Abstract
We studied whether volatiles released by putative host plants affect the antipredator response of an herbivorous mite, Tetranychus urticae, when the patch was invaded by Phytoseiulus persimilis. Tetranychus urticae laid a lower number of eggs on tomato leaves than on lima bean leaves, suggesting that lima bean is a preferred host food source for T. urticae. In addition, T. urticae preferred lima bean plant volatiles to tomato plant volatiles in a Y-tube olfactometer test. To investigate the antipredator response of T. urticae, we examined the migration of T. urticae from a lima bean leaf disc to a neighbouring leaf disc (either a tomato or lima bean leaf disc) when ten predators were introduced into the original lima bean disc. A Parafilm bridge allowed for migration between the leaf discs. No migrations occurred between leaf discs when there were no predators introduced to the original leaf disc. However, when predators were introduced migrations did occur. When the neighbouring leaf disc was upwind of the original disc, the migration rate of the mite from original lima bean leaf disc to a neighbouring tomato leaf disc was significantly lower than that to a neighbouring lima bean leaf disc. By contrast, when the neighbouring leaf disc was downwind of the original leaf disc, there was no difference in the migration rates between lima bean leaf discs and tomato leaf discs. The number of T. urticae killed by P. persimilis for each treatment was not different, and this clearly shows that the danger was the same in all treatments regardless of the decision made by T. urticae. From these results, we conclude that T. urticae change their antipredator response by evaluating the difference in host plant volatiles in the patch they inhabit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In predator–prey interactions, prey alter their morphology (Tollrian 1995; Tollrian and Harvell 1999; Relyea 2003; Kishida and Nishimura 2004), and behaviour (Lima and Dill 1990; Lima 1998, Losey and Denno 1998a, b; Magalhães et al. 2002) to escape from predators. Although these responses would reduce predation risk (Lima 1998; Tollrian and Harvell 1999), several studies have reported that such responses are costly (Abrahams and Dill 1989; Grand and Dill 1997; Matsumoto et al. 2002) and thus it is important for prey to assess their predation risk to minimize the cost of antipredator responses. For example, herbivorous arthropod preys stop feeding and leave a host-plant some time after perceiving the presence of predators. However, several factors have been shown to affect the timing of such a decision (Lima 1998). One example is starvation in pea aphids, when the energetic internal stress (i.e., starvation) of the pea aphid Acyrthosiphon pisum increases, the predominant antipredator response changes from walking away and dropping to kicking at the parasitoids (Villagra et al. 2002).
Other important aspects of the antipredator response are the relative quality of the host-plant (or patch) used by herbivores and the proximity of available host-plants when predators invade. Specifically, the detectability and/or availability of new host-plants would affect antipredator response of herbivorous arthropods, as they do not always find suitable host-plants near their current food source. Many herbivore species use intact plant volatiles as a cue for host-plant selection (Visser 1986; Bernays and Chapman 1994; Dicke 2000). Thus, plant volatiles are likely to be an important factor for herbivorous arthropods when predators invade and they leave to search for new host-plants. However, it is not known if herbivores change their antipredator responses when plant volatiles released from neighbouring plants indicate that they are less suitable hosts than the host plants where they are feeding. Therefore, the objective of this study is to determine if plant volatiles are critical factors in herbivorous arthropod antipredator responses.
We studied the effect of plant volatiles on the patch-leaving decision of a herbivore when the patch was invaded by its predators. Our predator–prey system consisted of the predatory mite, Phytoseiulus persimilis, and herbivorous mite, Tetranychus urticae. Tetranychus urticae is a polyphagous herbivore, with over 900 host-plant species recorded (Bolland et al. 1998). It has been reported that T. urticae avoid plant volatiles emitted by conspecific-infested lima bean plants (Dicke 1986; Horiuchi et al. 2003) and plants with predators (Pallini et al. 1999). However, it is unclear whether they distinguish intact plant volatiles among plant species. To test the effect of intact plant volatiles on the patch-leaving decision by T. urticae to escape from P. persimilis, we used lima bean plants and tomato plants. Tomato is reported to be an unsuitable host for T. urticae due to the presence of toxic phytochemicals in their leaves and glandular trichomes (Aina et al. 1972; Chatzivasileiadis and Sabelis 1997; Chatzivasileiadis et al. 1999). In this study, we address whether or not T. urticae change its patch-leaving behaviour to escape from P. persimilis (antipredator response) according to volatiles emitted from suitable and unsuitable host plants next to the patch. We also discuss the costs and benefits of the antipredator response of T. urticae to P. persimilis.
Materials and methods
Plants and mites
Lima bean plants (Phaseolus lunatus cv. Sieva) and tomato plants (Lycopersicon esculentum cv. Hausu-Momotaro) were grown in soil in a greenhouse at 25±2°C, 60–70% relative humidity (RH) and a photoperiod of 16L:8D. For the experiments, we used lima bean plants and tomato plants at 10–15 and 15–20 days after germination.
Herbivorous mites (T. urticae) were obtained from the Laboratory of Ecological Information, Graduate School of Agriculture, Kyoto University, in 2002, and reared on lima bean plants in an incubator (25±2°C, 60–70% RH, 16L:8D).
Predatory mites (Phytoseiulus persimilis) were purchased from Koppert (Berkel and Rodenrijs, The Netherlands). They were reared on detached lima bean leaves, heavily infested with T. urticae in an incubator (25±2°C, 60–70% RH, 16L:8D). Fresh T. urticae-infested leaves were added every other day.
Oviposition of T. urticae on a leaf disc
Using a clean razor blade, we made a 1×1 cm leaf disc from a primary leaf of a lima bean plant and from the fourth leaf from the ground of a tomato plant (n=50 for each plant). At that time, the tomato plants had 5–6 leaves. These discs were individually put on water-saturated cotton in a Petri dish (9 cm diameter, 1.4 cm height). For each replicate, an adult female T. urticae that had been randomly selected from the rearing colony was placed on a disc. The number of eggs laid by each mite was counted 3 days after the initiation of the experiment. These experiments were conducted in a climate-controlled room (25±2°C, 60–70% RH, 16L:8D). The data were analyzed using a t-test.
Response of T. urticae to plant odour
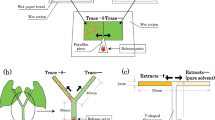
A Y-tube olfactometor (see Takabayashi and Dicke 1992 for setup details) was used to test whether T. urticae distinguishes between volatiles of intact lima bean and intact tomato plants. We placed a Y-shaped iron wire at the center of the olfactometer. Air that had been cleaned through an activated charcoal filter was pumped (2.5 l/min) to an odour source bottle that was connected to the arm of the olfactometer. For odour sources, we used plants that had been cut with a razor blade just above soil level. The cut area was covered with moist cotton wool. Two plants were used for an odour source. There was no significant difference in the weight of the lima bean and tomato plants that formed the odour sources (lima bean 7.782±1.343 g; tomato 7.579±1.297 g; P=0.8273, Mann–Whitney U-test).
For each assay, a single, randomly selected adult female T. urticae was positioned on the start of the iron wire. When the mite reached the end of one arm of the olfactometer, its choice was recorded. The maximum duration of each observation was 5 min. After every five bioassays, the odour sources on each arm were exchanged with that of the other arm to adjust for potential asymmetries in the experimental arena. As T. urticae left silken thread when they moved on the iron wire, the iron wire was carefully wiped with dry cotton wool after each bioassay. Individual mites were used only once and a total of 20 spider mites were used in 1 day. Bioassays were replicated 3 experimental days using different odour sources to avoid pseudoreplication. The tests were performed in a climate-controlled room (25±2°C, 60–70% RH, 16L:8D).
Data were analyzed with a binomial test to determine whether the distribution of mites over the two odour sources was significantly different from a 1:1 distribution.
Antipredator response of T. urticae
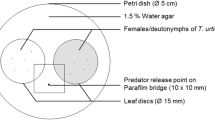
We made a 3.5×2.5 cm leaf disc from a primary leaf of a lima bean plant (hereafter called the original leaf disc), and put it on water-saturated cotton wool in a Petri dish (15 cm diameter, 1 cm height). Then, a 3.5×2.0 cm leaf disc (hereafter called the neighbouring leaf disc), made from either a primary leaf of a lima bean plant or a leaflet (fourth from the ground) of a tomato plant, was put in the same Petri dish, 5 cm from the original disc. Thirty, randomly selected, adult female T. urticae were placed on the original disc and allowed to lay eggs for 24 h. We then introduced randomly selected female P. persimili onto the original disc (0, 1, or 10 P. persimilis per disc). The neighbouring leaf disc was for T. urticae to escape from the predators in the original leaf disc (antipredator response). For the control experiments, P. persimilis were not introduced. We connected the two leaf discs with a Parafilm bridge (5 cm length, 0.5 cm width), and placed the Petri dishes holding the two discs in a wind tunnel (air flow 10 cm/s, size 40×40×80 cm). We placed three Petri dishes, each of which held a pair of leaf discs with different P. persimilis densities (0, 1, 10), as a group in the center of the wind tunnel. The neighbouring leaf discs were positioned upwind of the original leaf disc to expose T. urticae to the plant volatiles from the neighbouring leaf disc. The number of T. urticae on each neighbouring leaf disc, and the number of T. urticae killed by P. persimilis on each original leaf disc, were counted 24 h after the introduction of the Petri dishes into the wind tunnel. Care was taken not to expose T. urticae to plant volatiles from different Petri dishes. We arranged three dishes in a triangle in the wind tunnel. Under this condition, we confirmed that contamination of volatiles from one leaf disks set to another did not occur by checking the flow of smoke introduced into the wind tunnel. The position of each Petri dish in the wind tunnel was changed for every experiment to adjust for potential asymmetry in the experimental arena. Experiments were conducted in a climate-controlled room (25±2°C, 60–70% RH, 16L:8D).
We then tested whether the migration of T. urticae was also triggered by an increase in T. urticae density, and how T. urticae migrated in response to predators when they were not exposed to plant volatiles from the neighbouring leaf disc. To test the first possibility, 30 additional T. urticae adult females were placed on each original leaf disc 24 h after the initial 30 T. urticae adults were introduced onto the disc. As for the above experiments, a neighbouring leaf disc was connected to the original with a Parafilm bridge. The Petri dishes were placed in the wind tunnel, with the original leaf disc downwind to the neighbouring leaf disc. To test the second possibility, we used the same experimental design as for the initial experiment. In this experiment, however, the neighbouring leaf disc was positioned downwind in the wind tunnel so that T. urticae were not exposed to plant volatiles. The numbers of T. urticae adults that migrated to the neighbouring leaf disc were counted 24 h after placing the Petri dishes in the wind tunnel.
We calculated the proportion of migrated mites [i.e., the number of T. urticae that migrated to the neighbouring leaf disc/the number of T. urticae introduced into the original leaf disc (30)] for each replicate. We repeated the above experiments 18 times per treatment. The proportions of migrated T. urticae data were normalized by arcsine square root transformations and compared using a Tukey–Kramer test followed by two-way ANOVA (the effects of predator density and plant species on the migration rate). The numbers of T. urticae killed by P. persimilis were compared using a Tukey–Kramer test followed by two-way ANOVA (the effects of predator density and plant species on the mortality). The control data were excluded from the statistical analyses, because we focused on the number of spider mites that were preyed upon by the predatory mites.
Results
Performance and olfactory response of T. urticae to different plant species
Tetranychus urticae laid on average of about five times more eggs on lima bean leaf discs than on tomato leaf discs (mean±SE number of T. urticae eggs on lima bean leaf discs: 30.28±1.38; on tomato leaf discs: 6.50±0.48; P<0.0001, t-test). When T. urticae was offered a choice between lima bean plants and tomato plants in a Y-tube olfactometer, it preferred lima bean plants to tomato plants (number of T. urticae attracted to lima bean plant volatiles=40 and to tomato plant volatiles=20; P=0.0135, binomial test).
Antipredator response of T. urticae
When the neighbouring leaf discs were positioned either upwind or downwind of T. urticae in the wind tunnel, the proportions of T. urticae adults that migrated to the neighbouring leaf disc (lima bean and tomato) increased with the density of P. persimilis that invaded the original leaf disc (P<0.0001, two-way ANOVA; Table 1a, b; Fig. 1a, b). When ten P. persimilis were introduced on the original leaf disc, the proportions of T. urticae adults that migrated to lima bean leaf discs were significantly higher than those that migrated to tomato leaf discs (P=0.0033, two-way ANOVA; Table 1a), (P<0.05, Tukey–Kramer test; Fig. 1a). There were no significant differences in the proportions of T. urticae adults that migrated to the neighbouring leaf disc (lima bean or tomato) between the introduction of one and no P. persimilis on the original leaf disc (P>0.05, Tukey–Kramer test; Fig. 1a). The interaction between the density of P. persimilis and the plant species of the neighbouring leaf disc was significant in the migration from downwind to upwind (P=0.0153, two-way ANOVA; Table 1a), and marginally significant in the migration from upwind to downwind (P=0.0521, two-way ANOVA, Table 1b).
The proportions of T. urticae female adults that migrated from a colony patch to a lima bean leaf disc and a tomato leaf disc (mean±SE). a Migration from downwind to upwind. b Migration from upwind to downwind. The letters above each bar indicate significant differences among treatments by Tukey–Kramer test (P<0.05)
When T. urticae migrated to tomato leaf discs that were upwind of them in the wind tunnel, there was no significant difference in the proportion of migrants with the density of introduced P. persimilis (P>0.05, Tukey–Kramer test; Fig. 1a), while when T. urticae migrated to upwind lima bean leaf discs, there was significant difference (P<0.05, Tukey–Kramer test; Fig. 1a). In contrast, when the neighbouring leaf discs were positioned downwind of T. urticae in the wind tunnel, we found no significant difference in the proportions of T. urticae adults that migrated to the lima bean and tomato leaf discs (P=0.0735, two-way ANOVA; Table 1b; Fig. 1b).
To exclude the possibility that the density of the total numbers of T. urticae and P. persimilis affected the migration rates of T. urticae, we introduced 30 additional T. urticae onto the original discs to which 30 T. urticae had previously been introduced. The migration rate was very low, and did not differ significantly between the introduction of 30 and 60 T. urticae adults (migration rate for 30 T. urticae on the original leaf disc: 0.0278±0.0195, for 60 T. urticae: 0.0241±0.0134; P=0.870, t-test).
When one P. persimilis was introduced onto the original leaf disc, few T. urticae were killed by P. persimilis compared to with no P. persimilis. In contrast, the number of killed T. urticae increased when 10 P. persimilis were introduced onto the original leaf disc (P<0.05, Tukey–Kramer test; Fig. 2). The number of killed T. urticae increased with increasing density of introduced P. persimilis (P<0.0001, two-way ANOVA; Table 2; Fig. 2). We found no significant difference in the number of killed T. urticae between the two plant species of the neighbouring leaf disc (P=0.1458, two-way ANOVA; Table 2; Fig. 2), and the interaction between the density of P. persimilis and the plant species of the neighbouring leaf disc was not significant (P=0.2495, two-way ANOVA; Table 2).
Discussion
The proportion of T. urticae that migrated to the neighbouring leaf disc increased when ten P. persimilis were placed onto each original leaf disc (Fig. 1a, b). This increase could be explained simply by the increased density of arthropods (both spider mites and predatory mites) per colony patch. However, this explanation is unlikely because T. urticae did not migrate to the neighbouring leaf disc when 30 additional T. urticae were introduced onto the original leaf disc that had already been harbouring 30 T. urticae. Our data indicate that T. urticae migrated from the original to the neighbouring leaf disc as an antipredator response against 10 P. persimilis (Fig. 1a, b; Table 1a, b). The predation risk in a patch harbouring 10 P. persimilis was significantly higher than that in a patch harbouring one P. persimilis (Table 2; Fig. 2). In this study, we did not investigate the cues that triggered the migration of T. urticae in response to P. persimilis. However, it is reported that T. urticae avoids P. persimilis-exposed leaf discs, as well as pierced eggs (Grostal and Dicke 1999). It may be that T. urticae decides when to leave a predator-invaded patch using such cues.
The migration of T. urticae to a tomato leaf disc was significantly lower than to a lima bean leaf disc when the neighbouring disc was positioned upwind of the original disc in the wind tunnel (Fig. 1a; Table 1a). However, when the neighbouring leaf disc was positioned downwind of the original disc in the wind tunnel, there was no significant difference in the proportions of T. urticae that migrated to the two plant species of the neighbouring leaf discs (Fig. 1b). The predation risk on the original leaf disk did not differ with the species of the neighbouring leaf disc (Table 2), and yet more T. urticae were attracted to lima bean plants than to tomato plants in a Y-tube olfactometer. This attraction correlates with previous research that has shown that tomato plants are less suitable host-plants for T. urticae than lima bean plants (Aina et al. 1972; Chatzivasileiadis and Sabelis 1997; Chatzivasileiadis et al. 1999). These results suggest that T. urticae may changes its patch-leaving behaviour not only because of the potential predation risk but also in response to plant volatiles upwind. Further study is needed to clarify if changes in patch-leaving decisions lead to enhanced reproductive success.
Tetranychus urticae may migrate among their host-plants to escape from predators not only by walking, but also by wind (Kondo and Takafuji 1985; Li and Margolies 1993) and by phoresy (Athias-Binche 1993; Holte et al. 2001; Yano 2004). When they migrate by wind and phoresy, they have little to no control over their landing site and, under these circumstances, it is unlikely that plant volatiles in the patch play any role.
Although there are many plant species that can host T. urticae, their suitability as a food source varies. In this study, we showed that T. urticae changes its patch-leaving behaviour in response to plant volatiles around their feeding site, even with increased potential predation risk. It is risky for herbivores to leave their food source since they might not find a suitable alternative host plant nearby and this behaviour therefore entails a potential cost. Under such conditions, they need to decide whether to leave the host-plants by estimating both the predation risk in the patch and the availability of finding suitable host-plants nearby. Plant volatiles provide information that organisms can use from a distance in order to make critical antipredator responses. In conclusion, we showed that plant volatiles play an important role in the decision-making of antipredator responses by herbivores.
References
Abrahams M, Dill LM (1989) A determination of the energetic equivalence of the risk of predation. Ecology 70:999–1007
Aina OJ, Rodriguez JG, Knavel DE (1972) Characterizing resistance to Tetranychus urticae in tomato. J Econ Entomol 65:641–643
Athias-Binche F (1993) Dispersal in varying environments: the case of phoretic uropodid mites. Can J Zool 71:1793–1798
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman and Hall, London
Blackwood JS, Schausberger P, Croft BA (2001) Prey-stage preference in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environ Entomol 30:1103–1111
Bolland H, Gutierrez J, Flechtmann C (1998) World catalogue of the spider mite family. Brill, Leiden
Chatzivasileiadis EA, Sabelis MW (1997) Toxicity of methyl ketones from tomato trichomes to Teranychus urticae Koch. Exp Appl Acarol 21:473–484
Chatzivasileiadis EA, Boon JJ, Sabelis MW (1999) Accumulation and turnover of 2-tridecanone in Teranychus urticae and its consequences for resistance of wild and cultivated tomatoes. Exp Appl Acarol 23:1011–1021
Dicke M (1986) Volatile spider-mite pheromone and host-plant kairomone, involved in spaced-out gregariousness in the spider mite Tetranychus urticae. Physiol Entomol 11:251–262
Dicke M (2000) Chemical ecology of host-plant selection by herbivorous arthropods: a multiple perspectives. Biochem Syst Ecol 28:601–617
Grand TC, Dill LM (1997) The energetic equivalence of cover to juvenile coho salmon (Oncorhynchus kisutch): ideal free distribution theory applied. Behav Ecol 8:437–447
Grostal P, Dicke M (1999) Direct and indirect cues of predation risk influence behavior and reproduction of prey: a case for acarine interactions. Behav Ecol 10:422–427
Holte AE, Houck MA, Collie NC (2001) Potential role of parasitism in the evolution of mutualism in astigmatid mites: Hemisarcoptes cooremani as a model. Exp Appl Acarol 25:97–107
Horiuchi J, Arimura G, Ozawa R, Shimoda T, Dicke M, Takabayashi J, Nishioka T (2003) A comparison of the response of Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae) to volatiles emitted from lima bean leaves with different levels of damage made by T. urticae or Spodoptera exigua (Lepidoptera: Noctuidae). Appl Entomol Zool 38:365–368
Kishida O, Nishimura K (2004) Bulgy tadpoles: inducible defense morph. Oecologia 140:414–421
Kondo A, Takafuji A (1985) Resource utilization pattern of two species of tetranychid mites (Avarina: Tetranychidae). Res Popul Ecol 27:145–157
Li J, Margolies DC (1993) Quantitative genetics of aerial dispersal behaviour and life-history traits in Tetranychus urticae. Heredity 70: 544–552
Lima SL (1998) Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Study Behav 27:215–290
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Losey JE, Denno RF (1998a) The escape response of pea aphid to foliar-foraging predators: factors affecting dropping behaviour. Ecol Entomol 23:53–61
Losey JE, Denno RF (1998b) Interspecific variation in the escape responses of aphids: effect on risk of predation from foliar-foraging and ground-foraging predators. Oecologia 115:245–252
Magalhães S, Janssen A, Hanna R, Sabelis MW (2002) Flexible antipredator behaviour in herbivorous mites through vertical migration in a plant. Oecologia 132:143–149
Matsumoto T, Itioka T, Nishida T (2002) Fitness cost of parasitoid avoidance behavior in the arrowhead scale, Unaspis yanonensis Kuwana. Entomol Exp Appl 105:83–88
Pallini A, Janssen A, Sabelis MW (1999) Spider mites avoid plants with predators. Exp Appl Acarol 23:803–815
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Takabayashi J, Dicke M (1992) Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol Exp Appl 64:187–193
Tollrian R (1995) Predator-induced morphological defenses: costs, life history shifts, and maternal effects in Daphnia pulex. Ecology 76:1691–1705
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
Villagra CA, Ramírez CC, Niemeyer HM (2002) Antipredator responses of aphids to parasitoids change as a function of aphid physiological state. Anim Behav 64:677–683
Visser JH (1986) Host odor perception in phytophagous insects. Annu Rev Entomol 31:121–144
Yano S (2004) Does Tetranychus urticae (Acari: Tetranichidae) use flying insects as vectors for phoretic dispesal? Exp Appl Acarol 32:243–248
Acknowledgment
This study was supported by Core Research for Evolutional Science and Technology of the Japan Science and Technology Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stefan Scheu.
Rights and permissions
About this article
Cite this article
Choh, Y., Takabayashi, J. Predator avoidance in phytophagous mites: response to present danger depends on alternative host quality. Oecologia 151, 262–267 (2007). https://doi.org/10.1007/s00442-006-0590-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0590-1