Abstract
Rice blast disease caused by Magnaporthe grisea is a continuous threat to stable rice production worldwide. In a modernized agricultural system, the development of varieties with broad-spectrum and durable resistance to blast disease is essential for increased rice production and sustainability. In this study, a new gene is identified in the introgression line IR65482-4-136-2-2 that has inherited the resistance gene from an EE genome wild Oryza species, O. australiensis (Acc. 100882). Genetic and molecular analysis localized a major resistance gene, Pi40(t), on the short arm of chromosome 6, where four blast resistance genes (Piz, Piz-5, Piz-t, and Pi9) were also identified, flanked by the markers S2539 and RM3330. Through e-Landing, 14 BAC/PAC clones within the 1.81-Mb equivalent virtual contig were identified on Rice Pseudomolecule3. Highly stringent primer sets designed for 6 NBS-LRR motifs located within PAC clone P0649C11 facilitated high-resolution mapping of the new resistance gene, Pi40(t). Following association analysis and detailed haplotyping approaches, a DNA marker, 9871.T7E2b, was identified to be linked to the Pi40(t) gene at the 70 Kb chromosomal region, and differentiated the Pi40(t) gene from the LTH monogenic differential lines possessing genes Piz, Piz-5, Piz-t, and Pi-9. Pi40(t) was validated using the most virulent isolates of Korea as well as the Philippines, suggesting a broad spectrum for the resistance gene. Marker-assisted selection (MAS) and pathotyping of BC progenies having two japonica cultivar genetic backgrounds further supported the potential of the resistance gene in rice breeding. Our study based on new gene identification strategies provides insight into novel genetic resources for blast resistance as well as future studies on cloning and functional analysis of a blast resistance gene useful for rice improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is the staple food for more than half of the world population and is considered as the lifeline of Asia, where it originally evolved (Khush 2004). However, several biotic and abiotic stresses threaten the sustainable production of rice. Rice blast disease caused by the fungus Magnaporthe grisea (Hebert) Barr (anamorph Pyricularia grisea Sacc.) is one of the most destructive diseases of rice worldwide. It has been estimated that, each year, the disease kills enough rice to feed 60 million people (Zeigler et al. 1994). An outbreak of blast can devastate rice fields, completely destroying crops in the most extreme cases (Ou 1985). Host–plant resistance based on the hypothesis of gene-for-gene interaction is the most economical and environmentally safe approach to control the disease (Jia et al. 2000). However, the pathogenicity of M. grisea isolates is highly variable and sometimes a small section of the virulent isolate spreads rapidly and overcomes resistance genes of rice cultivars (Fukuoka and Okuno 2001; Wang et al. 1994).
During the past decade, the genetics of blast resistance has been extensively studied and nearly 40 resistance genes have been identified. Most of the resistance genes are dominant, except the recessive gene pi21, and some are quantitative in nature (Fukuoka and Okuno 2001; Hayashi et al. 2004; Zhou et al. 2004; Gowda et al. 2006). Most of the resistance genes are from landrace, of indica subspecies. The only resistance gene, Pi9, has originated from a wild species, O. minuta (Liu et al. 2002). Five resistance genes, Pib, Pita, Piz-5, Piz-t and Pi9, have been cloned and sequenced (Bryan et al. 2000; Qu et al. 2006; Wang et al. 1999; Zhou et al. 2006). The gene Pik-h has been fine mapped (Sharma et al. 2005). A few resistance genes have been used for cultivar development, but these are often not durable. Moreover, most of the resistance genes are race-specific (Deng et al. 2006; Mackill and Bonman 1992). It is imperative to identify broad-spectrum blast resistance genes for effective protection against dynamic blast isolates of M. grisea.
The resistance genes Pib, Pita, Piz-5, Piz-t and Pi9 produce NBS-LRR gene products that interact with the effecter gene of the pathogen and follow a gene-for-gene type of resistance (Bryan et al. 2000; Qu et al. 2006; Zhou et al. 2007). With the discovery of the complete sequence of the rice genome, it was found that NBS-LRR sequences are ubiquitous in the rice genome (http://rgp.dna.affrc.go.jp; http://www.genomics.org.cn). DNA markers within NBS-LRR motif sequences may provide additional links to look for new blast resistance genes.
The wild species of Oryza have rarely been used as sources for blast resistance except the only gene (Pi9) that was reported to have resistance to most of the South and Southeast Asian blast isolates (Lu et al. 2004). However, the Pi9 gene as well as several genes on the Piz locus express a low level of resistance or susceptibility to the dynamic blast pathogen population in South Korea (Kim et al. 2004). It is necessary to look for novel resistance gene(s) for blast that can express a broad spectrum of resistance not only in Korea but also in South and Southeast Asia.
In this study, we have made an attempt to search for a new resistance gene from an EE genome wild Oryza species (O. australiensis) and fine-map the new gene using genome sequence information of Nipponbare through e-Landing on rice chromosome 6. We report here the differentiation of the new R gene from other known Pi genes using gene-marker association analysis of monogenic differential lines for blast resistance and by accuracy of pathogenicity assays using diverse isolates of M. grisea. Our results led us to the identification and fine mapping of the novel resistance gene Pi40(t) using new molecular approaches that will be useful for developing durable blast-resistant cultivars for rice improvement.
Materials and methods
Plant materials and DNA extraction
Five rice cultivars, 2 breeding lines, 12 blast monogenic differential lines (Tsunematsu et al. 2000) of japonica cultivar Lijiangxintuanheigu (LTH) background, and one wild Oryza species, O. australiensis (Acc. No. 100882), were used in this study (Table 1). Breeding line IR31917-45-3-2 is the progenitor of the introgression line IR65482-4-136-2-2 (Jena et al. 1991). Two blast-susceptible japonica cultivars, Jinbubyeo and Junambyeo, were used as the recurrent parents of the mapping population and recipient lines for R gene introgression. Two cultivars, Co39 and IR50, were used as susceptible checks for pathogenicity assays using Philippines isolates. Seeds of elite japonica cultivars Jinbubyeo and Junambyeo were obtained from the Genetics and Breeding Division of the National Institute of Crop Science (NICS), Rural Development Administration (RDA), Republic of Korea, and seeds of other lines were obtained from the Genetic Resources Center of the International Rice Research Institute (IRRI), Los Baños, Philippines.
Ninety-four F2 and F2:3 progenies from a cross between Jinbubyeo and IR65482-4-136-2-2 were used for genetic studies of blast resistance and developing markers tightly linked to the target locus for MAS applications. Resistant seedlings of 36 BC2F4 and 19 BC3F3 families from the crosses of Jinbubyeo × IR65482-4-136-2-2 and Junambyeo × IR65482-4-136-2-2, respectively, were used for MAS validity test of the new blast resistance gene, Pi40(t). Total genomic DNA was extracted from young leaves according to Murray and Thompson (1980), with minor modifications.
Blast inoculation
Three-week-old seedlings were inoculated with blast isolate suspension as described by Kim et al. (2004). The fungal spore suspension concentration was adjusted as 1.5 × 105 spores/ml. Seedlings were spray inoculated with 20 ml of spore suspension using an electric motor sprayer to ensure an even and uniform distribution of spores and were kept for 24 h in a dew growth chamber at 25°C in darkness and then transferred to a greenhouse with 12/12 h (day/night) photoperiod at 90% relative humidity for 7 days. Disease reactions were scored 7 days post inoculation. For Korean blast isolates, disease reaction was scored using a numerical system similar to that of Campbell et al. (2004). Several Philippine isolates were used to differentiate lines containing genes Pi40(t) and Piz-t (see below). Overall means scored from 15 to 25 representative seedlings per line were used for statistical analysis. Percent diseased leaf area (DLA) scorings (Asaga 1981) obtained from ten randomly selected seedlings were used for the Philippine blast isolates.
Preliminary screening of PCR-based DNA markers
From STS markers provided by the Rice Genome Research Program (http://rgp.dna.affrc.go.jp/E/publicdata/caps/index.html) and high-resolution rice linkage map with SSR markers (McCouch et al. 2002), DNA markers evenly spaced on rice chromosomes were preselected to detect polymorphism between the parental lines, Jinbubyeo and IR65482-4-136-2-2. Four restriction endonuleases (AluI, HaeIII, HinfI, and RsaI; NEB, Beverly, MA, USA) were used on monomorphic STS-PCR products to detect latent polymorphism. A total of 38 polymorphic markers were applied to the F2 mapping population to conduct association analysis between marker genotypes of F2 progenies and mean resistance levels of segregating F2:3 progeny rows.
e-Landing on rice Pseudomolecule3 and Mirror Map construction
The concept of electronic chromosome landing (e-Landing) was adopted to determine the relative physical positions of DNA markers by projecting their primer sequences over a reference rice genome (Mirror Map). The primer sequences used the query sequences to localize on the rice Pseudomolecule3. The Sequence-BLAST menu at Gramene (http://www.gramene.org/Multi/blastview/BLA_SXIXXeafF) was used, where BLASTN and near-exact matches were the options for search tool and search sensitivity, respectively. When both primer sequences successfully recognized their physical locations to be annealed, the expected PCR product size was calculated to judge PCR products. Based on the defined physical locations, the BAC/PAC clones, including the primer annealing sites of the tested primers, were determined by using the Rice Pseudomolcule Information and Gene Search supported by TIGR (http://www.tigr.org/tdb/e2k1/osa1/pseudomolecules/). The determined cM position for each BAC/PAC clone was directly adopted from the TIGR database.
In addition to the 38 STS and SSR anchor markers, used for construction of a linkage map skeleton, 17 DNA markers previously reported tagging blast resistance genes as well as 10 STS markers tagging six NBS-LRRs on a PAC clone, P0649C11 (GenBank Acc. = AP005659), were used for e-Landing (see Table 4).
PCR amplification and the physical location of the Pi40(t) gene
Primers were synthesized by the Bioneer Company (Deajon, Korea). The DNA sequence data sets corresponding to the six NSB-LRRs on P0649C11 were used to design highly stringent primer sets. First, all possible oligonucleotide sequences were selected by using Primer3 (http://www.frodo.wi.mit.edu/primer3/) to have optimum melting temperature (Tm) of 65°C and 0.5–1.5 kb as their expected PCR products. Those oligonucleotides were then subjected to e-Landing on the rice Pseudomolecule3. PCR was performed in a total volume of 30 μl containing 10 ng of DNA template, 10 pmole of each primer, 1.5 mM of MgCl2, 0.2 mM of dNTPs, and 1 U of Taq polymerase (Nurotics, Deajon, Korea). PCR began with one cycle at 95°C for 3 min, followed by 35 cycles at 95°C for 30 s, at 65°C for 30 s (see Table 4 for references), and at 72°C for 1 min, with a final extension at 72°C for 10 min (MJ Research PTC-100 thermocycler; Waltham, MA, USA). The primer annealing temperature for SSR markers was 55°C.
Detailed haplotypes of nine genotypes were evaluated for the intervening physical region of 1.81 Mb between markers S2539 and RM3330 on the chromosome 6 short arm to differentiate the Pi40(t) gene from the other blast resistance genes, and also to confirm the putative nesting place of Pi40(t) within the six NBS-LRRs on PAC clone P0649C11. Marker allele types at each of the 19 tested loci were determined based on the unique band sizes as well as banding patterns derived from cleaved STS-PCR products by six frequent cutters (AluI, HaeIII, HinfI, RsaI, TaqI, and Tsp509), where 4 μl of PCR product was digested by 2.5 U of each restriction endonuclease in a 20 μl reaction volume for 2 h following the manufacturer’s instructions. Agarose gel (1.2%, 0.5× TBE, 150 V) and natural polyacrylamide gel (5% polyacrylamide, 0.5× TBE, 300 V) electrophoresis were used for STS-PCR and cleaved PCR products, respectively, and stained by ethidium bromide. Sequencing gel electrophoresis (5% polyacrylamide, 6 M urea, 1× TBE, 80 W) was used for SSR-PCR product separation and bands were visualized using Silver Sequence™ (Promega, Madison, WI, USA).
The F2 mapping population as well as resistant BC progenies from the cross between Jinbubyeo and IR65482-4-136-2-2 were genotyped with informative markers to narrow down the putative locus of the blast resistance gene. The most tightly linked markers, 9871.T7E and 9871.T7E2b, were used for a MAS validity test on blast-resistant BC progenies. Marker 9871.T7E2b was used to detect the introgression test of an O. australiensis segment at the target locus. Some 50 ng of O. australiensis DNA template was used for the PCR amplification.
Statistical analyses
MAPMAKER/EXP 3.0 was used to analyze the linkage between molecular markers and the target gene (Lincoln et al. 1992). The marker intervals were calculated by using the Haldane mapping function (Haldane 1919). Chi-square analysis was used for segregation tests. PROC GLM of the SAS statistical package (SAS Institute 2000) was used to estimate the relative contribution of tested loci for blast resistance. For the F test of loci, markers having a P value less than 0.05 were declared as significant empirically. The percentage of phenotypic variation explained (R 2), additive genetic effects, and degrees of dominance were then estimated for the declared loci within the mapping population.
Results
Genetic evaluation of blast resistance
We have identified IR65482-4-136-2-2 as a new source of blast resistance by evaluating it with 15 recommended blast isolates of Korea; resistance was contributed by a single locus (Jeung et al. 2003). The details of the materials used, disease reaction and association analysis are given in Tables 1, 2 and 3. In order to find out whether blast resistance was contributed by any known gene(s), the corresponding gene homologues in IR65482-4-136-2-2 and its recurrent parent, IR31917-45-3-2, were tested by using tightly linked markers for seven blast resistance genes (Pib, Pi9, Pi3, Pi5, Pi1, Pik, and Pita; see Table 4 for the primer sets used). Both IR65482-4-136-2-2 and IR31917-45-3-2 did not share marker allele types with Pi9, Pi3, Pi5, Pi1 and Pik (data not shown). DNA markers tagging the Pib and Pita genes amplified the same PCR products in terms of band size as well as band strength in IR65482-4-136-2-2, whereas IR31917-45-3-2 generated the same PCR product for the Pib locus only (Table 1). This result indicated that the Pita homologue in IR65482-4-136-2-2 might be integrated from the wild rice progenitor O. australiensis. However, due to susceptible reactions of the LTH monogenic differential lines with Pib and Pita genes to many Korean blast isolates (Table 2), we concluded that the resistance in IR65482-4-136-2-2 is unlikely to be contributed by either Pib or Pita genes.
We have screened 87 F 2:3 lines (N = 94) for blast resistance by using a specific Korean blast isolate, 01–01 (KJ105a). The frequency distribution of resistance levels of 87 F2:3 segregating progenies exhibited an abnormal distribution pattern (w = 0.8; P < 0.0001, skewness = 1.45), with 52 plant-progenies completely resistant (0 score), 20 resistant (1–2 score), 6 moderately resistant (2–3 score), and 9 progenies completely susceptible (4–5 score). The resistance levels of Jinbubyeo and IR65482-4-136-2-2 were 4 and 0, respectively. The disease reaction of the F2:3 segregating progenies indicated that blast resistance in IR65482-4-136-2-2 is controlled by major genetic factor(s), which could be easily localized on the rice chromosome following prompt DNA marker development.
Localization of the Pi40(t) gene on chromosome 6 via association analysis
Of the preselected 106 even-spacing STS and SSR markers, 38 markers (30 SSR and 8 STS) detected polymorphism between Jinbubyeo and IR65482-4-136-2-2. The mapping population was successively genotyped with those 38 markers using single-locus ANOVA to test the association between a marker locus and disease reaction phenotype of F 2:3 segregating progenies, two chromosomal regions were identified (App = 1 in Table 3). One genetic factor was located on the short arm of chromosome 6 distal to RM5963 and RM5745 (Fig. 1a) and the other was on the long arm of chromosome 9 tagged by RM0201, where the allele type of IR65482-4-136-2-2 and Jinbubyeo was favorable to increase blast resistance, respectively. Seven markers only for those two chromosomal regions were screened and eight additional informative markers (3 STS and 5 SSR) were applied for association analysis (App = 2 in Table 3). A region of chromosome 6 delimited by RM0527 and RM3330 (3.5 cM) was strongly associated with blast resistance, which explained 58.9–66.5% of the total phenotypic variation (Table 3). However, the other genetic factors localized on chromosome 9 identified by RM0215 were less effective (R 2 = 0.137).
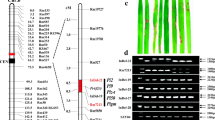
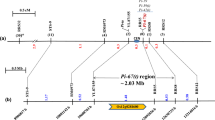
a Linkage map skeleton of the chromosome 6 short arm with six DNA markers used to narrow down the putative location of the R-gene. Markers were applied based on F-statistics from single-locus ANOVA results (App. application, see also Tables 3, 4). The genetic intervals are shown in centiMorgan (cM). The black rectangle indicates the centromere region. The PCR primer set for marker 9871.T7 (in boldface) tagging the resistance gene that originated from Nipponbare PAC clone P0649C11 was determined as the putative location of the Pi40(t) gene based on comparative haplotype analyses. b e-Landing-mediated high-resolution map of the Pi40(t) locus and determined physical positions of specific markers for Pi2 (Piz-5), Piz, Piz-t, and Pi9 reported earlier. e-Landing of three DNA markers, used for second application (S2539, RM527, RM3330; App. = 2 in a), on Rice Pseudomolecule3 delimited the corresponding 1.81 Mb virtual contig composed of 14 BAC or PAC clones. The physical positions of previously reported markers (italics) on the virtual contig were also evaluated via e-Landing except for SSR140 and pB8. c PAC clone P0649C11 (138,870 bp, GenBank Acc. AP005659), containing 6 NBS-LRR disease resistance protein homologues (black rectangles) out of 19 putative ORFs predicted by TIGR. Of the ten previously reported primer sets for Pi2 (Piz-5), Piz, Piz-t, and Pi9 loci (b and Table 4), applied to e-Landing procedures, only two SNP markers for the Piz loci, z60510 and zt6057 (italics), could be placed on the P0649C11 clone
The ANOVA confirmed that the blast resistance in the donor line (IR65482-4-136-2-2) was due to the gene on the short arm of chromosome 6, where at least four blast resistance genes (Piz, Piz-t, Piz-5, and Pi9) were previously reported (Conaway-Bormans et al. 2003; Hayashi et al. 2006; Jiang and Wang 2002; Liu et al. 2002). However, the monogenic differential lines with these resistance genes exhibited a differential reaction to the selected Korean blast isolates compared to line IR65482-4-136-2-2 (Table 2). This new blast resistance gene in IR65482-4-136-2-2 inherited from O. australiensis is designated as Pi40(t).
Candidate BAC/PAC clone identification using e-Landing and mirror-map
Through e-Landing, we eventually constructed a “Mirror Map” for which the relative physical positions of the markers could be projected over a reference rice genome, Nipponbare (Table 4). The 1.81-Mb virtual contig composed of 14 overlapping BAC/PAC clones was identified as the intervening physical regions for the three markers, S2539, RM0527, and RM3330, that exhibited significant associations between markers and phenotype (Fig. 1). Simultaneously, the physical positions of the markers tightly linked to Pi2 (Piz-5), Piz, Piz-t, and Pi9 were also determined and placed on the Mirror Map (Fig. 1b, Table 4). A PAC clone, P0649C11 (GenBank Acc. = AP005659), containing 19 putative open reading frames (ORFs) was identified by surveying TIGR annotated gene content of each BAC/PAC clone in which 6 ORFs were annotated as NBS-LRR disease-resistance protein homologues (Fig. 1b, c). The other clones did not contain any ORFs having putative functions related to disease resistance. The PAC clone (P0649C11) was thus selected as the most putative physical region containing the Pi40(t) gene. The markers, S2539 and zt6057-Nip, were placed within any putative ORF having a putative function, peroxidase on P0038C05 (Gene ID = 3007.T00010) and similar to NBS-LRR disease resistance on P0649C11 (Gene ID = 9871.T00014). The other DNA markers were placed in the intergenic regions or partially shared with ORFs. Interestingly, the clone P0649C11 flanked by clones P0502B12 and B1197G05 was composed of many putative retrotransposon proteins (Fig. 1b).
DNA markers for Pi40(t) via haplotyping of the target region
Highly stringent primer sets for each NBS-LRR homologue were used to compare the marker allele types not only between IR65482-4-136-2-2 and IR31917-45-3-2 but also among the monogenic differential lines for Piz-5, Piz-t, Piz, and Pi9 genes (Table 4; marker names having the Gene ID numbers ‘9871.T-‘I or E’ indicate the primer sequences derived from intron (I) and exon (E) regions, respectively). Two quality criteria were applied to ensure specific tagging of the NBS-LRR homologues during PCR amplification: (1) high annealing temperature and (2) singleton amplification. Twelve primer sets were synthesized and all successfully generated the expected target bands without serious false positives (Table 4; Fig. 1c, 2a).
PCR analysis using NBS-LRR tagging primer sets and differentiation of allele types by restriction endonucleases. a Confirmation of expected banding patterns using designed primer sets as detected by e-Landing and e-PCR (Table 4). Note that 9871.T9E3 detected insertions (1.8 kb in italics) on ID-1, 3, 4, 9, and 10 (IR31971-45-3-2, Jinbubyeo, Junambyeo, IRBLz-Fu, and IRBL9-w, respectively), whereas ID-2, 5, 7, and 8 (IR65482-4-136-2-2, LTH, IRBLz5-CA, and IRBL-zt-T, respectively) generated the exact PCR product (926 bp) as expected. However, the primer 9871.T14I1E2 generated the expected PCR product (965 bp) only in Junambyeo (ID-4), IR31917-45-3-2 (ID-1) generated a null allele, and the others showed a deleted allele type (0.6 kb). b Detection of latent polymorphism at the tagged locus using six restriction endonucleases. Expected band size of 1,639 bp was identified by the primer 9871.T7E in all tested genotypes except for ID-10 (IRBL9-w). The banding patterns generated by HaeIII and RsaI cleavages were considered as the same pattern. AluI cleavage, for example, generated three unique banding patterns (pattern I = ID-1, 3, 9; pattern II = ID-2, 7, 8; pattern III = ID-4, 5). However, two, three, and four unique banding patterns were identified by HinfI, TaqI, and Tsp509 I enzymes, respectively. The primer 9871.T7E (null at ID-10) within tested nine genotypes further discriminated two allele types into a total of five different allele types (Type A = ID-1; Type B = ID-2, 7, 8; Type C = ID-3; Type D = ID-4, 5; Type E = ID-9). Line IDs are listed in Table 1. Agarose gel was used for gel images having the DNA step marker lane ‘M’ (2-Log DNA ladder; NEB), and a natural polyacrylamide gel was used for those having ‘m’ (100 bp DNA ladder; Promega). In both cases, ethidium bromide staining was used for visualization
Detailed haplotypes of IR65482-4-136-2-2, IR31917-45-3-2, and the monogenic differential lines with Piz-5, Piz-t, Piz, and Pi9 genes for the 1.81-Mb physical segment flanked by S2539 and RM3330 were determined by using unique allele types derived from 19 DNA markers (Table 5; Fig. 2b). Identical haplotypes as well as pathotyping results between Jinbubyeo (ID-3) and IRBLz-Fu (ID-9) suggested that Jinbubyeo has the Piz gene in addition to the minor genetic factor identified on the long arm of chromosome 9 (Tables 2, 4, 5). This was also confirmed by checking ‘Fukunishiki’ as one of the progenitor lines of Jinbubyeo. The haplotype of IR31917-45-3-2 (ID-1) was fairly distinct from that of IR65482-4-136-2-2 (ID-2), and indicated integration of a 1.81-Mb DNA segment from O. australiensis (Table 5). The F2 mapping population was genotyped by using five NBS-LRR derived markers to conduct single-locus ANOVA by using the pathotyping results (Table 3). The homozygous and heterozygous alleles of IR65482-4-136-2-2 could not be distinguished at the 9871.T9E3 locus, and the genotype data at the locus were not collected (data not shown). Association analysis confirmed that the physical region including six NBS-LRR homologues was the most putative location of Pi40(t) (F = 104.3, R 2 = 0.713), which was likely to be an additive gene (degree of dominance = 0.49). However, the genetic intervals among the four DNA markers could not be estimated due to the small size of the mapping population (N = 94).
Fine mapping of the Pi40(t) gene
The marker allele types of the blast R genes (Piz-5, Piz-t, Piz, and Pi9) were compared to infer their putative precise locations (Table 5). The locus 9,871.T8E3 was considered as one of the putative locations for the Piz-5 (ID-7) and Piz-t (ID-8) genes because the inferred allele type of those two monogenic lines was different from that of LTH (ID-5). Under the assumption that the allele types of Pi40(t) could not be shared with any other R genes (Piz-5, Piz-t, Piz, or Pi9), due to their uniqueness in terms of gene source and reaction to a range of blast isolates tested (Tables 1, 2), the probable ORFs for the Piz gene could be one of three: 9871.T7, 9871.T10, or 9871.T14. Similarly, the possible ORFs for Pi9 (ID-10) could be either 9871.T7 or 9871.T14 (see Table 5). The ORF 9871.T5 could be excluded from consideration, because all tested genotypes showed a similar banding pattern even after applying six endonucleases to the 1,794-bp region tagged by 9871.T5I1 and 9871.T5E2 (see Table 5).
The number of marker allele types resolved by 12 specific primer sets varied from 1 (9871.T5I1 and 9871.T5E2) to 6 (9871.T7E, and 9871.T14E23) (Table 5). The locus 9871.T7E2b expressed seven marker allele types by which all tested genotypes could be differentiated except between IR65482-4-136-2-2 and IRBLzt-T (Table 5, Fig. 3a). We concluded that the 9871.T7 locus could be the most putative location for the Pi40(t) gene.
Confirmation of the novelty of the Pi40(t) gene. Line IDs, electrophoresis conditions, and DNA step markers are the same as in Fig. 2. a The primer, 9871.T7E2b, amplified a 642-bp fragment in all tested materials. ID-1 and ID-10 (IR31917-45-3-2 and IRBL9-w) were differentiated as a different allele type from another eight genotypes due to their weak band intensity. Combined interpretation over the banding patterns from the HinfI and Tsp509 I digested PCR products discriminated all LTH monogenic lines (ID-5, 7, 8, 9, 10 for LTH, Piz-5, Piz-t, Piz, Pi9, respectively) and also the blast resistance gene donor line (ID-2) from its recurrent parent (ID-1) and the two Korean japonica varieties used as recurrent parents of this study (ID-3 and ID-4). Note that the allele type of IR65482-4-136-2-2 could not be differentiated from that of IRBLzt-T (ID-8). b Comparisons on the PCR banding patterns from HinfI and Tsp509 I cleavages between the introgression line (ID-2) and O. australiensis (ID-20) indicate alleles different from IR31917-45-3-2 (a; ID-1) that originated from O. australiensis. c Highly susceptible reactions of IRBLzt-T (ID-8) to a selected Korean isolate, 90-002 (KI215), suggested that Pi40(t) is a new blast resistance gene having a broad spectrum of resistance against rice blast (see also Table 1)
Evidence of O. australiensis chromosome segment integration
The STS marker 9871.T7E2b amplified the predicted size of 642 bp band for both IR65482-4-136-2-2 and O. australiensis. The cleaved banding patterns by HinfI and Tsp509I supported the integration of O. australiensis DNA segment(s) into IR65482-4-136-2-2, which had occurred near the 9871.T7 locus (Fig. 3b).
MAS validity test and cross-evaluation of blast resistance level
The linkage between the Pi40(t) gene and PCR markers 9871.T7E and 9871.T7E2b was validated as complete linked marker by genotyping resistant BC2F4 or BC3F3 progenies in the genetic backgrounds of Jinbubyeo and Junambyeo. Blast reaction with the isolate 01–01 showed segregation of 36 and 19 lines as resistant out of 95 and 68 lines tested in the genetic background of Jinbubyeo and Junambyeo, respectively. Some 115 (Jinbubyeo BCs) and 77 (Junambyeo BCs) resistant individuals (scored as 0–2) randomly selected for amplification of 9871.T7E (data not shown) and 9871.T7E2b (Fig. 4) loci did not exhibit any homozygous susceptible marker allele type of Junambyeo. This result confirmed that the Pi40(t) gene was the essential genetic component for resistance against blast infection.
MAS validity tests of blast-resistant BC progenies in a susceptible japonica cultivar genetic background. Partial gel image of detected marker genotypes on the 9871.T7E2b locus among 38 randomly selected BC3F3 and 39 BC2F4 progenies from a cross between Junambyeo (ID-4) and IR65482-4-136-2-2 (ID-2). An endonuclease, Tsp509 I, was used to detect polymorphism between parental lines. Progenies marked with a closed circle are homozygous for the IR65482-4-136-2-2 allele and a star indicates heterozygous progenies. Note that none of the resistant BC progenies was homozygous for the Junmanbyeo allele. ‘m’ = 100 bp DNA ladder (Promega). A natural polyacrylamide gel was used for electrophoresis, followed by ethidium bromide staining
The IR65482-4-136-2-2 genotype was also evaluated for Philippine blast isolates along with the check lines, including IR31917-45-3-2 and IRBLzt-T. None of the tested isolates was compatible with IR65482-4-136-2-2, but IR31917-45-3-2 and IRBLzt-T were susceptible to several isolates (Table 2; Fig. 3c). Our results suggested that the Pi40(t) gene indeed has a broad spectrum of blast resistance as well as a different genetic value from that of Piz-t.
Discussion
Developing broad-spectrum durable resistance to blast pathogen is the primary objective of most rice breeding programs worldwide. However, to date, the available R genes are mostly race-specific and short-lived. The availability of sequence information of Nipponbare, novel genetic resources of alien introgression lines, and new strategies accelerating the fine mapping of R genes provides new ways to develop broad-spectrum blast resistance in rice.
Qualitatively interpreted disease reaction results are often used to infer genotypes of segregating progenies at the resistance locus. However, qualitatively expressed data may cause serious bias per se, due to phenotypic mis-scoring as well as environment fluctuations during evaluation (Tabien et al. 2002). Therefore, incorporation of the resistance genes using DNA markers of a high-density linkage map would be challengeable, especially because of the ambiguous judgment on heterozygous progenies (Jena et al. 2006). To overcome these practical problems, we made a quantitative interpretation of the disease reaction data, since it is less sensitive to even modest numbers of phenotypic mis-scores. Based on the principles of linkage disequilibrium (Remington et al. 2001), and observed abnormality of frequency distribution of resistance levels from F 2:3 segregating progenies, only 2–5 anchor markers representing each chromosome were initially tested to find the chromosomal location of an effective genetic factor. A locus, contributed by Jinbubyeo, was localized on the subterminal region of chromosome 9, where a QTL was reported in Lemont and Teqing recombinant inbred lines (Tabien et al. 2002). The presence of the Pi40(t) gene on chromosome 6 was indicated even by markers tagging telomere regions due to its strong allele substitution effect. In spite of the small population size (N = 87) for association analysis, with selective markers for the putative chromosomal regions, plotting of F values from single-locus ANOVA provided the unbiased locations of effective loci as well as estimated genetic effects. Our study demonstrated for the first time that merging of quantitative data (pathogenicity) and a restricted amount of qualitative (marker alleles) data via association analysis would be one of the most practical approaches not only to map unknown resistance genes but also to evaluate their informative genetics such as breeding value and degree of dominance.
We adopted here the concept of e-Landing (Jena et al. 2006), by which all anchor markers were preplaced on a physical map constructed in silico based on the contig map of BAC/PAC clones of Nipponbare supported by TIGR. Our e-Landing approach offered the substitution of previously reported DNA markers with well-evaluated pools of primer sets of STS and SSR anchor markers to test the possibility of known blast genes that might be involved in the broad-spectrum resistance of IR65482-4-136-2-2. After we identified the 1.81-Mb virtual contig as the most putative location of the Pi40(t) gene, it was realized that at least four blast resistance genes, Piz-5(Pi2), Piz, Piz-t, and Pi9, were previously placed on the same chromosomal location. However, the determined marker intervals are informative only within the mapping population used to differentiate marker positions.
To avoid the time-consuming efforts of tedious genotyping of a large number of segregating progenies, we proposed here a new concept of “Mirror-Map”. We searched the corresponding physical regions on the Nipponbare genome for DNA markers of each blast resistant gene, based on an assumption that there may be a high level of synteny relationships among the rice lines used for Piz-5, Piz, Piz-t and Pi9 tagging DNA markers as well as those used in our study. By projecting markers over the Nipponbare reference genome, their physical and genetic intervals were successfully elucidated. We could identify a PAC clone, P0649C11, that includes six NBS-LRR disease resistance protein homologues as the most likely nested place of five rice blast resistance genes, including Pi40(t). The haplotyping results suggest that the ORF orders among the recurrent parents, the donor line for resistance, and monogenic differential lines are collinear and that there is a high degree of synteny at the micro level. Recent studies on Pi2, Pi9, Piz, and Piz-t genes also confirmed that the putative physical regions for these blast resistance genes contained a cluster of the NBS-LRR gene homologues (Hayashi et al. 2006; Qu et al. 2006; Zhou et al. 2007).
The Nipponbare genome harbors about 600 NBS-LRR motifs and these are considered as candidate resistance genes (Monosi et al. 2004; Zhou et al. 2004). The structural features and resistance specificity to avirulence proteins of NBS-LRR have been investigated (Jia et al. 2000; Martin et al 2003; Qu et al. 2006; Zhou et al. 2006). Clusters of resistance genes have been identified in diverse plant species (Song et al. 1995; Sun et al. 2004; Qu et al. 2006; Wei et al. 2002), where NBS-LRR genes are the most prevalent class out of six distinct resistance gene classes. Clustering of genes suggest that the tandem repeats of resistance gene homologues at a locus may provide a variety of opportunities for plants to evolve new specificities of resistance when the corresponding avirulence gene in the pathogen has mutated (Dean et al. 2005). Although six NBS-LRR disease resistance protein homologues were identified within the PAC clone P0649C11, they expanded up to only 70-kb physical regions, which corresponded to a 0.1-cM genetic interval based on the Mirror Map (Fig. 1c; Table 4). In our study, the 70-kb physical region was dissected by 12 primer sets and haplotypes for nine genotypes, including monogenic differential lines for Piz-5, Piz-t, Piz, and Pi9 were determined (Table 5). The most likely locus/loci for each blast resistance gene on chromosome 6 could be successfully assigned. The reliability of our approach is also supported posteriori by standard map-based cloning of the Pi9 gene (Qu et al. 2006). The Pi9 gene was putatively localized to the genomic region containing Nbs2–Pi9, which is highly collinear to the 9871.T7 locus. In our study, 9871.T7 and 9871.T14 loci are predicted as the most putative ORFs for the Pi9 gene. It is noteworthy to mention that comparative analysis of determined haplotypes for LTH monogenic differential lines could make it possible to develop a PCR primer set for the 9871.T7E2b marker, by which all LTH monogenic differential lines were discriminated against each other.
Comparative analysis on determined haplotypes for the 1.81-Mb physical intervals suggested that micro-colinearity was highly conserved across all tested genotypes, even for IR65482-4-136-2-2 and IRBL9-w. IR65482-4-136-2-2 carrying Pi40(t) gene was developed as an introgression line through an interspecific cross between IR31917-45-3-2 and O. australiensis (Jena et al. 1991). The Pi9 gene, which was also introduced into O. sativa from a wild species, O. minuta (Liu et al. 2002). O. minuta and O. australiensis have two distinct genome types as well as genome sizes (Wing et al. 2005); therefore, the alien DNA segments, including Pi40(t) and Pi9 genes, might have precisely recognized their destination within the O. sativa genome during interspecific crosses. A recent study strongly suggested that the physical positions of alien DNA fragment integration might be dependent on the host genome structure (Wang et al. 2005). Our previous studies on another O. australiensis introgression line, IR65482-7-216-1-2, having the Bph18(t) gene also supported the hypothesis (Jena et al. 2006). We have detected an interesting genomic feature nearby the alien DNA integration for blast resistance. The flanking BAC/PAC clones of Nipponbare were the clones composed of many putative retroelements—P0502B12 and B1197G05 for the Pi40(t) and Pi9 genes.
The possible mechanism of alien gene introgression into O. sativa is by restricted reciprocal recombination as detected in this study and as reported earlier (Jena et al. 1992, 2006). However, our study indicates that the marker polymorphism-based judgment on integration events may lead to under- and overestimations on the total number of integration events and their fragment sizes, especially at the physical regions maintaining a high level of synteny between O. sativa and its wild relatives. An integration test for the 70-kb physical regions, including the Pi40(t) gene, revealed polymorphism between IR65482-4-136-2-2 and IR31917-45-3-2 at seven loci out of 13 loci tested (Table 5). Without two double crossovers within the 7.7-kb (between 9871.T8E3 and 9871.T9I1) and 18-kb (between 9871.T10I2 and zt6057-Nip) intervals, the monomorphic regions could not be interpreted as negative-for-integration events. Therefore, the actual size of the O. australiensis DNA segment in IR65482-4-136-2-2 around the Pi40(t) gene might be at least 1.1 Mb, which was flanked by S2539 and 9871.T14E23. The comparative genomics program of OMAP will provide comprehensive information not only on the genome structure in the region but also on the detailed sequence variation among the NBS-LRR family members (Wing et al. 2005).
In our study, advanced breeding lines derived from BC progenies were used to validate the markers 9871.T7E and 9871.T7E2b as markers completely associated with the Pi40(t) gene. They have passed meioses independently 3 or 4 times and rapidly regressed into the unique haplotypes of their recurrent parental lines. The occurrence of resistant BC progenies from two genetic backgrounds with resistance-specific marker alleles in either the homozygous or heterozygous state suggests that the breeding value of the Pi40(t) gene estimated by using progenies from Jinbubyeo × IR65482-4-136-2-2 could be stably maintained in other susceptible japonica cultivars.
References
Asaga K (1981) A procedure for evaluating field resistance to blast in rice varieties. J Cent Agric Stn 35:51–138
Bryan GT, Wu KS, Farrall L, Jia YL, Hershey HP, McAdams SA, Faulk KN, Donaldson GK, Tarchini R, Valent B (2000) A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12:2033–2046
Campbell MA, Chen D, Ronald PC (2004) Development of co-dominant amplified polymorphic sequence markers in rice that flank the Magnaporthe grisea resistance gene Pi7(t) in recombinant inbred line 29. Phytophathology 94:302–307
Conaway-Bormans CA, Marchetti MA, Johnson CW, McClung AM, Park WD (2003) Molecular markers linked to the blast resistance gene Pi-z in rice for use in marker-assisted selection. Theor Appl Genet 107:1014–1020
Dean RA, Talbot NJ, Ebbole DJ, Farman ML (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980–986
Deng Y, Zhu X, Shen Y, He Z (2006) Genetic characterization and fine mapping of the blast resistance locus Pigm(t) tightly linked to Pi2 and Pi9 in a broad-spectrum resistant Chinese variety. Theor Appl Genet 113:705–713
Fukuoka S, Okuno K (2001) QTL analysis and mapping of pi21, a recessive gene for field resistance to rice blast in Japanese upland rice. Theor Appl Genet 103:185–190
Gowda M, Barman-Roy S, Chatoo BB (2006) Molecular mapping of a novel blast resistance gene Pi38 in rice using SSLP and AFLP markers. Plant Breed 125:596–599
Haldane JBS (1919) The combination of linkage values and the calculation of distances between the loci of linked factors. J Genet 8:299–309
Hayashi K, Hashimoto N, Daigen M, Ashikawa I (2004) Development of PCR-based SNP markers for rice blast resistance genes at the Piz locus. Theor Appl Genet 108:1212–1220
Hayashi K, Yoshida H, Ashikawa I (2006) Development of PCR-based allele-specific and InDel marker sets for nine rice blast resistance genes. Theor Appl Genet 113:251–260
Jena KK, Multani GS, Khush GS (1991) Monogenic alien addition lines of Oryza australiensis and alien gene transfer. Rice Genet II:728
Jena KK, Khush GS, Kochert G (1992) RFLP analysis of rice (Oryza sativa L.) introgression lines. Theor Appl Genet 84:109–118
Jena KK, Jeung JU, Lee JH, Choi HC, Brar DS (2006) High-resolution mapping of a new brown planthopper (BPH) resistance gene, Bph18(t) and marker-assisted selection for BPH resistance in rice (Oryza sativa L.). Theor Appl Genet 112:288–297
Jeung JU, Han SS, Cho YC, Hwang HG, Choi HC, Moon HP, Lee MH, Brar DS, MacKill DJ, Jena KK (2003) Identification of a new source of resistance to blast isolates of Korea in an alien introgression line of rice. Rice Genet Newslett 20:92–93
Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19:4004–4014
Jiang J, Wang S (2002) Identification of a a118-kb DNA fragment containing the locus of blast resistance gene Pi-2(t) in rice. Mol Genet Genomics 268:249–252
Khush GS (2004) Feeding five billion rice consumers—the role of rice breeding. In: Lee KS, Jena KK, Heong KL (eds) Adv Rice Sci pp 3–15
Kim BR, Roh JH, Choi SH, Ahn SW, Han SS (2004) Durability of rice cultivars to blast in Korea by sequential planting method (in Korean). Korean J Breed 36:350–356
Lincoln S, Daly M, Lander ES (1992) Construction genetic maps with MAPMAKER/EXP 3.0. Whitehead Institute Technical Report, 3rd edn
Liu G., Lu G., Zeng L, Wang G.L (2002) Two broad-spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome 6. Mol Genet Genomics 267:472–480
Lu G, Jantasuriyarat C, Zhou B, Wang GL (2004) Isolation and characterization of novel defense response genes involved in compatible and incompatible interactions between rice and Magnaporthe grisea. Theor Appl Genet 108:525–534
Mackill DJ, Bonman JM (1992) Inheritance of blast resistance in near-isogenic lines of rice. Phytopathology 82:746–749
Martin GB, Bogdanove AJ, Sessa G (2003) Understanding the function of plant disease resistance proteins. Annu Rev Plant Biol 54:23–61
McCouch SR, Teytelman L, Xu YB, Lobos KB, Clare K, Walton M, Fu BY, Maghirang R, Li ZK, Xing YZ, Zhang QF, Kono I, Yano M, Fjellstom R, Declerck G, Scheider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
Monosi B, Wisser RJ, Pennill L, Hulbert SH (2004) Full-genome analysis of resistance gene homologues in rice. Theor Appl Genet 109:1434–1447
Murray MG, Thompson WF (1980) Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Ou SH (1985) Rice diseases, 2nd edn. Commonwealth Mycological Institute, Kew, Surrey, 380 pp
Remington DL, Thornsberry JM, Matsuoka Y, Wilson IM, Whitt SR, Doebley J, Kresovich S, Goodman MM, Buckler ES (2001) Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proc Natl Acad Sci USA 98:11479–11484
SAS Institute (2000) SAS language and procedure: Usage, Release & 01. SAS Inst., Carry, NC
Sharma TR, Madhav MS, Singh BK, Shanker P, Jana TK, Dalal V, Pandit A, Singh A, Gaikwad K, Upreti HC, Singh NK (2005) High resolution mapping, cloning and molecular characterization of the PiK h gene of rice, which confers resistance to M. grisea Mol Gen Genomics 274:569–578
Song WG, Wang GL, Chen LL, Kim HK, Pi LY et al (1995) The rice disease resistance gene, Xa21, encodes a receptor-like protein kinase. Science 270:1804–1806
Sun XL, Cao YL, Yang ZF, Xu CG, Li XH, Wang SP, Zhang QF (2004) Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J 37:517–527
Tabien RE, Li Z, Paterson AH, Marchetti MA, Stansel JW, Pinson SRM (2002) Mapping QTLs for field resistance to the rice blast pathogen and evaluating their individual and combined utility in improved varieties. Theor Appl Genet 105:313–324
Tsunematsu H, Yanoria MJT, Ebron LA, Hayashi N, Ando I, Kato H, Imbe T, Khush GS (2000) Development of monogenic lines of rice blast resistance. Breeding Sci 50:229–234
Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, Han B, Wang GL (2006) The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172:1901–1914
Wang GL, Mackill DJ, Bonman JM, McCouch SR, Champox MC, Nelson RJ (1994) RFLP mapping of genes conferring complete and partial resistance to blast in a durably resistant rice cultivar. Genetics 136:1421–1434
Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19:55–64
Wang YM, Dong ZY, Zhang ZJ, Lin XY, Shen Y, Zhou D, Liu B (2005) Extensive de novo genomic variation in rice induced by introgression from wild rice (Zizamia latifola Griseb.). Genetics 170:1945–1956
Wei F, Wing RA, Wise RP (2002) Genome dynamics and evolution of the Mla (powdery mildew) resistance locus in barley. Plant Cell 14:1903–1917
Wing RA, Ammiraju JSS, Luo M, Kim HR, Yu Y, Kudrna D, Goicoechea JL et al (2005) The Oryza map alignment project: the golden path to unlocking the genetic potential of wild species. Plant Mol Biol 59:53–62
Zeigler RS, Thome J, Nelson J, Levy M, Correa-Victoria FJ (1994) Lineage exclusion: a proposal for linking blast population analysis to resistance breeding. Rice blast disease. CAB International, Wallingford, pp 267–292
Zhou T, Wang Y, Chen JQ, Araki H, Jing Z, Jiang K, Shen J, Tian D (2004) Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol Gen Genomics 271:402–415
Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Bellizzi M, Wang GL (2006) The eight aminoacid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant Microbe Interact 19:1216–1228
Zhou B, Dolan M, Sakai H, Wang GL (2007) The genomic dynamics and evolutionary mechanism of the Pi2/9 locus in rice. Mol Plant Microbe Interact 20:63–71
Acknowledgments
We are grateful to the Rural Development Administration (RDA), Suwon, Korea, for financial support for this study. We thank Darshan S. Brar (plant breeder, IRRI) and Guo-Liang Wang, Ohio State University, USA for critical review of the manuscript, Y. Fukuta (plant breeder, IRRI) for providing blast monogenic differential lines and C. M. Veracruz (plant pathologist, IRRI) for evaluating the lines against Philippines blast isolates. We are thankful to Ms. Kang Min-Hae for technical assistance and Bill Hardy (science editor, IRRI) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Sasaki
Rights and permissions
About this article
Cite this article
Jeung, J.U., Kim, B.R., Cho, Y.C. et al. A novel gene, Pi40(t), linked to the DNA markers derived from NBS-LRR motifs confers broad spectrum of blast resistance in rice. Theor Appl Genet 115, 1163–1177 (2007). https://doi.org/10.1007/s00122-007-0642-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-007-0642-x