Abstract
Backcross substitution of Brassica juncea (2n = 36; AABB) nucleus into the cytoplasm of a wild crucifer, Brassica fruticulosa helped in development of a new cytoplasmic male sterility (CMS) system. Male sterility was complete, stable, and expressed as rudimentary anthers containing sterile pollen grains. There was no impact on other floral as well as vegetative characters. All the natural B. juncea genotypes evaluated maintained the sterility. Gene for fertility restoration could be successfully introgressed from cytoplasm donor species. Genomic in situ hybridization studies revealed B. fruticulosa intogression in at least three chromosomes of the recipient species. Genetic studies carried out on F1, F2 and test cross progenies derived from hybridizing cytoplasmic male sterile and fertility restoring genotypes revealed a monogenic dominant control for the fertility restoration. Bulked segregant analysis with 588 SSR and 30 ISSR primers allowed identification of putative primers associated with fertility restoration. Co-segregation analysis of ten such primers with Rff gene revealed that Rf was flanked by two markers, namely cnu_m316 and nia_ m22, which were located 27.1 (LOD = 3.0) and 19.7 cM (LOD = 3.0) respectively around the gene in question. Distinctness of new CMS system from the ogura CMS was also demonstrated. This newly developed CMS-fertility-restorer system has a significant potential for hybrid seed production programs in mustard as an alternative to currently popular ogura CMS system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytoplasmic male sterility (CMS) is an important outcome of nucleo-cytoplasmic incompatibilities that prevents production of functional pollen grain, with or without impacting female fertility. Specific dominant nuclear genes, named restorers of fertility (Rf), are able to restore male function of genotypes carrying male sterilizing mitochondria (Maureen et al. 2004). Majority of CMS systems, characterized till date, arose from alterations in chimeric open reading frames (ORFs) in the mitochondrial genome (Städler and Delph Stadler and Delph 2002; Duroc et al. 2006; Kim et al. 2007). In B. juncea mitochondria, targeted expression of CMS-associated orf220 gene reportedly caused male sterility through retrograde regulation of nuclear gene expression (Yang et al. 2010). Such nuclear–mitochondrial gene interactions governing CMS were previously believed to be unique to each system. However, recent investigations in three CMS lines in Brassica juncea carrying mitochondrial genomes from Diplotaxis berthautii, D. catholica, or D. erucoides have demonstrated that male fertility in these CMS systems was restored by a common fertility restorer introgressed from Moricandia arvensis. Altered atpA transcription was associated with CMS having conserved atpA coding and downstream sequences. A novel orf108, co-transcribing with atpA, was detected in male sterile flowers of CMS lines. In presence of the restorer gene, bicistronic orf108–atpA transcript was cleaved within orf108 to yield a monocistronic atpA transcript resulting in male fertility (Kumar et al. 2012).
CMS-restorer systems have been harnessed as useful pollination control tools to exploit heterosis in several crops such as maize, sunfower, rice, sorghum, onion, sugar beet and oilseed Brassica (Banga and Banga 1998). Cytoplasmic substitution or alloplasmic line is a major option to generate new cytoplasmic male sterility systems. Outcome in such nucleo-cytoplasm combinations is normally a deep male sterility for which fertility restorers are required to be sourced from a donor of male sterilizing cytoplasm (Banga et al. 2003; Deol et al. 2003; Janeja et al. 2003). There is also an inherent element of biological penalty that impacts manifestation of heterosis in such CMS based hybrids. In contrast, if evolutionary divergence between cytoplasm donor and recipient species is less, it may not result into cytoplasmic male sterility or result in a shallow male sterility that is easy to restore but difficult to maintain.
Indian mustard (Brassica juncea (L.) Czern & Coss.) is a premier oilseed crop of Indian subcontinent. It also has niches and adaptation in Russia, eastern Europe and China. Cytoplasms from several wild species, namely Brassica oxyrrhina (oxy), Trachystoma balli (trachy), Moricandia arvensis (mori), Diplotaxis catholica (cath), D. siifolia (sii), D. erucoides (eru), D.berthautii (berth), Enarthrocarpou lyratus (lyr), Erucastrum canariense (can) and Raphanus sativus (ogu), have been introgressed into B. juncea using sexual or somatic hybridization (Prakash et al. 2010). Limitations, however, exist in the form of chlorophyll deficiencies (ogu, oxy, mori), poor female fertility (trachy, lyr) and the yield penalties (Kaur et al. 2004). Chloroplast substitutions or mitochondrial recombinations have helped improve deficiencies associated with many CMS systems (Kirti et al. 1995; Prakash et al. 2010).
In this communication, we report the synthesis of a new CMS-fertility restorer system for B. juncea. This CMS was caused by the male sterilizing cytoplasm from a wild crucifer, B.fruticulosa. B.fruticulosa is a valuable genetic resource for resistance to mustard aphid (Kumar et al. 2011; Atri et al. 2012) and Sclerotinia stem rot (Garg et al. 2010). We also preferred B. fruticulosa for developing alloplasmic line of B. juncea because it is considered close to Brassica A genome (Chandra et al. 2004). This was done to avoid the occurrence of deep sterility likely to be associated with a highly diverged alien cytoplasmic donor and to facilitate easy introgression of a fertility restorer gene(s) from a cytoplasm donor species.
Materials and methods
Development of a cytoplasmic male sterility-fertility restoration system
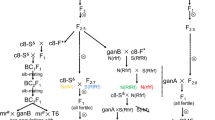
The CMS line of B. juncea (2n = 36; AABB) with B. fruticulosa cytoplasm was synthesized. To facilitate this, B. fruticulosa (2n = 16, FF) was hybridized as female with B. rapa (2n = 20; AA), the A-genome donor species for B. juncea. It was followed by chromosome doubling to develop a synthetic amphiploid (2n = 36; AAFF) (Chandra et al. 2004). Synthetic amphiploid was crossed with B. juncea, followed by four cycles of backcrossing with four genotypes of B.juncea. Only one B.juncea genotype cv. Pusa Jai Kisan was used until BC2. We followed a previously described crossing scheme (Banga et al.2003) for simultaneous synthesis of alloplasmic CMS lines and introgressing fertility restorer gene(s). To develop a CMS line, only male sterile plants were used as the female parent for each generation of backcrossing. For introgression of the fertility restorer (Rf) gene(s), only male fertile segregants were used for each generation of backcrossing until we achieved fully fertile alloplasmic plants with the euploid chromosome number expected for Brassica juncea.
Cytological analysis
For these studies, anthers were fixed in Carnoy’s–II solution for 24 h before squashing in acetocarmine (2 %). Pollen grain fertility was also studied by staining with acetocarmine. Female fertility of the CMS lines was estimated from seed set following cross-pollination as a percent of the cross seed set in the corresponding euplasmic maintainer line.
Genomic in situ hybridization (GISH) studies
Protocol as proposed by Schwarzacher and Heslop-Harrison (2000) was used with minor modifications for GISH. To prepare GISH probe, sheared DNA of a desired length from B. fruticulosa was labelled with orange fluorescein dUTP using a nick-translation kit. A 200-fold excess of sheared genomic DNA (300–500 bp) of B. juncea was added to the hybridization solution to prevent non-specific inter-genomic cross-hybridization. For preparing chromosome spreads, anthers of appropriate size were first washed in a citrate buffer for 30 min and then incubated in the enzyme mixture containing 2 % (v/w) cellulase and 20 % (v/v) pectinase in four mmol/l citrate buffer (pH 4.8) for about 1 h at 37 °C. The treated anthers were stirred with a micropipette tip to disperse the pollen mother cells (PMC’s) and centrifuged for 3 min. at 600-800 g, followed by 45 min. treatment in 150 mmol/l KCl for 20 min. One drop of 10 µl of PMC’s suspension was released on acid cleaned chilled slide from a height of 50 mm to spread the PMC’s on the slide. Subsequently, 40 µl of hybridization mixture containing 50 % formamide, 2X SSC, 10 % dextran sulphate, 0.025 µg salmon sperm DNA, 1.25 mM EDTA, 0.1255 % acetic acid; 200 ng labeled probe and 200 fold blocking DNA (sheared genomic DNA of B. juncea) was applied on chromosome spreads on the slides. These slides were then incubated at 80 °C for 4 min for simultaneous denaturating of labelled probe and chromosomes, and then hybridized overnight at 37 °C. Slides were then dehydrated through an ethanol series (70, 90 and 96 % ethanol, 2 min. each) and air-dried. Chromosomes were counterstained with DAPI by incubating slides with 100 µl DAPI solution (4 µg/ml in McIlvaine’s buffer) under a plastic cover slip at room temperature for 10–30 min. in the dark. The slides were rinsed in detection buffer and two drops of anti-fade was added. Fluorescence was visualized using Zeiss/Metasystems automated karyotyping workstation.
Molecular characterization of CMS source
For molecular characterization, we used gene-based markers designated as CMS–ORF. These were developed from the sequence information of CMS genes or predicted ORFs in rapeseed. To facilitate molecular investigations, genomic DNA was extracted from young leaves of fruti and ogura CMS lines using the cetyl trimethyl ammonium bromide (CTAB) method (Doyle and Doyle 1990). PCR amplifications were carried out using the protocol as described earlier (Kaur and Banga 2015). PCR products were analyzed by an automated capillary electrophoresis system (CaliperLabChip GX version 3.0.618.0). The PCR products were sized relative to the internal standard. Each band position was considered a single allele. Amplifications were confirmed in replicate assays.
Genetics of male fertility restoration
Identified fertility restoring genotypes were hybridized as pollen parents with CMS (fruti) B. juncea plants to develop several sets of F1 combinations. These were selfed to generate F2 progenies. F1’s were also crossed as male with corresponding CMS lines to produce test crosses. Segregation for male fertility and sterility was recorded in each F2 and test cross progenies. The data were subjected to goodness of fit to the hypothesis of monogenic dominant inheritance for fertility restoration using χ 2 analysis.
Tagging of the Rf gene
One F2 population with 50 plants and segregating in 3(F):1(S) ratio constituted the base material for molecular analysis. This was obtained by hybridizing stable male sterile lines with an introgressed fertility restoring genotype. Method for DNA isolation and genotyping assays has been described earlier in this paper. A and B genome chromosome specific SSR primers were used for initial genotyping assays. Primers detecting polymorphism between fertile and sterile bulks and showing sympatric expression with fertility restoration were tested for linkage analysis. Association of SSR markers with a fertility restorer gene was determined by analyzing the co-segregation data using MAPMAKER version 3.0 (Lander et al. 1987). All markers were allocated to linkage groups through pair-wise analysis at a threshold LOD score of 3 and using the “error detection” command operative in the MAPMAKER program. The order of markers on the linkage group (frame) was calculated by multipoint analysis and reconfirmed using the “ripple” command.
Results
Completely male sterile progenies were available in BC3 generation of the backcross substitution programme. Male sterility in CMS (fruti) B. juncea was characterized by flowers with vestigial anthers, narrow petals and normal nectaries (Fig. 1a–i). Rudimentary anthers harboured only a few small and unstained pollen grains in contrast to the deeply stained and large pollen grains of the euploid parent. Male sterility was complete and stable throughout the crop season. There was no seed set on selfing by bagging male sterile inflorescence. Alloplasmic plants were morphologically identical to the euplasmic B. juncea. Female fertility in CMS plants was normal(Table 1). Cytological investigations revealed the normal euploid B. juncea chromosome number, with regular 18IIs during metaphase I and 18-18 separation during anaphase I in CMS plants. The male-sterility expression for fruti CMS was monitored for several crop seasons and at two locations (approximately 247 ft at Ludhiana and 11,500 ft above mean sea level at Keylong) under both short-day and long-day length conditions. No influence of the prevailing environmental conditions was observed. SPAD values for chlorophyll content in alloplasmic lines were statistically identical with that of euplasmic nuclear donors (Table 2). Mitochondria specific primers, CMS–ORF 222 and CMS–ORF 263/288 could differentiate fruti and ogura CMS systems. This was confirmed by amplification of orf 222 and orf 263/288 specific 400 and 600 bp DNA fragment in fruti male sterile line, no PCR product was detected for the ogura CMS line of the corresponding size.
Flower morphology of the euplasmic fertile and fruti alloplasmic lines. a–c Flowers of euplasmic fertile, vestigial male-sterile and fertility restored F1 plants; d–f anthers of fertile, male sterile and fertility restored flowers; g–i nectaries of of fertile, male sterile and fertility restored flowers
Identification of male sterility maintainers and introgressing gene(s) for fertility restoration
To identify maintainers of male fertility, CMS plants were crossed with 104 genotypes of B. juncea, All the F1 combinations were completely male sterile. These genotypes were categorized as sterility maintainers for fruti CMS system. To develop fertility restorers, we used male-fertile alloplasmic plants, identified in BC2 progenies of crosses involving synthetic amphiploid (2n 36; AAFF), with B. juncea. Further cycles of backcrossing with B. juncea, selection for increased pollen fertility and the euploid chromosome number (2n = 36) was carried out. Cytogenetic analysis of BC5 male-fertile alloplasmic plants led to identification of few plants with 18II chromosome configuration (Fig. 2a). Pollen grains from the plant having the maximum male fertility were used to develop F1 combinations in crosses with fruti CMS lines. Pollen grain fertility in most F1 combinations was above 80 %. Such plants were classified as restorers of fertility. These were named as SRF 1, 2, 3, 4 etc.
a–b Meiotic configurations showing 18II in the PMCs of fertility restorer for fruti CMS in B. juncea. c GISH preparation in the PMC of B. fruticulosa showing 8II with fruticulosa specific red signals. d, e Meiotic configurations showing 18II in the PMCs of fertility restorers, SRF 3 and SRF 8 showing two bivalents each with red signals representing B.fruticulosa chromosome introgressions at diakinesis; (f) PMC of fertility restorer, SRF 9 with five bivalents showing B. fruticulosa introgressions. (Color figure online)
Genomic in situ hybridization
GISH studies were conducted to confirm the ‘F’ genome introgressions in fertility restoring genotypes. Occurrence of red signals in otherwise uniform blue DAPI background was construed as evidence for ‘F’ genome presence, since B. fruticulosa DNA was used to construct the probe. Fertility restorers namely SRF 3, SRF 8 and SRF 6, were selected for GISH studies. GISH preparations of SRF 3 and SRF 8 showed two bivalents each with red signals (Fig. 2b, c), representing ‘F’ chromosome fragment substitutions, during diakinesis. SRF 9,which showed distorted segregation in cross with fruti CMS and revealed five bivalents with B.fruticulosa introgression (Fig. 2d).
Genetics of fertility restoration
Most of the F1 progenies developed by hybridizing identified male fertility restoring genotypes produced male fertile progeny with near normal pollen grain fertility(>85 %). This indicated the dominance of fertility restoration. Self-generations obtained from selfing of fertile F1 plants revealed segregation for fertility with a ratio of 3:1 (fertile: sterile) (Table 3). χ 2 (3:1) in the test combinations involving SRF 2,3,4 was insignificant, confirming the model of monogenic dominant inheritance. Test cross analysis supported the inferences drawn from F2 generation(s). The genotype of a fully fertile homozygous fertility restorer was designated as Rff Rff. There were some combinations which deviated from 3:1 segregation.
Molecular tagging of the fertility restorer gene
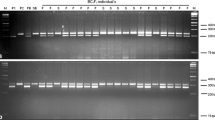
DNA from two pairs of bulks was used as a template in PCRs with around 588 SSR and 30 ISSR primers. Of these, 286 (54 %) SSR primers did not show any amplification and were discarded. For the remaining primers, DNA fragments ranging from 1 to 4 were amplified and 11 out of 332 primers showed polymorphism among the parents. The size of amplified alleles varied from 0.15 to 0.70 kb for SSR’s and 0.15 to 0.90 kb for ISSR’s. Based on the bulked segregant analysis (BSA), 11 primers showing reproducible polymorphism between male sterile and male fertile bulks were identified. These primers generated a total of twenty polymorphic alleles, which were surveyed between 45 individual F2 plants (Table 3; Fig. 3) to observe their cosegregation with the Rff locus. Linkage analysis of polymorphic markers was used to assign markers to the linkage group carrying the male fertility restoring locus. Mapping analysis revealed that Rff was flanked by two markers, namely cnu_m316 and nia_m22 which were 27.1 (LOD = 3.0) and 19.7 cM (LOD = 3.0) away from Rff, respectively (Fig. 3).
Discussion
F1 hybrid cultivars are now commercially available in Indian mustard. These are currently based on mori, ogu and 126I CMS systems. Mori CMS has gametophytic fertility restorer system, and it is sometimes difficult to differentiate between CMS plants and fertile contaminants in the seed production plots. Although not much is known about 126 I CMS system, it seems to be constrained by the availability of sterility maintainers. Ogura CMS system is now increasingly becoming popular in B. juncea due to stability of the CMS expression. Ogura CMS system was originally discovered in Japanese radish (Raphanus sativus) (Ogura 1968). Fertility restoring genes were also discovered from some European radish cultivsars (Bannerot et al. 1977). European scientists introduced ogura CMS into B. napus by intergeneric hybridization and repeated back-crossing (Bannerot et al. 1974, Heyn 1976). However, alloplasmic CMS lines of B. napus showed leaf chlorosis and yellowing at temperatures <below 15 °C. This abnormality was corrected through protoplast fusion (Pelletier et al. 1983). In B.juncea, ogura CMS system in India was first developed through backcross transfer of ogura sterilizing cytoplasm from CMS B. napus to B. juncea (Labana and Banga 1989). Leaf chlorosis associated with this CMS was subsequently resolved through somatic hybridization (Kirti et al. 1995).Although a number of hybrids based on ogura CMS system are commercially available, issues remain about the transmission of the introgressed fertility restorer gene in B. juncea. This results in a high frequency of male sterile plants in most of the F1 mustard hybrids currently available to Indian growers. Efforts to develop new CMS sources are thus continuing not only to improve agronomic performance of the F1 hybrids but also to avoid genetic vulnerability associated with worldwide usage of ogura CMS in oilseed Brassica hybrids.
Back cross substitution of B.juncea genome in the background of B. fruticulosa allowed development of cytoplasmic male sterile line characterized by vestigial anthers containing only a few sterile pollen grains. Rudimentary anther type of male sterility has been previously reported in several alloplasmic lines such as (Diplotaxis muralis)—B. rapa (Hinata and Konno 1979); (B. nigra)—B. oleracea (Pearson 1972); (R. sativus)—B. juncea (Kirti et al. 1995), (Erucastrum. canariense)—B. juncea (Prakash et al. Prakash 2001), (Enarthocarpou lyratus)—B.juncea (Deol et al. 2003; Banga et al. 2003). Unlike many other alloplasmic Brassica CMS systems (e.g. nigra, ogu, mori), fruti CMS was not associated with any chlorophyll abnormalities or other vegetative growth reductions. The male sterility was stable across a range of environmental conditions and revealed excellent outcross seed set following open pollination. Female fertility was not impacted in any way, and nectaries were normal. Molecular studies using CMS gene-based markers from mapped CMS genes or predicted ORFs in Brassica. Mitochondrial specific primer CMS–ORF 222 and CMS–ORF 263/288 could differentiate fruti and ogura CMS systems. It is possible that male sterility in fruti CMS was associated with orf222 or orf 263/288.
Like most other alloplasmic male-sterile lines in crop Brassica (Prakash et al. 2009), it was not possible to identify fertility restoring genotypes in high frequencies in Indian as well as exotic mustard germplasm resources. Commercial success of a CMS line is aided greatly by the absence of associated yield penalty and abundant occurrence of sterility maintainers in the primary gene pool of the species in question. In the present context, all the tested genotypes of natural and resynthesized B. juncea acted as sterility maintainers. To overcome paucity of fertility restorers, gene(s) for fertility restoration could be transferred from sterilizing cytoplasm donor species of B. fruticulosa into B. juncea using the protocol described earlier (Deol et al. 2003). Success of introgressed fertility restoration from cytoplasm donor species is a theoretical certainty for a CMS of alloplasmic origin. The euplasmic recipient species act as a male-sterility maintainer because recessive plasmon sensitive alleles are expected to occur with a high frequency in populations with a normal plasmotype. Contrarily, fertility restoring gene(s) are known to be present at higher frequency in populations carrying the male sterilizing plasmotypes (Banga and Labana 1985). F genome introgressions could be confirmed for two bivalents each in fertility restorers SRF 3 and SRF 8.
Genetic investigation revealed the involvement of a single dominant gene in a large number of cross combinations involving different CMS and restorer genotypes. Introgressed Rf gene in SRF 3 and SRF 8, appeared to be stable with least linkage drag. This was apparent from completely male fertile progenies in F1 of their crosses involving fruti CMS lines. Segregation distortions were also recognized in some test crosses involving SRF 6, 7, 9, 16. These deviations may be ascribed to varied size or multiplicity of introgressed alien chromosome segment(s). GISH studies confirmed the multiplicity of introgressed alien chromosome fragments in several fertility restorers (e.g. SRF9). Preferential loss/recovery of a specific allele in the self- or cross-generations may result from low transmission frequency that impacts the normal transmission of the heredity determinants from meiocytes to zygotes. Lethality of gametes can be caused by presence of introgressed lethal gene(s) or the size and number of introgressed chromosome fragments. Keeping these factors in view, it may be prudent to use fertility restorers showing stable Rf introgression and inheritance (e.g. SRF 2, 3, 4, 8 etc.) as a basic germplasm resource for use in hybrid breeding programmes.
We used BSA for gene mapping using an F2 population segregating for male fertility restoration. This F2 population itself was generated from parental genotypes differing for the Rff gene as well as the background genotypes. Very low polymorphism was apparent from identification of only 10 primers that showed reproducible polymorphism between male sterile and male fertile bulks. This was expected as the chance occurrence of shared homozygosity at specific unlinked chromosomal regions tends to limit the efficiency of BSA (Jean et al. 1997). Linkage analysis helped in the identification of a region that contained 10 markers spanning a total length of 302 cM of which only a fragment of 34 cM carrying Rff locus may be considered of significance because a marker placed beyond 30 cM was not considered to be linked. In this part of the linkage group, Rff was flanked with two markers, namely cnu_m316 and nia_m22 which were 27.1 (LOD = 3.0) and 19.7 cM (LOD = 3.0) away from Rff, respectively. Paucity of markers co segregating with Rff and their poor distribution across a relatively wide genomic region may be the reason for our failure to identify markers closely associated with the gene for fertility restoration. We hope to saturate this region with more SSR markers, possibly by using B. fruticulosa specific markers from our ongoing work on low coverage genome sequencing of this wild species. New B. juncea SSRs will also be added. In conclusion, development of this new CMS system, easy availability of sterility maintainers and successful introgression of fertility restorer gene will be beneficial for the development of an alternate hybrid seed production system for oilseed Brassicas, especially B. juncea.
References
Atri C, Kumar B, Kumar H, Kumar S, Sharma S, Banga SS (2012) Development and characterization of Brassica juncea—fruticulosa introgression lines exhibiting resistance to mustard aphid (Lipaphis erysimi Kalt). BMC Genet. doi:10.1186/1471-2156-13-104
Banga SS, Banga SK (1998) Hybrid cultivar development. Springer/Narosa Publishing House New Delhi, New Delhi
Banga SS, Labana KS (1985) Male sterility in Indian mustard (Brassica juncea (L.) Coss.). Genetics and cytology of MS-4. Can J Genet Cytol 27:487–490
Banga SS, Deol JS, Banga SK (2003) Alloplasmic male sterile Brassica juncea with Enarthrocarpus lyratus cytoplasm and the introgression of gene(s) for fertility restoration from cytoplasm donor species. Theor Appl Genet 106:1390–1395
Bannerot H, Boulidard L, Cauderon Y, Temp J (1974) Transfer of cytoplasmic male sterility from Raphanus sativus to Brassica oleracea. Eucarpia Meet Cruciferae Crop Sect 25:52–54
Bannerot H, Boulidard L, Cauderon Y (1977) Unexpected difficulties met with the radish cytoplasm. Eucarpia Cruciferae Newslett 2:16
Chandra A, Gupta ML, Banga SS, Banga SK (2004) Production of an interspecific hybrid between Brassica fruticulosa and B. rapa. Plant Breed 123:497–498
Deol JS, Shivanna KR, Prakash S, Banga SS (2003) Enarthrocarpus lyratus based cytoplasmic male sterility and fertility restorer system in Brassica rapa. Plant Breed 122:438–440
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Duroc Y, Gaillard C, Hiard S, Tinchant C, Berthomé R, Pelletier G, Budar F (2006) Nuclear expression of a cytoplasmic male sterility gene modifies mitochondrial morphology in yeast and plant cell. Plant Sci 170:755–767
Garg H, Atri C, Sandhu PS, Kaur B, Renton M, Banga SK, Singh H, Singh C, Barbetti MJ, Banga SS (2010) High level of resistance to Sclerotinia sclerotiorum in introgression lines derived from hybridization between wild crucifers and the crop Brassica species B. napus and B. juncea. Field Crop Res 117:51–58
Heyn FW (1976) Transfer of restorer genes from Raphanus to cytoplasmic male sterile Brassica napus. Cruciferae Newslett 1:15–16
Hinata K, Konno N (1979) Studies on a male-sterile strain with a Brassica campestris nucleus and Diplotaxis muralis cytoplasm. The breeding process and some characteristics of the strain. Jpn J Breed 29:305–311
Janeja HS, Banga SK, Bhaskar PB, Banga SS (2003) Alloplasmic male sterile Brassica napus with Enarthrocarpus lyratus cytoplasm. Introgression and molecular mapping of lyratus chromosome segment carrying fertility restoring gene. Genome 46:792–797
Jean M, Brown GG, Landry BS (1997) Genetic mapping of nuclear fertility restorer gene for the ‘polima’ cytoplasmic male sterility in canola (Brassica napus L) using DNA markers. Theor Appl Genet 95:321–328
Kaur H, Banga SS (2015) Discovery and mapping of Brassica juncea Sdt 1 gene associated with determinate plant growth habit. Theor Appl Genet 128:235–245
Kaur G, Banga SK, Gogna KPS, Joshi S, Banga S (2004) Moricandia arvensis cytoplasm based system of cytoplasmic male sterility in Brassica juncea: reappraisal of fertility restoration and agronomic potential. Euphytica 138:271–276
Kim DH, Kang JG, Kim BD (2007) Isolation and characterization of the cytoplasmic male sterility-associated orf456 gene of chilli pepper (Capsicum annuum L.). Plant Mol Biol 63:519–532
Kirti PB, Banga SS, Prakash S, Chopra VL (1995) Transfer of ogu cytoplasmic male sterility to Brassica juncea and improvement of the male sterile through somatic cell fusion. Theor Appl Genet 91:517–521
Kumar S, Atri C, Sangha MK, Banga SS (2011) Screening of wild crucifers for resistance to mustard aphid, Lipaphis erysimi (Kaltenbach) and attempt at introgression of resistance gene (s) from Brassica fruticulosa to Brassica juncea. Euphytica 179:461–470
Kumar P, Vasupalli N, Sirinivasan R, Bhat SR (2012) An evolutionarily conserved mitochondrial orf108 is associated with cytoplasmic male sterility in different alloplasmic lines of Brassica juncea and induces male sterility in transgenic Arabidopsis thaliana. J Exp Bot 63:2921–2932
Labana KS, Banga SK (1989) Transfer of Ogura cytoplasmic male sterility of Brassica napus into genetic background of Brassica juncea. Crop Improv 16:82–83
Lander E, Green P, Abrahamson J, Barlow A, Daly MJ, Lincon SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Maureen R, Hanson L, Stephane Bentolia S (2004) Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 16:154–169
Ogura H (1968) Studies on the new male-sterility in Japanese radish with special reference to the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima Univ 6:39–78
Pearson OH (1972) Cytoplasmically inherited male-sterility characters and flavour components from the species cross Brassica nigra (L.) Koch -B. oleracea L. J Am Soc Hort Sci 97:398–402
Pelletier G, Primard C, Vedel F, Chétrit P, Rémy R, Rousselle P, Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferae by protoplast fusion. Mol Gen Genet 191:244–250
Prakash S (2001) Utilization of wild germplasm of Brassica allies in developing cytoplasmic male sterility/fertility restoration systems in Indian mustard—Brassica juncea. In: Liu H, Fu TD (eds) Proceedings of the international symposium rapeseed Science. Science Press, New York, pp 63–68
Prakash S, Bhat SR, Quiro´s CF, Kirti PB, Chopra VL (2009) Brassica and its close allies: cytogenetics and evolution. Plant Breed Rev 31:21–187
Prakash S, Bhat SR, Quiros CF, Kirti PB, Chopra VL (2010) Brassica and its close allies: cytogenetics and Evolution. Plant Breed Res 3:2–187
Schwarzacher T, Heslop-Harrison P (2000) Practical In situ Hybridization. BIOS Scientific Publishers Oxford. p 203
Stadler T, Delph LF (2002) Ancient mitochondrial haplotypes and evidence for intragenic recombination in a gynodioecious plant. Proc Natl Acad Sci USA 99:11730–11735
Yang JH, Liu XY, Yang XD, Zhang MF (2010) Mitochondrially-targeted expression of a cytoplasmic male sterility-associated orf220 gene causes male sterility in Brassica juncea. BMC Plant Biol 10:231. doi:10.1186/1471-2229-10-231
Acknowledgments
The research was supported by Indian Council of Agricultural Research in the form of ICAR National Professor Chair project “Broadening the genetic base of Indian mustard (Brassica juncea) through alien introgressions and germplasm enhancement” awarded to SSB. We are also thankful to anonymous reviewers for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atri, C., Kaur, B., Sharma, S. et al. Substituting nuclear genome of Brassica juncea (L.) Czern & Coss. In cytoplasmic background of Brassica fruticulosa results in cytoplasmic male sterility. Euphytica 209, 31–40 (2016). https://doi.org/10.1007/s10681-015-1628-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1628-4