Abstract
When adult females of the herbivorous mite, Tetranychus urticae, were exposed to the predatory mite, Phytoseiulus persimilis, they laid fewer eggs than females that had not been exposed to P. persimilis when transferred onto a new leaf patch. However, when T. urticae females were exposed to either products of P. persimilis or artificially damaged conspecific eggs on a leaf patch, the number of T. urticae eggs on a new leaf patch did not differ significantly from the control. The reduced oviposition was neither due to the feeding activity on the leaf patch with P. persimilis nor to that on the new leaf patch. There was also no significant difference between the number of T. urticae eggs produced on a new leaf patch following exposure to the odours of a neighbouring leaf patch where there had previously been either P. persimilis or T. urticae adults. However, female T. urticae that had been exposed to odours from neighbouring leaf patches on which both T. urticae and P. persimilis had been placed produced significantly fewer eggs on a new leaf patch than those that had not been exposed to such odours. Neither odours from neighbouring intact leaf patches on which T. urticae eggs were preyed on by P. persimilis, nor odours from a neighbouring Parafilm patch on which T. urticae was preyed on by P. persimilis affected the oviposition of T. urticae. These data suggest that the presence of T. urticae, P. persimilis and a leaf patch are needed for the emission of odours to reduce oviposition in T. urticae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predator-prey interactions do not always result in the consumption of prey by predators (Sih 1980, 1984; Lima and Dill 1990; Lima 1998; Peacor and Werner 2000; Brodin and Johansson 2002; Pangle and Peacor 2006; Werner and Peacor 2006). Prey can escape from predators by reducing their own performance such as growth rate (Lima and Dill 1990; Lima 1998; Peacor and Werner 2000; Brodin and Johansson 2002; Pangle and Peacor 2006; Werner and Peacor 2006) and/or changing their behaviour (Lima 1998; Losey and Denno 1998; Magalhães et al. 2002; Choh and Takabayashi 2007). However, escape from currently invading predators would not be the only goal for predator avoidance for prey over its lifetime, as the prey could encounter predators of the same or different species after the first avoidance. It is widely reported that previous experience with predators affects the subsequent antipredator responses in prey (Chivers et al. 1996; Alvarez and Nicieza 2006; Ferrari et al. 2006; Turner et al. 2006; Dalesman et al. 2006).
Predators attack not only the mobile stages of prey that show avoidance response/behaviour, but also the immobile stages, such as eggs and pupae, which are in most cases more vulnerable to predation than mobile prey. Thus, a reduction in oviposition may be one antipredator behavior for prey animals. It is reported that some females avoid ovipositing near their predators (Faraji et al. 2001; Agarwala et al. 2003; Nomikou et al. 2003), and retain eggs inside of their body in the presence of predators (Montserrat et al. 2007). For example, females of the phytophagous mite Tetranychus urticae have been reported to reduce oviposition in the presence of the predatory mite Phytoseiulus persimilis (Škaloudová et al. 2007), and to increase migration from a patch with predators to avoid predation (Choh and Takabayashi 2007). However, little is known about whether prior experience of a prey with its predator affects oviposition after escape from the predator. In this study, we examined the oviposition of T. urticae in a new patch after experience with P. persimilis, and investigated potential cues that could affect the oviposition by T. uritcae.
Materials and methods
Plants and mites
Lima bean plants (Phaseolus lunatus cv. Pole Sieva) were grown in soil in a greenhouse at 25 ± 2°C and 60–70% relative humidity, under a 16:8 h light:dark photoperiod. For all experiments, plants were 10–15 days post germination. Herbivorous mites (T. urticae) were obtained from the Laboratory of Ecological Information, Graduate School of Agriculture, Kyoto University, in 2002 and reared on lima bean plants in a climate-controlled room (25 ± 2°C, 60–70% r.h., 16:8 h L:D). Predatory mites (P. persimilis) were purchased from Koppert BV (Berkel en Rodenrijs, The Netherlands). They were reared on detached lima bean leaves that were heavily infested with T. urticae under the same climate conditions. Fresh T. urticae-infested leaves were added every other day.
General experimental conditions
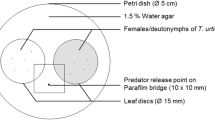
Leaf patches were prepared for experiments as follows. We cut a piece of leaf from the primary leaf of a lima bean plant with scissors and further cut the piece into ‘leaf patches’ of three sizes (4 × 5, 2.5 × 4 and 1 × 1 cm). Each piece was placed on water-saturated cotton wool in a Petri dish (9 cm in diameter, 1.4 cm high).
To obtain newly emerged adult female T. urticae for oviposition experiments, 50 quiescent female deutonymphs were selected from T. urticae cultures and introduced onto a leaf patch (4 × 5 cm). One day after the introduction, 50 adult males were selected from T. urticae cultures and introduced onto the leaf patch for mating. For the experiments, we randomly selected newly emerged (2 days post eclosion) T. urticae females from the leaf patch. All experiments were performed in a climate-controlled room (25 ± 2°C, 60–70% r.h., 16:8 h L:D).
Effect of the presence of Phytoseiulus persimilis on the oviposition of Tetranychus urticae
Thirty female T. urticae were introduced onto a leaf patch (4 × 5 cm). Ten adult female P. persimilis were randomly selected from the culture, and introduced onto the same leaf patch 24 h after the introduction of T. urticae. Phytoseiulus persimilis individuals were allowed to predate on T. urticae and their eggs for 24 h. We observed that a mean (±SE) of 3.08 ± 0.91 T. urticae were killed by P. persimilis in this period. One day after the introduction of P. persimilis, we randomly selected a T. urticae individual from the survivors and transferred it onto a new leaf patch (1 × 1 cm). As control, we kept 30 T. urticae on a leaf patch (4 × 5 cm) without P. persimilis for 2 days, and randomly selected a T. urticae female for the new leaf patch. We counted the eggs laid by T. urticae on the new leaf patch 3 days after introduction. To exclude the effect of changes in feeding behaviour by T. urticae in the presence of P. persimilis on the oviposition rate in the new leaf patch, we also exposed 30 female T. urticae to 10 P. persimilis on a Parafilm patch (4 × 5 cm) for 24 h in the absence of food. As a control, 30 female T. urticae were kept on the Parafilm patch in the absence of both P. persimilis and food for 24 h. After this, a T. urticae female was randomly selected from the Parafilm patch and put on a leaf patch (1 × 1 cm). Each test mite was carefully checked under a stereo microscope to make sure that all body parts were intact. The eggs laid by each test female over 3 days were counted. The experiments were repeated 12 times per treatment. The numbers of eggs were compared with a Mann–Whitney U-test.
The leaf area consumed by a female T. urticae was examined using individuals that had been exposed to zero and 10 P. persimilis on the same leaf patch, as described above. We placed a randomly selected T. urticae onto a 1 × 1 cm leaf patch. Each replicate patch was scanned digitally pre and 3 days post introduction of the mite (Kant et al. 2004). Each scan included a 1 cm2 reference of paper patch. The scans were processed in Photoshop CS2 (Adobe Systems, Mountain View, CA, USA) as follows. First, the background was selected and deleted. Second, the colored pixels were transformed to black-and-white using the threshold tool, so that all damaged areas were set to white and the remaining undamaged leaf-area was set to black. The histogram tool was used to count the white pixels (chlorotic lesions) and the black pixels (undamaged area) of each treated and reference patch. The consumed leaf area was calculated by the difference in the white area pre and 3 days post T. urticae-infestation. The experiments were repeated 12 times per treatment. The leaf areas consumed were compared using a Mann–Whitney U-test.
Effects of the presence of Phytoseiulus persimilis products on the oviposition of Tetranychus urticae
Ten P. persimilis females were placed on an intact leaf patch (4 × 5 cm). After 24 h, the predatory mites were removed from the patch, but their associated products, such as feces, were not removed. The patch was called a ‘predator-exposed’ leaf patch. We then placed 30 adult female T. urticae on the predator-exposed leaf patches and kept them for 24 h. As the control, we placed 30 adult female T. urticae on an intact leaf patch (4 × 5 cm) and kept them for 24 h. We randomly selected a T. urticae female from each of the predator-exposed and control patches, placed them on a separate intact leaf patch (1 × 1 cm) and counted the eggs after 3 days. The experiment was repeated 12 times per treatment. The numbers of eggs were compared with a Mann–Whitney U-test.
Effect of the presence of artificially damaged Tetranychus urticae eggs on the oviposition of T. urticae
Thirty adult female T. urticae were placed on a leaf patch (4 × 5 cm) and allowed to lay eggs for 24 h, resulting in more than 300 eggs on the leaf patch. We carefully pierced 50 of these eggs on the leaf patch with a fine needle to imitate P. persimilis predation. The female T. urticae and damaged eggs were kept on the leaf patch for a further 24 h. As a control, 30 adult female T. urticae were kept on a leaf patch of the same size for 48 h, without having damaged any of the eggs. We randomly selected a T. urticae female from the patch with damaged eggs and from the control patch, placed them on a separate intact leaf patch (1 × 1 cm) and counted the eggs after 3 days. The experiment was repeated 12 times per treatment. The numbers of eggs were compared with a Mann–Whitney U-test.
Effect of predation-related odours on the oviposition of Tetranychus urticae
We examined the effects of odours from a leaf patch with either T. urticae or P. persimilis, and a leaf patch with both T. urticae and P. persimilis, on the oviposition of T. urticae. Two leaf patches (2.5 × 4 cm) were placed 0.5 cm apart in a Petri dish. Thirty adult female T. urticae and 10 adult female P. persimilis were placed on one of the leaf patches. To prevent the migration of mites to the other leaf, a tanglefoot barrier was made around the edge of the leaf patch. As control, 30 adult female T. urticae were placed on one of the leaf patches. Thirty adult female T. urticae were introduced onto the other leaf patch without a tanglefoot barrier (hereafter called the exposed patch). Each pair of leaf patches was covered with a plastic cup (12 cm diameter, 6 cm height), which had an air hole (1 × 3 cm, 1.4 cm above the base; see Oku et al. 2003), and kept for 24 h. Under these conditions, T. urticae on the exposed leaf patch received odours from the other leaf patch. We randomly selected a T. urticae female from the patch that had been placed next to patch with T. urticae alone, and P. persimilis alone, and the patch with both T. urticae and P. persimilis, and counted the eggs after 3 days on a separate new intact leaf patch (1 × 1 cm). The experiment was repeated 12 times per treatment. The egg numbers were compared with Steel–Dwass test (Dwass 1960; Steel 1960) following a Kruskal–Wallis test.
We examined the effects of odours from eggs killed by P. persimilis on the oviposition of T. urticae using the set-up described above. Thirty female T. urticae were placed on a leaf patch (2.5 × 4 cm) for 24 h. We then removed all T. urticae and placed 10 P. persimilis on the leaf patch, and these preyed on the T. urticae eggs. This leaf patch was used as the odour source. Thirty T. uricae were placed on a separate leaf patch (2.5 × 4 cm), as described above, and received odours from the odour source patch. A leaf patch with unpredated T. urticae eggs was used as the odour source for the control patch. After exposure to odours for 24 h, we randomly selected a T. urticae from each of the exposed and control patches, placed them on a separate new intact leaf patch (1 × 1 cm) and counted the eggs after 3 days. The experiment was repeated 12 times per treatment. The egg numbers were compared with a Mann–Whitney U-test.
We also examined the effect of odours from a Parafilm patch (2.5 × 4 cm) with T. urticae and P. persimilis on the oviposition of T. urticae. Parafilm patches were used to exclude any volatiles of leaf-patch origin. Thirty female T. urticae and 10 P. persimilis were placed on the Parafilm patch as an odour source. Female T. urticae on the exposed leaf patch (2.5 × 4 cm) received odours from the Parafilm patch for 24 h. As the control, we used a Parafilm patch with T. urticae as the odour source. We randomly selected a T. urticae from each of the exposed and control patches, placed them on a new intact leaf patch (1 × 1 cm) and counted the eggs after 3 days. The experiment was repeated 12 times per treatment. The numbers of eggs were compared with a Mann–Whitney U-test.
Results
Effect of the presence of Phytoseiulus persimilis on the oviposition of Tetranychus urticae
Tetranychus urticae that had been previously exposed to P. persimilis on the same leaf patch laid significantly fewer eggs than T. urticae that had not been exposed to P. persimilis (U = 26.50, df = 1, P = 0.0084; Fig. 1a). When T. urticae females were placed on a Parafilm patch without food, they laid fewer eggs when exposed to P. persimilis than when unexposed to the predator (U = 11.50, df = 1, P = 0.0005; Fig. 1b). There was no significant difference in the consumed leaf area between T. urticae females that had been exposed and unexposed to P. persimilis on a leaf patch (unexposed: 0.028 ± 0.0045 cm2, exposed: 0.035 ± 0.0061 cm2, U = 40, df = 1, P = 0.45).
Mean (±SE) number of eggs laid by T. urticae females on clean leaf patches over 3 days following being kept with and without (control) the predatory mite P. persimilis for 1 day on a a leaf patch and b Parafilm patch. The significance of differences were evaluated with a Mann–Whitney U test; **P < 0.01, ***P < 0.001
Effects of Phytoseiulus persimilis products and of artificially damaged Tetranychus urticae eggs on the oviposition of T. urticae
There was no significant difference in the oviposition rate by T. urticae on P. persimilis-exposed and clean leaf patches (U = 60.50, df = 1, P = 0.51; Fig. 2a). There was also no significant difference in oviposition rate by T. urticae on patches with and without artificially damaged conspecific eggs (U = 56.50, df = 1, P = 0.37; Fig. 2b).
Effect of predation-related odours on the oviposition of Tetranychus urticae
There were significant differences in the oviposition rate of T. urticae that had been exposed to odours from adjacent leaf patches of different treatments (T. urticae; 45.33 ± 2.01, P. persimilis; 44.33 ± 2.04, T. urticae + P. persimilis; 31.42 ± 3.13, H 2 = 11.25, P < 0.01, Kruskal–Wallis test). There was no significant difference in oviposition rate by T. urticae with previous exposure to odours from a leaf patch with P. persimilis compared to that from a leaf patch with conspecifics (P > 0.05, Steel–Dwass test). However, there was a significant decrease in oviposition rate by T. urticae following exposure to odours from a leaf patch with both P. persimilis and T. urticae (P < 0.05, Steel–Dwass test). There was no difference in oviposition rate by T. urticae following exposure to odours from a leaf patch with T. urticae eggs and P. persimilis, and from a leaf patch with only T. urticae eggs (U = 61.50, df = 1, P = 0.54; Fig. 3a). Furthermore, there was no significant difference in oviposition rate between T. urticae that had been exposed to odours from a Parafilm patch with T. urticae and P. persimilis, and from a Parafilm patch with only T. urticae (U = 71.00, df = 1, P = 0.95; Fig. 3b).
Discussion
In this study, T. urticae that had previously been exposed to P. persimilis showed reduced oviposition on a predator-free leaf patch (Fig. 1a). It has been reported that, in the presence of P. persimilis, T. urticae reduced its feeding time (Janssen et al. 1997) and increased its moving time (Škaloudová et al. 2007). Such changes in feeding by T. urticae might affect oviposition rate on clean leaf patches. In the present study, however, T. urticae that had been exposed to P. persimilis in the absence of food also reduced oviposition on clean leaf patches (Fig. 1b). These results suggest that feeding changes by T. urticae during exposure to P. persimilis do not affect oviposition on a new leaf. It is reported that the oviposition of T. urticae is related to feeding (Agrawal et al. 2002). If T. urticae reduced feeding on clean leaf patches after exposure to P. persimilis, they might reduce oviposition. However, our data indicate that reduced oviposition does not result from reduced feeding, because there was no difference in the consumed leaf area between T. urticae that had been exposed and not exposed to P. persimilis. We already reported that T. urticae disperse from a currently inhabiting leaf patch with P. persimilis to a new intact leaf patch (Choh and Takabayashi 2007). In this study, we showed that T. urticae reduce oviposition on the new patch. Here, some individuals may leave the current patch earlier than the others, and if so, an effect of the duration of the exposure to P. persimilis on the oviposition of T. urticae in a new patch might be expected. Further studies are needed to explore this possibility.
We examined which cues resulted in the reduced oviposition of T. urticae. Škaloudová et al. (2007) reported that T. urticae reduced oviposition on a P. persimilis-exposed leaf patch. Furthermore, it has been reported that T. urticae avoids leaf patches previously exposed to P. persimilis or with injured conspecifics (Kriesch and Dicke 1997; Grostal and Dicke 1999). However, we found that T. urticae did not reduce oviposition on a clean leaf patch after being kept on a P. persimilis-exposed leaf patch or on a leaf patch with damaged conspecific eggs (Fig. 2). In the damaged egg experiment, eggs were pierced in a short time (within 10 min), which is different from the time taken for egg predation. Such a difference might be a factor affecting the reduced oviposition. We then examined whether reduced oviposition was induced by airborne cues such as predator- and predation-related odours. Although T. urticae did not reduce oviposition after exposure to odours from a leaf patch with either predators or conspecific eggs killed by P. persimilis, oviposition was reduced after exposure to odours from a leaf patch with both predators and T. urticae. Furthermore, odours from leaf patches with T. urticae and from Parafilm patches with T. urticae and P. persimilis did not reduce the oviposition of T. urticae. These results suggest that odours from leaf patches, T. urticae and P. persimilis can reduce oviposition. It is important, therefore, to identify the origin and chemical nature of the active component(s) affecting oviposition by T. urticae.
Tetranychus urticae eggs are more vulnerable to predation by P. persimilis compared to the adult and larvae/nymph stages (Blackwood et al. 2001). It has been reported that female arthropods avoid ovipositing near their predators to reduce the risk of egg predation (Faraji et al. 2001; Agarwala et al. 2003; Nomikou et al. 2003). A T. urticae individual that has had experience with predators might reduce oviposition in a predator-free patch to reduce the potential predation risk of their eggs. Further studies are needed to clarify whether the reduced oviposition of T. urticae is a strategy to protect offspring.
References
Agarwala BK, Yasuda H, Kajita Y (2003) Effect of conspecific and heterospecific feces on foraging and oviposition of two predatory ladybirds: role of fecal cues in predator avoidance. J Chem Ecol 29:357–376
Agrawal AA, Vala F, Sabelis MW (2002) Induction of preference and performance after acclimation to novel hosts in a polyphagous spider mite: adaptive plasticity? Am Nat 159:553–565
Alvarez D, Nicieza AG (2006) Factors determining tadpole vulnerability to predators: can prior experience compensate for a suboptimal shape? Evol Ecol 20:523–534
Blackwood JS, Schausberger P, Croft BA (2001) Prey-stage preference in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environ Entomol 30:1103–1111
Brodin T, Johansson F (2002) Effects of predator-induced thinning and activity changes on life history in a damselfly. Oecologia 132:316–322
Chivers DP, Wisenden BD, Smith RJF (1996) Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet. Anim Behav 52:315–320
Choh Y, Takabayashi J (2007) Predator avoidance in phytophagous mites: response to present danger depends on alternative host quality. Oecologia 151:262–267
Dalesman S, Rundle SD, Coleman RA, Cotton PA (2006) Cue association and antipredator behaviour in a pulmonate snail, Lymnaea stagnalis. Anim Behav 71:789–797
Dwass M (1960) Some k-sample rank-order tests. In: Olkin I (ed) Contributions to probability and statistics. Stanford University Press, California, pp 198–202
Faraji F, Janssen A, Sabelis MW (2001) Predatory mites avoid ovipositing near counterattacking prey. Exp Appl Acarol 25:613–623
Ferrari MCO, Capitania-Kwok T, Chivers DP (2006) The role of learning in the acquisition of threat-sensitive responses to predator odours. Behav Ecol Sociobiol 60:522–527
Grostal P, Dicke M (1999) Direct and indirect cues of predation risk influence behavior and reproduction of prey: a case for acarine interactions. Behav Ecol 10:422–427
Janssen A, Bruin J, Jacobs G, Schraag R, Sabelis MW (1997) Predators use volatiles to avoid prey patches with conspecifics. J Anim Ecol 66:223–232
Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol 135:483–495
Kriesch S, Dicke M (1997) Avoidance of predatory mites by two-spotted spider mite Tetranychus urticae: the role of infochemicals. Proc Exp Appl Entomol 8:121–126
Lima SL (1998) Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Study Behav 27:215–290
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Losey JE, Denno RF (1998) The escape response of pea aphid to foliar-foraging predators: factors affecting dropping behaviour. Ecol Entomol 23:53–61
Magalhães S, Janssen A, Hanna R, Sabelis MW (2002) Flexible antipredator behaviour in herbivorous mites through vertical migration in a plant. Oecologia 132:143–149
Montserrat M, Bas C, Magalhães S, Sabelis MW, de Roos AM, Janssen A (2007) Predators induced egg retention in prey. Oecologia 150:699–705
Nomikou M, Janssen A, Sabelis MW (2003) Herbivore host plant selection: whitefly learns to avoid host plant that harbour predators of their offspring. Oecologia 136:484–488
Oku K, Yano S, Osakabe M, Takafuji A (2003) Spider mites assess predation risk by using the odour of injured conspecifics. J Chem Ecol 29:2609–2613
Pangle KL, Peacor SD (2006) Non-lethal effects of the invasive predator Bythotrephes longimanus on Daphnia mendotae. Freshwater Biol 51:1070–1078
Peacor SD, Werner EE (2000) Predator effects on an assemblage of consumers through induced changes in consumer foraging behaviour. Ecology 81:1998–2010
Sih A (1980) Optimal behavior: can foragers balance two conflicting needs? Science 210:1041–1043
Sih A (1984) The behavioral response race between predators and prey. Am Nat 123:143–150
Škaloudová B, Zemek R, Křivan V (2007) The effect of predation risk on an acrine system. Anim Behav 74:813–821
Steel RGD (1960) A rank sum test for comparing all pairs of treatments. Technometrics 2:197–207
Turner AM, Turner SE, Lappi HM (2006) Learning, memory and predator avoidance by freshwater snails: effects of experience on predator recognition and defensive strategy. Anim Behav 72:1443–1450
Werner EE, Peacor SD (2006) Lethal and nonlethal effects on an herbivore guild mediated by system productivity. Ecology 87:347–361
Acknowledgments
This study was supported in part by grants of priority area (S) by Junji Takabayashi from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Global COE program A06 of Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choh, Y., Uefune, M. & Takabayashi, J. Predation-related odours reduce oviposition in a herbivorous mite. Exp Appl Acarol 50, 1–8 (2010). https://doi.org/10.1007/s10493-009-9277-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-009-9277-8