Abstract
Prey perceiving predation risk commonly change their behavior to avoid predation. However, antipredator strategies are costly. Therefore, according to the threat-sensitive predator avoidance hypothesis, prey should match the intensity of their antipredator behaviors to the degree of threat, which may depend on the predator species and the spatial context. We assessed threat sensitivity of the two-spotted spider mite, Tetranychus urticae, to the cues of three predatory mites, Phytoseiulus persimilis, Neoseiulus californicus, and Amblyseius andersoni, posing different degrees of risk in two spatial contexts. We first conducted a no-choice test measuring oviposition and activity of T. urticae exposed to chemical traces of predators or traces plus predator eggs. Then, we tested the site preference of T. urticae in choice tests, using artificial cages and leaves. In the no-choice test, T. urticae deposited their first egg later in the presence of cues of P. persimilis than of the other two predators and cue absence, indicating interspecific threat-sensitivity. T. urticae laid also fewer eggs in the presence of cues of P. persimilis and A. andersoni than of N. californicus and cue absence. In the artificial cage test, the spider mites preferred the site with predator traces, whereas in the leaf test, they preferentially resided on leaves without traces. We argue that in a nonplant environment, chemical predator traces do not indicate a risk for T. urticae, and instead, these traces function as indirect habitat cues. The spider mites were attracted to these cues because they associated them with the existence of a nearby host plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is a strong selective force shaping the behavior, morphology, and life history traits of prey individuals and as such has important structuring effects on higher organization levels within ecosystems (Lima and Dill 1990; Peacor and Werner 1997; Kats and Dill 1998; Lima 1998; Lind and Cresswell 2005). Consequently, prey organisms developed a wide range of morphological, physiological, and/or behavioral adaptations to avoid being detected by predators or to defend themselves against predators upon detection (e.g., Lima and Dill 1990; Kats and Dill 1998). Behavioral antipredator strategies may include changed habitat use, changes in oviposition behavior, increased vigilance, escaping or avoidance behaviors, changed movement patterns, and/or changed feeding activities (Lima and Dill 1990, for review). Commonly, responding to predation threat has fitness costs for prey (e.g., Rhoades and Blumstein 2007). Therefore, in many animal taxa, prey evolved abilities to assess the degree of predation risk and to modulate the intensity of their antipredator response according to the magnitude of risk. This concept is called threat-sensitive predator avoidance hypothesis (Sih 1982; Helfman 1989) and has been extensively studied in both aquatic (Rochette et al. 1997; Engström-Öst and Lehtiniemi 2004; Blanchet et al. 2007) and terrestrial (Edut and Eilam 2003; Amo et al. 2004; Monclús et al. 2009; Walzer and Schausberger 2011) ecosystems. Most pertinent studies were concerned with intraspecific threat sensitivity, such as discrimination between different densities or life stages of the same predator species (e.g., Ferrari and Chivers 2009), while only a few dealt with interspecific threat sensitivity, i.e., discrimination between different species and the associated risk (e.g., Walzer and Schausberger 2011).
Prey organisms may perceive their predators or predator-associated cues through different sensory modalities, such as olfaction, taste, vision, audition, physical contact, or combinations thereof (Kiesecker et al. 1996; Kats and Dill 1998; Dicke and Grostal 2001). Arthropods such as insects and mites most commonly use volatile and tactile chemosensory cues via olfaction and taste, respectively, to assess predation risk (Kats and Dill 1998; Dicke and Grostal 2001). Such cues—as other types of cues that indicate predation risk—may emanate from either a direct or an indirect source. Direct cues of predation are signals produced by the predators themselves, such as their body odors, pheromones, exuviae, eggs, excreta, sounds, movements, etc. Indirect cues are signals not produced by the predators themselves and may include any other perceivable environmental factor that is more or less reliably associated with predation risk, such as pheromones released by alarmed, injured, or dead conspecific individuals (e.g., Thorson et al. 1998; Dicke and Grostal 2001).
Here, we studied the antipredator behavior of a globally distributed herbivore, the two-spotted spider mite Tetranychus urticae Koch, to direct chemosensory cues of three predatory mite species, Phytoseiulus persimilis Athias-Henriot, Neoseiulus californicus (McGregor) and Amyblyseius andersoni Chant. T. urticae is one of the most important herbivorous pest species worldwide with more than 1,000 different host plant species (Helle and Sabelis 1985a; Bolland et al. 1998). All three predatory mites are natural enemies of herbivorous mites and insects and used for biological control in various greenhouse and outdoor crops (e.g., Helle and Sabelis 1985b; McMurtry and Croft 1997; Hoy 2011). Several previous studies showed that antipredator behaviors of spider mites can be induced or mediated by predatory mite-associated chemosensory cues but do not require physical presence of the predators (Grostal and Dicke 1999; Škaloudova et al. 2007; Kroon et al. 2008). However, it is unknown whether the spider mites show interspecific threat sensitivity in their antipredator responses. Our first objective was thus to determine whether T. urticae is able to detect traces of each of the three predatory mite species and adjust its antipredator responses accordingly. The three predator species differ in the threat posed to T. urticae, with P. persimilis—due to its specialization on spider mite prey—representing a greater threat than the other two species, which may feed on a range of mite and insect prey and plant-derived substances such as pollen (e.g., McMurtry and Croft 1997; Castagnoli and Simoni 2003; Gerson and Weintraub 2007; Pozzebon and Duso 2008). Traits making P. persimilis a high risk predator for the spider mites are, among others, its well-developed searching and detection ability, well-developed ability to cope with the spider mite webs, high aggressiveness and voracity, persistent presence inside the webs, and favorable functional and numerical responses. The relative risk posed by the generalists N. californicus and A. andersoni to T. urticae and whether one or the other should be considered a higher risk predator depends on circumstances such as the host plant species, prey density, and the trait used to judge predation risk. All three predator species are commercially available for biological control of T. urticae. Predation risk also commonly varies with space and time, and the information content of signals may depend on the environmental background. Consequently, antipredator responses are context dependent (Lima and Dill 1990; Kats and Dill 1998) and may, for example, vary with the features of the patch, site, or habitat where chemosensory cues or other predator-associated cues are perceived (e.g., Sih and Kats 1991; Kats and Dill 1998; Rodriguez et al. 2001; Heithaus et al. 2009; Wirsing et al. 2010). Hence, our second objective was to assess whether the response of T. urticae to predator cues depends on the spatial context. Specifically, we were interested in the response of T. urticae to predator cues on a plant, representing the feeding and reproductive sites of both the herbivorous and the predatory mites, and in a nonplant environment, representing the transitory sites during interplant traveling of the herbivores and their predators. In such transitory sites, the predation risk for the spider mites should be much lower than in their intrinsic habitat, the plant.
Materials and methods
Plants and mites
The laboratory population of T. urticae was maintained on common bean plants, Phaseolus vulgaris L., grown in a substrate mixture consisting of 50 % soil, 25 % sand, and 25 % peat moss, under room conditions at 20–25 °C, 50–80 % RH, and 16:8 h L/D. All spider mites were predator naïve, i.e., did not have any experience with predators or their cues before experiments. The predatory mites, N. californicus, P. persimilis, and A. andersoni, used for the experiments were taken from populations reared on T. urticae prey on separate artificial arenas kept in an environmental chamber at 25 ± 1 °C, 60 ± 5 % RH, 16:8 h L/D or in an air-conditioned room at 25 ± 2 °C, 60 ± 10 % RH, natural daylight. The laboratory populations of all three predators had been originally founded with specimens collected in Sicily, Italy (Walzer and Schausberger 2011). Each predator-rearing unit consisted of a plastic tile resting on a water-soaked foam cube (15 × 15 × 4 cm) placed in a plastic box (20 × 20 × 6 cm) half-filled with tap water. The edges of the tile were covered with moist tissue paper to prevent the predators from escaping. The predators were fed with all life stages of the spider mites brushed off detached bean leaves onto arenas (for A. andersoni), or by adding fresh detached spider mite-infested leaves onto arenas in two to three day intervals (for P. persimilis and N. californicus). All spider mite females and predatory mite females used in experiments were randomly withdrawn from their respective rearing units. Each spider mite female, each predatory mite female, and each leaf disc and cage were used only once in experiments. All experimental units were stored in an environmental chamber at 25 ± 1 °C, 60 ± 5 % RH, and 16:8 h L/D.
Response to chemical traces and eggs of predatory mites (no choice, leaf)
In the first experiment, we assessed the general activity (moving or resting) and oviposition of T. urticae in response to cues from gravid females of the three predatory mite species. Cues were chemical traces such as metabolic waste products and, possibly, chemical footprints left by the predatory mite females on the leaf surface with and without their eggs. Treatments with chemical traces of the predators with and without their eggs were considered representing different risks to the spider mites because the eggs give rise to potential future predators. Each experimental unit consisted of a circular disc (ø 13 mm), punched out from a trifoliate leaf of a noninfested bean plant, floating on top of a water column inside a cylindrical compartment (ø 15 mm) of a plastic cartridge. The compartment was completely filled with tap water, ensuring that the water surface was slightly above that of the upper edge of the plastic cartridge. Before the experiment, a gravid predatory mite female was placed on the leaf disc for 12 h or the leaf disc was left without a predator for control. After 12 h, the predatory mite female was gently removed from the disc using a fine brush and eggs laid by the female were either removed (for treatments with only chemical traces) or two eggs left on the disc (for treatments with chemical traces plus eggs). Thereafter, a gravid T. urticae female, randomly taken from the laboratory population, was released on the disc using a fine brush. The activity (moving or resting) and oviposition of the spider mite female was recorded every 10 min during the first hour, then every 20 min during the second to sixth and then again 24 h after release. Each treatment (chemical traces of the three predatory mite females with or without their eggs and the respective controls) was replicated 27–33 times. Treatments within a given cue type (traces or traces plus eggs) were conducted in parallel but, according to cue type, allocated to two separate experimental series, which were run immediately after each other. To allow joint analysis, a control treatment (blank leaf) was included in both series, enabling to distinguish effects caused by the difference in the offered cue types from artifacts caused by the series.
Response to chemical traces of predatory mites (choice, artificial cage)
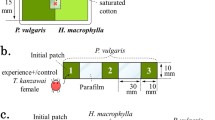
In the second experiment, we examined the residence preference of T. urticae given a binary choice between sites with and without chemical traces left by females of the three predatory mite species in an artificial cage, i.e., a nonplant environment. Each choice cage consisted of a T-shaped maze drilled into a 3-mm thick rectangular acrylic plate (35 × 80 mm). The maze consisted of two large circular chambers (ø 15 mm), located at either end of the horizontal bar of the T (length, 15 mm), and a smaller circular chamber (ø 5 mm) at the base of the vertical bar of the T. The T connecting the three chambers consisted of a 2-mm wide aisle. The bottom of the cages was closed with fine gauze to allow air exchange, while the upper side was closed by a microscope slide held in place with a rubber band (Schausberger and Hoffmann 2008). Before the experiment, a gravid predatory mite female was caged in one of the two large chambers, while the other remained empty. To avoid that the predator female moved to the other side of the choice cage, the aisle connecting the two large chambers was blocked with an inert plastic piece. After 12 h, the predatory mite female was removed from the chamber, the plastic piece blocking the aisle was removed, and the experiment started by releasing one gravid T. urticae female in the small chamber at the bottom end of the T. During the next 4 h, the position of the spider mite female (inside the chamber with or without predator traces or somewhere else, considered the neutral zone) was recorded every 30 min. Each choice situation (blank vs. cues of P. persimilis, N. californicus, or A. andersoni), and for control, a choice situation without predatory mite cues (blank vs. blank) was replicated 20–23 times, taking care that different choice situations were carried out in parallel.
Response to chemical traces of predatory mites (choice, leaf)
In the third experiment, we examined the residence preference of T. urticae given a binary choice between leaves with and without chemical traces left by females of the three predatory mite species. The spatial arrangement was similar to the artificial choice cages. Each experimental unit consisted of two leaf discs (ø 15 mm), spaced apart 15 mm, resting on top of a water-soaked foam cube covered by a tissue paper and connected by a T-shaped wax bridge (∼5 mm wide). The wax bridge was created by dripping hot wax from a nonfragrant candle onto the tissue covering the foam (Walzer et al. 2006). Before the experiment, a gravid predatory mite female was placed on one of the two leaf discs for 12 h, while the other remained predator-free. To prevent the predatory mite from moving to the other disc, the wax bridge was blocked by a strip of moist tissue paper partially covered with glue. After 12 h, the predatory mite female was removed from the leaf disc, the paper strip blocking the bridge was removed, and the experiment started by releasing a gravid T. urticae female at the bottom end of the T-shaped wax bridge. During the next 4 h, the position of the spider mite female (on the disc with or without predator traces or on the neutral wax bridge) was recorded every 30 min. Each choice situation (blank vs. cues of P. persimilis, N. californicus, or A. andersoni), and for control, a choice situation without predatory mite cues (blank vs. blank) was replicated 20–24 times, taking care that different choice situations were carried out in parallel.
Statistical analyses
SPSS 15.0.1 (SPSS Inc., Chicago, USA, 2006) was used for all statistical analyses. In the first experiment (no choice), generalized linear models (GLM; gamma distribution, log link) and post hoc least significant difference (LSD) tests were used to compare the time of the first egg laid and total oviposition within 24 h among the four treatments (no cues, or cues of A. andersoni, N. californicus, or P. persimilis) and between cue types (only chemical predator traces or traces plus eggs). If the first egg was not laid within the initial 6 h of the experiment but eggs were found after 24 h, for statistical analyses, we assumed that the first egg was laid after 7 h. To compare the activity (moving or resting) of T. urticae among treatments and between cue types, GLM (counts of events, binomial distribution, logit link) was used. For GLM on activity, the data were aggregated before analysis, i.e., the number of times out of 21 observations in total the mites were observed moving was calculated. In the second (choice, cage) and third (choice, leaf) experiment, Wilcoxon signed-rank tests were used to analyze the position of T. urticae on the blank side and cue side (no cues, i.e., blank, or cues of A. andersoni, N. californicus, or P. persimilis), respectively, within each choice situation (assuming equal distribution if choice was random). GLMs (count of events, binomial distribution, logit link) and post hoc LSD tests were used to compare the residence of T. urticae on the blank side among choice situations (blank vs. blank, or vs. cues of A. andersoni, N. californicus, or P. persimilis). In the second and third experiment, the residence data were aggregated before analyses, i.e., the number of times the spider mite female was found on the blank side and the alternative side were calculated.
Results
Response to chemical traces and eggs of predatory mites (no choice, leaf)
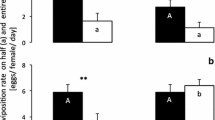
The time of the first egg laid by T. urticae was affected by treatment (no cues, or cues of A. andersoni, N. californicus, or P. persimilis) (GLM; Wald \( \chi_3^2=13.739 \), P = 0.003) but not cue type (traces or traces plus eggs) (Wald \( \chi_1^2=0.484 \), P = 0.487) and the interaction of treatment and cue type (Wald \( \chi_3^2=4.363 \), P = 0.225; Fig. 1). The spider mites laid their first egg significantly later on leaf discs harboring cues of P. persimilis than on leaf discs harboring cues of N. californicus or A. andersoni or leaf discs without predator cues (LSD; P < 0.05 for every pairwise comparison). The time of the first egg laid did not differ between leaf discs harboring cues of A. andersoni or N. californicus or without predator cues (P > 0.05 for every pairwise comparison; Fig. 1).
The total number of eggs produced by T. urticae within 24 h was affected by treatment (GLM; Wald \( \chi_3^2=18.427 \), P < 0.001) but not cue type (Wald \( \chi_1^2=3.091 \), P = 0.079) and the interaction of treatment and cue type (Wald \( \chi_3^2=6.421 \), P = 0.093) (Fig. 2). The spider mites laid fewer eggs on leaf discs harboring cues of P. persimilis and A. andersoni than on leaf discs harboring cues of N. californicus or without predator cues (LSD; P < 0.05).
The relative time spent moving was significantly influenced by treatment (GLM; Wald \( \chi_3^2=37.497 \), P < 0.001) and cue type (Wald \( \chi_1^2=19.882 \), P < 0.001) but not the interaction of treatment and cue type (Wald \( \chi_3^2=1.599 \), P = 0.660; Fig. 3). The spider mites moved less on blank leaves than on leaves harboring predatory mite cues (LSD; P < 0.05 for every pairwise comparison). Moving did not differ among discs harboring predatory mite cues (P > 0.05 for every pairwise comparison). T. urticae spent more time moving in presence of predatory mite traces plus eggs than only predatory mite traces. However, this was apparently an artifact of conducting treatments with traces plus eggs and traces alone in two separate series because the difference was also true for blank leaf discs (Fig. 3). An exclusive effect of cue type would have been evident by a significant interaction term and lacking difference on blank leaf discs, which was not the case.
Response to chemical traces of predatory mites (choice, artificial cage)
T. urticae females given a choice between a blank cavity (without predatory mite cues) and a cavity containing chemical traces of predatory mites (P. persimilis or A. andersoni or N. californicus) preferentially resided in the cavity containing the predatory mite traces (Wilcoxon signed-rank tests; blank vs. A. andersoni cues Z = −2.397, P = 0.017; blank vs. N. californicus cues Z = −2.836, P = 0.005; blank vs. P. persimilis cues Z = −3.356, P = 0.001; Fig. 4a). The spider mites did not have a preference in the control choice situation (blank vs. blank Z = −0.272, P = 0.785). GLM (Wald \( \chi_3^2=20.637 \), P < 0.001) and post hoc LSD tests revealed that the residence preference differed between the control choice situation (blank vs. blank) and the choice situations with predatory mite cues (P < 0.05 for every pairwise comparison) but did not differ among choice situations with predatory mite cues (P > 0.05 for every pairwise comparison).
Residence of T. urticae females given a choice between a blank side and a side containing cues (chemical traces) of the predatory mites A. andersoni, N. californicus, or P. persimilis or no predator cues for control (blank). The P values refer to Wilcoxon signed-rank tests within each choice situation assuming random choice (indicated by the broken lines). a Choice experiment using artificial cages. b Choice experiment using leaf discs (N = 20–24 for each choice situation of a and b)
Response to chemical traces of predatory mites (choice, leaf)
T. urticae females given a choice between a blank leaf disc (without predatory mite cues) and a leaf disc containing chemical traces of predatory mites (P. persimilis or A. andersoni or N. californicus) preferentially resided on the blank leaf disc in every choice situation (Wilcoxon signed-rank tests; blank vs. A. andersoni cues Z = −2.474, P = 0.013; blank vs. N. californicus cues Z = −2.664, P = 0.008; blank vs. P. persimilis cues Z = −2.152, P = 0.031; Fig. 4b). The spider mites did not have a residence preference in the control choice situation (blank vs. blank Z = −0.894, P = 0.371). GLM (Wald \( \chi_3^2=9.178 \), P = 0.027) and post hoc LSD tests revealed that the preference differed between the control choice situation (blank vs. blank) and the choice situations containing the predatory mite cues (P < 0.05 for every pairwise comparison) but did not differ among choice situations with predatory mite cues (P > 0.05 for every pairwise comparison).
Discussion
The first, no choice, experiment suggests that the two-spotted spider mite T. urticae is able to assess the predation risk of different predatory mite species through chemical traces left by the predators on the leaf surface and to adjust its antipredator response accordingly. Depending on the behavioral trait, T. urticae discriminated between the cues of the high risk predator P. persimilis and the other two lower risk predators or no predator cues, which is in accordance with the threat-sensitive predator avoidance hypothesis (Sih 1982; Helfman 1989). Threat-sensitivity did not manifest in every trait, probably indicating trait-dependent benefit–cost ratios. The spider mites delayed oviposition of their first egg only in presence of P. persimilis cues, while there were no differences among the other two predatory mite cues and the control. Moreover, total oviposition of T. urticae within 24 h was lower in presence of cues of P. persimilis and A. andersoni than in presence of cues of N. californicus or without cues. Interspecific threat sensitivity was not reflected in the moving activity of the spider mites, which was generally higher in presence than absence of predator cues.
Several previous studies are consistent with the hypothesis that T. urticae is able to discriminate between cues associated with different levels of risk but did not test for or did not show interspecific threat sensitivity. For example, Škaloudová et al. (2007) demonstrated that oviposition of T. urticae decreased with increasing predation risk within the same predator species, P. persimilis. Grostal and Dicke (2000) investigated the variability in the response of T. urticae to cues from a range of enemy and nonenemy mite species. In their choice experiments, T. urticae avoided oviposition on leaflets harboring cues of a wide range of predatory and parasitic mite species, but was unaffected by cues of exclusive fungivores and pollen-feeding mites. Likewise, in their choice experiments the proportion of eggs laid by the spider mites was lower on leaf discs harboring cues of predatory mites previously fed on spider mites than on those harboring cues of pollen-fed ones. Grostal and Dicke (2000) concluded that the behavioral changes of prey were triggered by the differences in metabolic waste products of zoophagous and nonzoophagous predator species and individuals, respectively. Walzer and Schausberger (2011) provided evidence for interspecific threat sensitivity in oviposition site selection within a guild of predatory mites sharing T. urticae as prey.
Regarding oviposition and activity levels, our no-choice experiment principally corroborates the findings of previous related studies on spider mite antipredator behaviors but the results differ in several aspects. For example, in a similar set-up to ours, Grostal and Dicke (1999) did not find an effect of P. persimilis cues on oviposition of T. urticae, while Škaloudová et al. (2007) observed decreased spider mite oviposition in response to increased predation risk by P. persimilis. Similarly, Choh et al. (2010) observed that T. urticae laid fewer eggs when it had previously experienced P. persimilis on the same leaf or when it was exposed to the predator’s odors from an adjacent leaf. Differences among these studies could be due to the variety of set-ups and procedures used. Grostal and Dicke (1999) tested the oviposition response of a single spider mite on a leaf disc, while Škaloudová et al. (2007) and Choh et al. (2010) tested groups of spider mites placed together on the same leaf disc. In the latter two studies, the spider mites could have responded to indirect predator-associated cues, i.e., alarm signals from their conspecifics (Dicke and Grostal 2001; Oku et al. 2003), possibly inducing or intensifying their anti-predator responses. Unlike our experimental procedure, introducing the spider mite female onto the leaf disc immediately after removing the predatory mite and its eggs, Grostal and Dicke (1999) introduced them only 2 h later. Due to partial volatility of the chemical cues of the predators (Dicke and Grostal 2001), their concentration on the leaf disc and subsequent perceptibility for the spider mites probably decreased over time. The fact that T. urticae spent more time moving in the presence of predator cues may indicate an attempt of the spider mites to escape from the dangerous environment associated with the predator cues, which is a widespread antipredator response (Lima and Dill 1990 for review). Such escape or avoidance responses of spider mites have been frequently observed (Pallini et al. 1999; Magalhães et al. 2002; Oku et al. 2004; Škaloudová et al. 2007; Choh and Takabayashi 2007). The increased moving activity in presence of predatory mite cues is also a possible explanation for the oviposition reduction of T. urticae (Oku et al. 2004; Škaloudová et al. 2007). Our experimental set-up did not allow the spider mites to leave the leaf discs but to explore them and walk around, searching for an exit (Janssen et al. 1997) or a suitable egg laying site (Hoffmeister and Roitberg 1997). This behavior may have resulted in less time spent feeding, in turn negatively affecting the number of eggs produced. However, this explanation does not explain the delay in the first egg laid in presence of P. persimilis cues because the moving activity of the spider mites did not differ among the cues of different predatory mite species. The delay in time of the first egg laid could be due to egg retention by the spider mites. Similar egg retention behaviors have been reported for predatory mites in intraguild predation situations (Montserrat et al. 2007; Abad-Moyano et al. 2010).
In the two choice experiments, we assessed the residence preference of T. urticae in nonplant and plant environments with and without cues of the three predatory mite species. In all choice situations of the experiment using the artificial cages (experiment 2), the spider mites consistently preferred the chamber harboring the predatory mite cues. This result is at first glance counter-intuitive (Stamps and Krishnan 2005) and in contradiction to most previous findings showing that, in choice situations, prey, including herbivorous mites such as T. urticae, try to escape from sites where they perceive direct or indirect cues of their predators (Lima and Dill 1990; Grostal and Dicke 1999; Pallini et al. 1999; Magalhães et al. 2002; Choh and Takabayashi 2007). Moreover, in the no-choice experiment, the spider mites were more active in presence of predator cues, which may be interpreted as attempts to get away from a site perceived to be dangerous. However, the third experiment using leaf discs suggests that the information conveyed by the predator cues depends on the spatial context and is functionally reversed—from repulsion to attraction—when perceived on plants versus in nonplant environments. On the leaf discs, the spider mites may have perceived the chemosensory predator cues, possibly together with cues emitted by the plants, by both olfaction and taste through piercing the leaf tissue, whereas in the artificial cage they could only perceive the predator cues by olfaction. Therefore, not only the information conveyed but also the sensory modalities involved may have differed between the plant and nonplant environments. Ultimately, in the nonplant environment, the artificial cage, T. urticae may have searched for and may have been attracted to signals indicating a host plant or plant tissue. Considering that all three predatory mites had fed on spider mites before the experiment, which in turn had fed on plants, their cues may have contained plant signals. In the nonplant environment, the spider mites could thus have associated the predatory mite cues with the existence of a nearby host plant. In natural settings, following these cues could guide the spider mites to a new host plant because the predators themselves are plant dwelling and thus have to travel to new host plants. We therefore argue that predatory mite cues perceived in a nonplant environment are not considered dangerous by the spider mites because they are only indicative of predators traveling to new host plants and thereby functioning as indirect habitat cues (Stamps and Krishnan 2005). The possibility of a functional reversal of the information conveyed by chemosensory signals in dependence of the spatial context, on a plant vs. nonplant substrate, has important bearings for the experimental design and interpretation of future studies on signaling and communication through chemosensory means in plant-dwelling arthropods.
References
Abad-Moyano R, Urbaneja A, Schausberger P (2010) Intraguild interactions between Euseius stipulatus and the candidate biological control agents of Tetranychus urticae in Spanish clementine orchards: Phytoseiulus persimilis and Neoseiulus californicus. Exp Appl Acarol 50:23–34. doi:10.1007/s10493-009-9278-7
Amo L, López P, Martín J (2004) Wall lizards combine chemical and visual cues of ambush snake predators to avoid overestimating risk inside refuges. Anim Behav 67:647–653. doi:10.1016/j.anbehav.2003.08.005
Blanchet S, Bernatchez L, Dodson JJ (2007) Behavioural and growth responses of territorial fish (Atlantic salmon, Salmo salar L.) to multiple predator cues. Ethology 113:1061–1072. doi:10.1111/j.1439-0310.2007.01410.x
Bolland HR, Gutierrez J, Flechtmann CHW (1998) World catalogue of the spider mite family (Acari, Tetranychidae). Brill, Leiden
Castagnoli M, Simoni S (2003) Neoseiulus californicus (Mc Gregor) (Acari Phytoseiidae): survey of biological and behavioural traits of a versatile predator. Redia 86:153–164
Choh Y, Takabayashi J (2007) Predator avoidance in phytophagous mites: responses to present danger depends on alternative host quality. Oecologia 151:262–267. doi:10.1007/s00442-006-0590-1
Choh Y, Uefune M, Takabayashi J (2010) Predation-related odours reduce oviposition in a herbivorous mite. Exp Appl Acarol 50:1–8. doi:10.1007/s10493-009-9277-8
Dicke M, Grostal P (2001) Chemical detection of natural enemies by arthropods: an ecological perspective. Annu Rev Ecol Syst 32:1–23. doi:10.1146/annurev.ecolsys.32.081501.113951
Edut S, Eilam D (2003) Rodents in open space adjust their behavioral response to the different risk levels during barn-owl attack. BMC Ecol 3:10. doi:10.1186/1472-6785-3-10
Engström-Öst J, Lehtiniemi M (2004) Threat-sensitive predator avoidance by pike larvae. J Fish Biol 65:251–261. doi:10.1111/j.1095-8649.2004.00448.x
Ferrari MCO, Chivers DP (2009) Temporal variability, threat sensitivity and conflicting information about the nature of risk: understanding the dynamics of tadpole antipredation behaviour. Anim Behav 78:11–16. doi:10.1016/j.anbehav.2009.03.016
Gerson U, Weintraub PG (2007) Mites for the control of pests in protected cultivation. Pest Manag Sci 63:658–676. doi:10.1002/ps
Grostal P, Dicke M (1999) Direct and indirect cues of predation risk influence behavior and reproduction of prey: a case for acarine interactions. Behav Ecol 10:422–427. doi:10.1093/beheco/10.4.422
Grostal P, Dicke M (2000) Recognising one’s enemies: a functional approach to risk assessment by prey. Behav Ecol Sociobiol 47:258–264. doi:10.1007/s002650050663
Heithaus MR, Wirsing AJ, Burkholder D, Thomson J, Dill LM (2009) Towards a predictive framework for predator risk effects: the interaction of landscape features and prey escape tactics. J Anim Ecol 78:556–562. doi:10.1111/j.1365-2656.2008.01512.x
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behav Ecol Sociobiol 24:47–58. doi:10.1007/BF00300117
Helle W, Sabelis MW (1985a) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier Science, Amsterdam
Helle W, Sabelis MW (1985b) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier Science, Amsterdam
Hoffmeister TS, Roitberg BD (1997) Counterespionage in an insect herbivore–parasitoid system. Naturwissenschaften 84:117–119. doi:10.1007/s001140050358
Hoy MA (2011) Agricultural acarology. Introduction to integrated mite management. CRC, Boca Raton
Janssen A, Bruin J, Jacobs G, Schraag R, Sabelis MW (1997) Predators use volatiles to avoid prey patches with conspecifics. J Anim Ecol 66:223–232. doi:10.2307/6024
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kiesecker JM, Chivers DP, Blaustein AR (1996) The use of chemical cues in predator recognition by western toad tadpoles. Anim Behav 52:1237–1245. doi:10.1006/anbe.1996.0271
Kroon A, Veenendaal RL, Bruin J, Egas M, Sabelis MW (2008) “Sleeping with the enemy”—predator-induced diapause in a mite. Naturwissenschaften 95:1195–1198. doi:10.1007/s00114-008-0442-4
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–659. doi:10.1139/z90-092
Lima SL (1998) Nonlethal effects in the ecology of predator–prey interactions: what are the ecological effects of anti-predator decision-making? Bioscience 48:25–34
Lind J, Cresswell W (2005) Determining the fitness consequences of antipredation behavior. Behav Ecol 16:945–956. doi:10.1093/beheco/ari075
Magalhães S, Janssen A, Hanna R, Sabelis MW (2002) Flexible antipredator behaviour in herbivorous mites through vertical migration in a plant. Oecologia 132:143–149. doi:10.1007/s00442-002-0950-4
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 43:291–321. doi:10.1146/annurev.ento.42.1.291
Monclús R, Palomares F, Tablado Z, Martínez-Fontúrbel A, Palme R (2009) Testing the threat-sensitive predator avoidance hypothesis: physiological responses and predator pressure in wild rabbits. Oecologia 158:615–623. doi:10.1007/s00442-008-1201-0
Montserrat M, Bas C, Magalhães S, Sabelis MW, de Roos AM, Janssen A (2007) Predators induce egg retention in prey. Oecologia 150:699–705. doi:10.1007/s00442-006-0527-8
Oku K, Yano S, Osakabe M, Takafuji A (2003) Spider mites assess predation risk by using the odor of injured conspecifics. J Chem Ecol 29:2609–2613. doi:10.1023/A:1026395311664
Oku K, Yano S, Takafuji A (2004) Nonlethal indirect effects of native predatory mites, Amblyseius womersleyi Schicha (Acari: Phytoseiidae), on the phytophagous mite Tetranychus kanzawai Kishida (Acari: Tetranychidae). J Ethol 22:109–112. doi:10.1007/s10164-003-0102-2
Pallini A, Janssen A, Sabelis MW (1999) Spider mites avoid plants with predators. Exp Appl Acarol 23:803–815. doi:10.1023/A:1006266232714
Peacor SD, Werner EE (1997) Trait-mediated indirect interactions in a simple aquatic food web. Ecology 78:1146–1156. doi:10.2307/2265865
Pozzebon A, Duso C (2008) Grape downy mildew Plasmopara viticola, an alternative food for generalist predatory mites occurring in vineyards. Biol Control 45:441–449. doi:10.1016/j.biocontrol.2008.02.001
Rhoades E, Blumstein DT (2007) Predicted fitness consequences of threat-sensitive hiding behavior. Behav Ecol 18:937–943. doi:10.1093/beheco/arm064
Rochette R, Dill LM, Himmelman JH (1997) A field test of threat sensitivity in a marine gastropod. Anim Behav 54:1053–1062. doi:10.1006/anbe.1997.0488
Rodriguez A, Andren H, Jansson G (2001) Habitat-mediated predation risk and decision making of small birds at forest edges. Oikos 95:383–396. doi:10.1034/j.1600-0706.2001.950303.x
Schausberger P, Hoffmann D (2008) Maternal manipulation of hatching asynchrony limits sibling cannibalism in the predatory mite Phytoseiulus persimilis. J Anim Ecol 77:1109–1114. doi:10.1111/j.1365-2656.2008.01440.x
Sih A (1982) Foraging strategies and the avoidance of predation by the aquatic insect Notonecta hoffmanni. Ecology 63:768–796. doi:10.2307/1936799
Sih A, Kats LB (1991) Effects of refuge availability on the responses of salamander larvae to chemical cues from predatory green sunfish. Anim Behav 42:330–332. doi:10.1016/S0003-3472(05)80569-X
Škaloudova B, Zemek R, Krivan V (2007) The effect of predation risk in an acarine system. Anim Behav 74:813–821. doi:10.1016/j.anbehav.2007.02.005
Stamps J, Krishnan VV (2005) Non-intuitive cue use in habitat selection. Ecology 86:2860–2867. doi:10.1890/05-0290
Thorson JM, Morgan RA, Brown JS, Norman JE (1998) Direct and indirect cues of predatory risk and patch use by fox squirrels and thirteen-lined ground squirrels. Behav Ecol 9:151–157. doi:10.1093/beheco/9.2.151
Walzer A, Paulus HF, Schausberger P (2006) Oviposition behavior of interacting predatory mites. J Insect Behav 19:305–320. doi:10.1007/s10905-006-9025-4
Walzer A, Schausberger P (2011) Threat-sensitive anti-intraguild predation behavior: maternal strategies to reduce offspring predation risk in mites. Anim Behav 81:177–184. doi:10.1016/j.anbehav.2010.09.031
Wirsing AJ, Cameron KE, Heithaus MR (2010) Spatial responses to predators vary with prey escape mode. Anim Behav 79:531–537. doi:10.1016/j.anbehav.2009.12.014
Acknowledgments
We thank Andreas Walzer, Stefan Peneder, and David J. Patiňo-Ruiz for comments on an earlier version of the manuscript. M. Celeste Fernández-Ferrari was partially funded by an Erasmus Mundus stipend.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Fernández Ferrari, M.C., Schausberger, P. From repulsion to attraction: species- and spatial context-dependent threat sensitive response of the spider mite Tetranychus urticae to predatory mite cues. Naturwissenschaften 100, 541–549 (2013). https://doi.org/10.1007/s00114-013-1050-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-013-1050-5