of Western Australian for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. F. Quiros.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2007_673_MOESM1_ESM.doc
Table S1 RFLP patterns of CMS and maintainer lines. Numbers in bold indicate the banding patterns from each probe and restriction enzyme combination. Size of bands is indicated in kb (DOC 58 KB).
Rights and permissions
About this article
Cite this article
Wan, Z., Jing, B., Tu, J. et al. Genetic characterization of a new cytoplasmic male sterility system (hau) in Brassica juncea and its transfer to B. napus . Theor Appl Genet 116, 355–362 (2008). https://doi.org/10.1007/s00122-007-0673-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-007-0673-3