Abstract
Many endotherms save energy during food and water shortage or unpredictable environment using controlled reductions in body temperature and metabolism called torpor. In this study, we measured energy metabolism and water turnover in free-ranging grey mouse lemurs Microcebus murinus (approximately 60 g) using doubly labelled water during the austral winter in the rain forest of southeastern Madagascar. We then compared patterns of thermal biology between grey mouse lemurs from the rain forest and a population from the dry forest. M. murinus from the rain forest, without a distinct dry season, entered daily torpor independent of ambient temperature (T a). There were no differences in torpor occurrence, duration and depth between M. murinus from the rain and dry forest. Mouse lemurs using daily torpor reduced their energy expenditure by 11% in the rain forest and by 10.5% in the dry forest, respectively. There was no significant difference in the mean water flux rates of mouse lemurs remaining normothermic between populations of both sites. In contrast, mean water flux rate of individuals from the dry forest that used torpor was significantly lower than those from the rain forest. This study represents the first account of energy expenditure, water flux and skin temperature (T sk) in free-ranging M. murinus from the rain forest. Our comparative findings suggest that water turnover and therefore water requirement during the austral winter months plays a more restricting role on grey mouse lemurs from the dry forest than on those from the rain forest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Animals from seasonal environments exhibit a variety of physiological and behavioral characteristics enabling them to cope with the temporal fluctuations in climate, food and water availability. Endotherms are known to adjust their metabolism and thermal biology to unpredictable environments and seasonality through adjustments of their basal metabolic rates and thermal conductances (McNab 1969, 1979; Downs and Perrin 1995; Lovegrove 2000a; Carey et al. 2003). The most extreme adaptations to cope with seasonal energy shortages and low temperatures are daily torpor and hibernation. Daily and prolonged torpor is characterised by a controlled reduction of body temperature (T b) and metabolic rate that reduces both energy expenditure and water flux rate (Wang 1989). Daily torpor is usually maintained during the daily rest phase and lasts only for several hours, whereas hibernation is characterised by a sequence of prolonged torpor bouts with a mean bout duration of 2 weeks (see Hudson 1973; Wang 1989; Heldmaier and Ruf 1992; Geiser and Ruf 1995 for reviews).
Although widespread among mammals and birds, torpor is rare among primates and has been reported only for the Family Cheirogaleidae (Microcebus, mouse lemurs and Cheirogaleus, dwarf lemurs) restricted to the island of Madagascar (reviewed by Dausmann 2008). The grey mouse lemur, Microcebus murinus, is a small (approximately 60 g), nocturnal primate that feed on fruits, small animals, gum and insect secretions (Martin 1973; Corbin and Schmid 1995). The distribution of M. murinus ranges from the dry deciduous forests in western and northwestern Madagascar to populations found in evergreen littoral rain forests and spiny forests in the south (Mittermeier et al. 2006). Hence, the environments they inhabit cover a wide range of resource productivity/seasonality, T a and rainfall. In the dry forest, they sleep alone or in groups in tree holes, and the size of sleeping groups varies seasonally (Martin 1973; Radespiel et al. 1998; Schmid 1998; Radespiel et al. 2003). In contrast, mouse lemurs in the littoral forest prefer sleeping alone in open vegetation (Lahann 2008). In both dry and rain forest, body mass of M. murinus varies seasonally, but populations from the rain forest were on average heavier than those from the dry forest (Lahann et al. 2006). The most striking difference between populations that occur in the rain and dry forests involves reproduction. In the rain forest, the females reproduce at least twice annually (Lahann et al. 2006), whilst in the dry forest, they have only a single litter each year (Fietz 1999; Eberle and Kappeler 2004). Environmental conditions such as resource availability and/or climate therefore seem to be reflected in population-specific differences in behavioural and life history traits.
Previous studies of the energetics of free-ranging mouse lemurs have been limited to M. murinus and Microcebus berthae from the Kirindy/CFPF forest, a dry deciduous forest in western Madagascar, for which there is now very detailed information (Schmid 1996, 2000, 2001; Ortmann et al. 1997; Schmid et al. 2000; Schmid and Speakman 2000). However, nothing is currently published concerning the energetics of free-living M. murinus from the evergreen rain forest. M. murinus in the dry forests may face a lack of free surface water for weeks or even months at a time. Hence, strategies that save energy and water could be important for survival in these seasonally unproductive habitats. In contrast, populations of M. murinus that occur in the littoral rain forests where there is no distinct dry season may show different physiological and behavioural adaptations. We previously reported that the use of torpor in animals in the dry forest appeared to have a negligible effect on energy conservation but a large impact on water use (Schmid and Speakman 2000). We suggested that the primary function of torpor use in these small primates might therefore be to conserve water rather than energy. If this is the case, we would predict less use of torpor among lemurs inhabiting the more mesic rain forest habitat.
In this study, we describe daily energy expenditure (DEE), water flux rate and skin temperature (T sk) of free-living M. murinus in the evergreen littoral rain forest in southern Madagascar during the austral winter. We then compared our results with previously published data on energy budgets of M. murinus in the dry deciduous forest of Kirindy/CFPF.
Materials and methods
Study site
Grey mouse lemurs (M. murinus) were studied at two forests remnants of Mandena, some 12 km northeast of Tolagnaro (Fort Dauphin) at an altitude of 0–20 m (24°56.9′ S, 46°59.7′ E; for a detailed description of the study site, see Ramanamanjato and Ganzhorn 2001; Schad et al. 2005). Mandena is a 10- to 15-m-high evergreen littoral rain forest with a thick understorey. The average yearly rainfall in the Fort Dauphin region is approximately 1,600 mm/year and there is no distinct dry season. The average T a per month ranges from 20°C to 27°C with an annual mean of 23°C (Ramanamanjato and Ganzhorn 2001). The coolest months per year are June, July and August. The first study site (M16) was a slightly degraded primary forest fragment of about 75 ha which lies in direct neighborhood of M15, a larger fragment of approximately 133 ha with similar degree of degradation. Marshland and plantations of Eucalyptus and Pinus surround both fragments. The second study site (M5) was also a slightly degraded secondary forest fragment but of only 28 ha. It has been isolated at least since 1957 and is bordered by swamps without forest vegetation. The two sites were approximately 4 km apart and populations were unlikely to move between the two forest remnants (for further descriptions of the study area, see Ganzhorn et al. 2007). Our study was conducted in July 2001 (winter).
For comparisons between sites, we used already published data on energy expenditure of a population of M. murinus from Kirindy, a dry deciduous forest in western Madagascar (data from Schmid and Speakman 2000, Table 2). The primary forest plot CS7 is located in the forestry concession (CFPF; general information is given by Ganzhorn and Rohner 1996). Kirindy/CFPF is characterised by a pronounced seasonality with a hot rainy season between December and March and a cool dry season of 7–8 months with virtually no precipitation. The average monthly T a ranges from 21°C to 27°C with an annual mean of 24.7°C. T a during the dry season shows extreme diurnal variation with cold nights (4°C) and hot days (32°C; Sorg and Rohner 1996).
Trapping and telemetry
Mouse lemurs were caught using Sherman live traps (7.7 × 7.7 × 30.5 cm) baited with banana and set approximately every 25 m 1–2 m above the ground. Captured individuals were taken to the research station where they were individually marked by subdermal injection of a transponder (Trova, Weilerswist, Germany), sexed, weighed using a 100-g spring balance and examined for reproductive state. For skin temperature (T sk) measurements, we used pre-calibrated radio collars (SS-2 button cell tags, accuracy ±1°C) with attached temperature sensitive sensors (Biotrack, Wareham, Dorset, UK) to monitor the physiological state of the mouse lemurs (for details, see Schmid and Speakman 2000). The mean weight of the transmitters was 2.5 g and is below 5% of the animal’s body mass. The external radio collars were placed in direct contact with the animal’s skin of the neck. These T sk data from collar transmitters precisely reflect core body temperature of resting individuals over a wide range of ambient temperature (Dausmann et al. 2005). After release at their point of capture, we located mouse lemurs using a manual TR-4-receiver (Telonics, Mesa, AZ, USA) with a flexible two-element Yagi antenna and then recorded pulse intervals approximately every 20 min. We determined T sk by averaging three timings of the interval required for ten transmitter pulses and then using the transmitter-specific calibration curves. Because of the thick vegetation and the lack of trail systems in the experimental areas, it was impossible to follow the radio-collared mouse lemurs during the night. To monitor physiological state as accurately as possible, we took T sk recordings 1 h after the animals had been released in the forest. In addition, we took mobile daytime readings of T sk when the mouse lemurs were located early in the morning in their sleeping sites until they restarted their activity at dusk (approximately 0700–1900 hours). We also recorded T sk of recaptured individuals when found in the traps at their site of capture before taking them back to the camp for the final blood sample. Although T sk recordings were fragmentary, this method allowed us to distinguish between mouse lemurs that entered torpor and those that remained completely normothermic. During the period of each experiment, we measured ambient temperature at the site of capture, 1–2 m above the ground in the forest (T a) to the nearest 0.5°C using data loggers (Thermochron iButtons®, Model DS1921). Monthly precipitation from the rain forest in July 2001 during which our experiments were carried out was 107.0 mm (Vincelette et al. 2007).
Daily energy expenditure
We measured the DEE (kJ/day) using the doubly labelled water (DLW) technique (Lifson and McClintock 1966; Speakman 1998). This method has been previously validated by comparison to indirect calorimetry (e.g. Speakman and Krol 2005) and provides an accurate measure of daily energy expenditure over periods of several days (Speakman et al. 1994; Berteaux et al. 1996). We only selected males and females that were not reproductively active. Mouse lemurs selected for measurement were injected intraperitoneally with 0.1 ml isotopically characterised doubly labelled water containing H2 18O (18O 618,834 ppm) and D2O (2H 172,491 ppm; O/H ratio, 3.59). Mouse lemurs were held for 1 h in Sherman traps to allow uniform equilibration of the isotopes in the body water (Krol and Speakman 1999). Animals were anaesthetised for 10–20 min with 0.03 ml Ketavet (100 mg ml−1) and an initial blood sample of 50–100 μl was taken by vena puncture of the ear veins. Samples were flame-sealed in calibrated capillaries (Vitrex, Camlab, Cambridge, UK). Body mass was measured and the animals were fitted with radio collars. We set ten to 15 traps within 25 m to the sleeping site to recapture animals for the second blood sample. If animals were not recaptured within the second night after injection, we continued mobile daytime recordings until the animals were caught (up to four successive days). Recaptures were timed as close as feasible to whole 24-h periods (Speakman and Racey 1988) to estimate isotope elimination rates. Upon recapture, mouse lemurs were weighed, a second blood sample was taken and the radio collar was removed. Mouse lemurs were released at their site of capture on the same day at dusk. To determine background isotope levels, we took blood samples from 15 mouse lemurs that were not labelled (method C: Speakman and Racey 1988). Capillaries that contained the blood samples were vacuum-distilled (Nagy 1983), and water from the resulting distillate was used to produce CO2 and H2 (methods in Speakman et al. 1990 for CO2 and Ward et al. 2000 for H2). The isotope ratios 18O/16O and 2H/1H were analysed using gas source isotope ratio mass spectrometry (Optima, Micromass IRMS and Isochrom μG, Manchester, UK). Isotope enrichments were converted to values of CO2 production using a single pool model as recommended for this size of animal by Speakman (1993). There are several alternative approaches for the treatment of evaporative water loss in the calculation (Visser and Schekkerman 1999). We chose the assumption of a fixed evaporation of 25% of the water flux (Eq. 7.17 in Speakman 1997), which has been established to minimise error in a range of conditions (Visser and Schekkerman 1999; Van Trigt et al. 2002). We determined the error in individual estimates using the iterative procedures outlined in Speakman (1995). Conversion to energy expenditure was made by means of an assumed RQ of 0.8. We used the Natureware DLW software for calculations (Speakman and Lemen 1999).
A total of 12 mouse lemurs from fragment M16 and eight animals from fragment M5 were injected with D2 18O and successfully recaptured for blood sampling. Body masses obtained at initial and final capture were averaged for each individual. We calculated a mean T sk for each mouse lemur during the entire period of the DLW measurement. The corresponding T a readings were also averaged. For mouse lemurs that were found torpid at their sleeping sites and in the traps, respectively, we determined the onset of torpor using the calculated cooling rate for M. murinus of 3.4°C h−1 (Schmid 2000; Schmid and Speakman 2000). Mouse lemurs were considered to be torpid when the T sk was equal to or lower than 30°C (Geiser and Baudinette 1988). The total length of each torpor episode was defined as the time between the torpor onset when T sk fell below 30°C and the end of the torpor bout when T sk reached normothermic levels again. Descriptive statistics and statisticial tests were performed using SPSS 13.0. If not stated otherwise, numeric values are given as mean ± SD for the number of individuals ‘N’. The Kolmogorov–Smirnov (K–S) goodness of fit test was used to test the distribution of the data for normality.
Results
Body mass and thermoregulatory status
Mean body mass of M. murinus measured in M16 (53.6 ± 8.1 g, N = 12) did not differ from the mean mass of animals from M5 (58.3 ± 10.1 g, N = 8; t 18 = −0.65, P = 0.52). Furthermore, when both sites were combined, there was no significant difference in the mean body mass of females (54.3 ± 9.8 g, N = 10) and males (59.1 ± 7.4 g, N = 10; t 18 = −1.227, P = 0.24). For the entire set of 20 animals examined in the dry season, seven animals entered torpor and 13 individuals remained normothermic over the period of the doubly labelled water experiments. Body mass of animals that entered torpor averaged 57.6 (±7.4 g, N = 7), which was not significantly different from the mean value of normothermic individuals (56.2 ± 9.8 g, N = 13; t 18 = −0.334, P = 0.74).
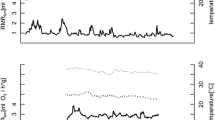
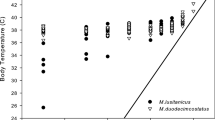
Figure 1 shows typical patterns of change in T sk over an entire doubly labelled water experiment for an individual that remained normothermic and one that employed daily torpor. The mean torpor bout length was 3.82 h (±2.5 h, N = 7). The shortest individual daily torpor bout recorded was 1.06 h (animal M-1990) and the longest was 8.5 h (animal M-B407), respectively (Table 1). The mean T sk across the entire DLW measurement period of mouse lemurs employing torpor was significantly lower than the mean T sk of animals remaining normothermic (T sk torpor, 32.1 ± 2.0°C, N = 7; T sk normothermic, 34.7 ± 0.9°C, N = 13; t 18 = 4.08, P = 0.001). The lowest individual T sk during a torpor bout was 19.0°C, which we measured for M-B407 at a T a of 16.6°C.
Body temperature (filled circles) and ambient temperature (unfilled circles) of two M. murinus during a normothermia (animal F-C40E) and b daily torpor (animal M-201F) recorded in the rain forest. The arrow marks the point when the lemur was trapped for the final blood sample. The black bars indicate nighttime
Daily energy expenditure in free-living mouse lemurs
The period of time between the first and second blood sample averaged 2.0 ± 0.9 days (range 1.0–3.8 days), summarised for all measurements carried out in the rain forest (Table 1). Energy expenditure was not significantly correlated with mean body mass during the experiment (Spearman rank correlation, R s = 0.16, N = 20, P = 0.49), and consequently, comparisons were made on whole animal rates of energy expenditure.
Thermoregulatory status (using torpor vs remaining normothermic; P = 0.323) was not significantly correlated with DEE for M. murinus measured in the rain forest. For the subsample of the seven mouse lemurs that used daily torpor during parts of the field measurement, the mean DEE was 62.85 ± 17.52 kJ day−1 at a mean T a of 16.8°C (±1.6°C), ranging from 47.9 to 98.45 kJ day−1 (Table 1). The ratio between DEE and estimated resting metabolic rate (RMR) ranged from 1.47 to 2.84 and was, on average, 1.86 ± 0.49 (RMR measured at T a of 25°C; data from Perret et al. 1998; Table 1). We found that DEE of mouse lemurs using daily torpor (N = 7) was not significantly correlated with torpor bout length (linear regression: R 2 = 0.38, P = 0.14; Fig. 2a). In addition, the mean temperature difference between T sk and T a (ΔT) had no significant effect on DEE of mouse lemurs using torpor (R 2 = 0.01, P = 0.87; Fig. 2b). The mean DEE of the animals that remained completely normothermic (N = 13) was 70.61 kJ day−1 (±15.66 kJ day−1) at a mean T a of 18.1°C (±1.2°C). The average DEE of mouse lemurs using torpor was 62.85 ± 17.52 kJ day−1 at a mean T a of 16.8°C (±1.6°C). The mean ratio between DEE and estimated RMR was 2.13 ± 0.44 and ranged from 1.2 to 2.81. Comparisons between lemurs using torpor and animals, which remained normothermic, revealed neither a significant difference in the mean DEE (t 18 = 1.02, P = 0.32) nor in the mean estimated RMR (t 18 = 1.23, P = 0.23). DEE did not change significantly in relation to ΔT (R 2 = 0.24, P = 0.09). We found that mean T a did not differ significantly between experimental periods of the animals that used torpor and the individuals that remained normothermic (t 18 = 1.97, P = 0.06).
Water flux rate
Water flux rates were highly variable between individuals and ranged from 11.8 to 36.14 ml day−1 (Table 1). Mean water flux rates did not differ between animals using daily torpor (18.71 ± 6.37 ml day−1, N = 7) and lemurs remaining normothermic (21.9 ± 6.33 ml day−1, N = 13; t 18 = 1.07, P = 0.30). There was no significant correlation between water flux rate and torpor bout length (linear regression: R 2 = 0.37, P = 0.15). Furthermore, water flux rate of animals was neither correlated with T a (R 2 = 0.17, P = 0.07) nor with the mean temperature difference between T sk and T a (ΔT; R 2 = 0.01, P = 0.69).
Inter-site comparison with M. murinus from the dry forest
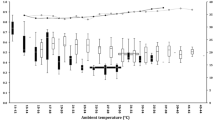
The mean T a during the doubly labelled water experiments for the rain forest (17.6 ± 1.4°C, N = 20) was significantly lower than the mean T a for the dry forest (19.6 ± 2.7°C, N = 23; t 41 = −3.02, P = 0.004). In contrast to the monthly precipitation of 107.0 mm from the rain forest (Vincelette et al. 2007), there was no rainfall at all during the month of experiments in the dry forest (J. Schmid, unpublished data). In the dry forest, 12 out of 23 mouse lemurs measured entered torpor during the doubly labelled water experiments, whereas in the rain forest, torpor episodes were observed in seven of 20 studied individuals. There was no significant difference in the propensity to enter daily torpor among examined mouse lemurs between the two sites (chi-square test: χ 2 1 = 0.29, P = 0.59). Mean torpor bout length did not differ between sites (rain forest, 3.83 ± 2.51 h; dry forest, 3.09 ± 1.9 h; t 17 = 0.73, P = 0.48). Mean T sks during torpor were similar in the rain forest (32.1 ± 2.0°C) and in the dry forest (33.7 ± 2.3°C; t 17 = −1.55, P = 0.14). Two-way analyses of variance revealed that DEE of mouse lemurs was significantly influenced by site (F 1,39 = 30.11, P < 0.001), but not by the thermoregulatory status (torpid or normothermic; F 1,39 = 1.63, P = 0.21). In contrast, water flux rate was affected by both site (F 1,39 = 6.34, P = 0.02) and the occurrence of torpor (F 1,39 = 8.62, P = 0.006). The mean DEE of M. murinus from the rain forest was significantly lower than at the dry forest, both for animals remaining normothermic (t 22 = −5.04, P < 0.001) and using torpor (t 17 = −3.02, P = 0.008; Fig. 3). Compared to the mean DEE of mouse lemurs remaining normothermic, the average DEE of animals using torpor was reduced by 11% for the rain forest and by 10.5% for the dry forest, respectively (for the mean values, see Fig. 3). There was no significant difference in the mean water flux rates between M. murinus remaining normothermic at both sites (t 22 = −1.14, P = 0.27; Fig. 4). In contrast, in lemurs using daily torpor, the mean water flux rate of individuals from the dry forest was significantly lower than in mouse lemurs using torpor from the rain forest (t 17 = −2.41, P = 0.03; Fig. 4). The average reduction of water flux rate of animals due to the use of torpor was 14.5% for the rain forest and 33.1% for the dry forest, respectively.
Daily energy expenditure of M. murinus determined in the rain forest (squares) in comparison to M. murinus measured in the dry forest (circles). Specifically, mouse lemurs that maintained normothermia (unfilled symbols) were compared with those using torpor (filled symbols). Values represent mean ± SD; Student’s t test was used for statistical analyses
Water flux rate of M. murinus determined in the rain forest (squares) in comparison to M. murinus measured in the dry forest (circles). Specifically, mouse lemurs that maintained normothermia (unfilled symbols) were compared with those using torpor (filled symbols). Values represent mean ± SD; Student’s t test was used for statistical analyses; ns not significant
Discussion
Our field study provides the first quantitative analysis of thermal physiology and patterns of daily torpor in one species of Microcebus across different habitats: comparing an evergreen rain forest and a dry deciduous forest population. We found that free-ranging M. murinus exposed habitats characterised by different climates may utilise torpor differently in terms of the physiological consequences (i.e. energy expenditure and water turnover rate, respectively).
M. murinus from the western dry forest use torpor only during the dry season and some individuals even appear to hibernate for some portion of the dry season (Ortmann et al. 1996; Schmid 2000, 2001; Schmid et al. 2000; Schmid and Speakman 2000; Schmid and Ganzhorn 2009). We found that M. murinus in the rain forest also utilised torpor during the austral winter months despite there being no distinct dry season. Lahann et al. (2006) demonstrated that body mass of M. murinus from the rain forests of Mandena was lowest in the austral winter months (May to August) compared to the other months, presumably a consequence of low food abundance during this part of the year. Food scarcity is known to influence the use of torpor and many heterotherms predominantly employ torpor when lean (Heldmaier 1989; Geiser and Ruf 1995; Genin and Perret 2003; Westman and Geiser 2004). Several other studies on primates have also shown that variation in food abundance can elicit numerous behavioural and physiological responses (Hemingway and Bynum 2005 for reviews). In our study, however, there was no evidence that lean mouse lemurs use torpor more often than fatter individuals.
Frequency of torpor did not differ between the two sites. It has been demonstrated that southwest Madagascar (i.e. the area where Kirindy is located) is characterised by extremely high unpredictability of annual rainfall totals and may experience major droughts more frequently compared to other regions in the tropics with similar average rainfall (Dewar and Wallis 1999). Highly unpredictable environments are suggested to be associated with a high incidence of daily torpor (Lovegrove 2000a, b). We also observed previously that use of torpor conferred a much greater effect on water balance than on energy balance. We suggested therefore that torpor in this species might primarily serve to conserve water resources rather than energy (Schmid and Speakman 2000). On this basis and given the more unpredictable habitat at the dry forest, we predicted therefore that the animals in the dry forest would more often use daily torpor than those from the evergreen forest where there is rainfall all over the year and no distinct dry season. However, this was not the case.
RMR is the energy expenditure of an endotherm during its rest phase and correlates with the energy expenditure of free-living animals (Nagy 1987; Ricklefs et al. 1996). The rain forest DEE of normothermic grey mouse lemurs investigated during the austral winter months (equals dry season) was 2.1 times the predicted RMR, which is clearly lower than the mean value of 3.4 (field metabolic rate, FMR/RMR) calculated by Speakman (Speakman 2000) based on 60 small mammalian species weighing less than 4 kg. In comparison, in M. murinus from the dry forest, the wet season FMR was 2.4 the RMR, whereas the dry season FMR (equals austral winter) was 3.2 times the RMR (Schmid and Speakman 2000). This variation in the seasonal FMR/RMR ratio in mouse lemurs has also been observed in many other species from a wide diversity of habitats and climatic zones (for example, common wombats (Vombatus ursinus), Evans et al. 2003; red squirrels (Tamasciurus hudsonicus), Humphries et al. 2005; golden moles (Ambysomus hottentottus longiceps), Scantlebury et al. 2005; field voles (Microtus agrestis), McDevitt and Speakman 1994; dusky antechinus (Antechinus swainsonii), Green and Crowley 1989 and antelope ground squirrels (Ammospermophilus leucurus), Karasov 1981) and suggests that using simple multiples of RMR as an index of FMR should be done with extreme caution because seasonal effects can be profound.
Levels of energy expenditure should be linked to duration of torpor and body temperature during torpor (Bartness and Wade 1984; Geiser and Kenagy 1988; Körtner und Geiser 2009). Nonetheless, DEE of mouse lemurs from the rain forest was neither significantly correlated with torpor bout length nor with the differential between T sk and T a during torpor. This is surprising because in M. murinus, the metabolic rate during torpor is reduced by almost 80% (Schmid 2000). A possible explanation for this disagreement could be that the mean T sks of torpid mouse lemurs of 32.1°C (mean value of torpid animals, N = 7) was not sufficient to account for a significant decrease of the daily energy budget. This would also explain why we did not find a significant difference between the mean DEE of torpid mouse lemurs and that of animals remaining completely normothermic. We assume that the duration of torpor was insufficient to reduce the overall expenditure. Savings are therefore minor, and since DEE includes the sum of metabolic rates during an entire day, they may have used up the energy savings of torpor for other activities or processes during the period when they were active, which finally led to total energy expenditures that were not reduced.
The torpor pattern was similar for both populations of grey mouse lemurs despite climatic differences between their habitats. Our results indicate that DEE values of torpid animals were slightly lower than values obtained for mouse lemurs remaining normothermic (11% reduction). This corresponds to the findings from the dry forest where the mean DEE of M. murinus using daily torpor was reduced by 10.5% compared to animals remaining normothermic (Schmid and Speakman 2000, see Fig. 4). Nevertheless, DEE of torpid and normothermic mouse lemurs was affected by site. This pattern is consistent with the findings of Scantlebury et al. (2003) who found that common spiny mice (Acomys cahirinus) from different habitats differed in their energy expenditure. In addition, Corp et al. (1997) documented variations in time energy budgets of wood mice (Apodemus sylvaticus) from two contrasting habitats.
In the present study, the difference in energy expenditure between the two forest types with higher values found in the dry forest is rather surprising and at least three explanations for this difference are possible. First, it has been argued that T a is an important factor influencing the energy budget with increasing values associated with decreasing temperatures (Geiser and Ruf 1995; Lovegrove et al. 2001; Warnecke et al. 2008). The data at hand, however, do not support this argument. On the contrary, the mean T a during the doubly labelled water study was significantly lower in the rain forest than that of the dry forest, and we would expect that mouse lemurs from the rain forest have higher energy expenditures to remain normothermic. It therefore appears that little of the daily energy costs of mouse lemurs in the present study are for thermoregulation, which was also recorded in some bat species (Geiser et al. 1996; Geiser and Coburn 1999). Second, the fact that the average DEE of M. murinus from the dry forest versus the rain forest was significantly higher may be based upon lower RMRs of the rain forest population. However, this remains an assumption because no data on RMR of M. murinus from the rain forest are available. Finally, based on Warren and Crompton (1998) observation that the cost of locomotion is an important factor influencing the energy budget of animals, nightly traveled distances and home ranges of mouse lemurs might be different at both sites. Recent comparative investigations in the rain and dry forest have shown that despite inter-sexual differences due to reproduction and mating system, mean home range of M. murinus was similar in the rain and dry forest (Schmid and Speakman 2000; Lahann 2008). Because we did not measure nightly traveled distances in our study, the argument that locomotion costs are responsible for the higher field metabolic rate at the dry forest remains speculative.
A benefit of torpor, in addition to energy saving, is conservation of water (Nagy et al. 1991; Schmid 2001; Peinke and Brown 2003). Our study in the rain forest revealed that water turnover rates of torpid mouse lemurs were only slightly reduced compared to normothermic animals. However, water flux rates of mouse lemurs were affected by site and the occurrence of torpor. Comparison between sites revealed that there was no difference in the water balance of M. murinus remaining normothermic. In contrast, mean water flux rates of torpid individuals differed substantially between rain and dry forest populations, which was lower in the dry than in the rain forest. In other words, although individuals of both populations used torpor with similar patterns, the physiological consequences differed markedly between sites. Because there is virtually no precipitation during the dry season in the dry forest (Sorg and Rohner 1996), the savings in their water budgets may be a significant factor influencing the utilisation of torpor. It has been documented for several other small mammals that torpor is used in response to water restriction or deprivation (Thomas and Cloutier 1992; Song et al. 1997). M. murinus exposed to dry deciduous environments may have adapted differently compared to populations of the rain forest and used daily torpor with emphasis on water savings instead of energy savings.
In a previous study, we suggested that use of torpor in the dry forest could be an adaptive strategy to deal with unpredictable and harsh environmental conditions, particularly by reducing water requirements. We predicted therefore that in the rain forest, M. murinus would not use torpor. However, contrasting this prediction, we found no differences in torpor occurrence, duration and depth between the two sites. Nevertheless, there were differences in the physiological consequences of daily torpor between the two populations of M. murinus from the rain and dry forests. The function and physiology of daily torpor may differ in different populations of the same species. This indicates that a full understanding of the adaptive consequences of torpor needs to include not only documentation of the use of torpor but also its physiological impact.
Abbreviations
- DEE:
-
daily energy expenditure
- ΔT :
-
mean temperature difference
- RMR:
-
resting metabolic rate
- T a :
-
ambient temperature
- T sk :
-
skin temperature
References
Bartness TJ, Wade GN (1984) Photoperiodic control of body weight and energy metabolism in Syrian Hamsters (Mesocricetus auratus): role of pineal gland, melatonin, gonads and diet. Endocrinology 114:492–498
Berteaux D, Thomas DW, Bergeron J-M, Lapierre H (1996) Repeatability of daily field metabolic rate in female Meadow Voles (Microtus pennsylvanicus). Funct Ecol 10:751–759
Carey HV, Andrews MT, Martin SL (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83:1153–1181
Corbin GD, Schmid J (1995) Insect secretions determine habitat use patterns by a female lesser mouse lemur (Microcebus murinus). Am J Primatol 37:317–324
Corp N, Gorman ML, Speakman JR (1997) Ranging behaviour and time budgets of male wood mice Apodemus sylvaticus in different habitats and seasons. Oecologia 109:242–250
Dausmann KH (2008) Hypometabolism in primates: torpor and hibernation. In: Lovegrove BG, McKechnie AE (eds) Hypometabolism in animals: hibernation, torpor and cryobiology. Interpak Books, Pietermaritzburg, pp 327–336
Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G (2005) Hibernation in the tropics: lessons from a primate. J Comp Physiol B 175:147–155
Dewar RE, Wallis JR (1999) Geographical patterning of interannual rainfall variability in the tropics and near tropics: an L-moments approach. J Climate 12:3457–3466
Downs CT, Perrin MR (1995) The thermal biology of three southern African elephant-shrews. J Therm Biol 20(6):445–450
Eberle M, Kappeler PM (2004) Sex in the dark: determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav Ecol Sociobiol 57:77–90
Evans M, Green B, Newgrain K (2003) The field energetics and water fluxes of free-living wombats (Marsupialia: Vombatidae). Oecologia 137:171–180
Fietz J (1999) Mating system of Microcebus murinus. Am J Primatol 48:127–133
Ganzhorn JU, Rohner J-P (1996) Ecology and economy of a tropical dry forest in Madagascar. German Primate Center, Göttingen
Ganzhorn JU, Andrianasolo T, Andrianjazalahatra T, Donati G, Fietz J, Lahann P, Ramarokoto REAF, Randriamanga S, Rasarimanana S, Rakotosamimanana B, Ramanamanjato J-B, Randria G, Rasolofoharivelo MT, Razanahoera-Rakototmalala M, Schmid J, Sommer S (2007) Lemurs in Evergreen Littoral Forest Fragments. In: Alonso A (ed) Biodiversity, ecology and conservation of littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort Dauphin). SI/MAB Series, Washington, DC, USA, pp 223–235
Geiser F, Baudinette RV (1988) Daily torpor and thermoregulation in the small dasyurid marsupials Planigale gilesi and Ningaui yvonneae. Aust J Zool 36:473–481
Geiser F, Coburn DK (1999) Field metabolic rates and water uptake in the blossom-bat Syconycteris australis (Megachiroptera). J Comp Physiol B 169(2):133–138
Geiser F, Kenagy GJ (1988) Torpor duration in relation to temperature and metabolism in hibernating ground squirrels. Physiol Zool 61:442–449
Geiser F, Ruf T (1995) Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool 68:935–966
Geiser F, Coburn DK, Körtner G, Law BS (1996) Thermoregulation, energy metabolism, and torpor in blossom-bats, Syconycteris australis (Megachiroptera). J Zool 239:583–590
Genin F, Perret M (2003) Daily hypothermia in captive grey mouse lemurs (Microcebus murinus): effects of photoperiod and food restriction. Comp Biochem Physiol B 136:71–81
Green K, Crowley H (1989) Energetics and behaviour of acive subnivean insectivores Antechinus swainsonii and Antechinus stuartii (Marsupialia: Dasyuridae) in the snowy mountains (New South Wales, Australia). Aust Wildl Res 16:509–516
Heldmaier G (1989) Seasonal acclimazation of energy requirements in mammals: Functional significance of body weight control, hypothermia, torpor and hibernation. In: Wieser W, Gnaiger E (eds) Energy transformations in cells and organisms. Georg Thieme, Stuttgart, pp 130–139
Heldmaier G, Ruf T (1992) The impact of daily torpor on energy requirements in the Djungarian hamster, Phodopus sungorus. Physiol Zool 65:994–1010
Hemingway CA, Bynum N (2005) The influence of seasonality on primate diet and ranging. In: Brockman DK, van Schaick CP (eds) Seasonality in primates. Studies of living and extinct human and non-human primates. Cambridge University Press, Cambridge, pp 57–104
Hudson JW (1973) Torpidity in mammals. In: Whittow GC (ed) Comparative physiology of thermoregulation. Academic, London, pp 97–165
Humphries MM, Boutin S, Thomas DW, Ryan JD, Selman C, McAdam AG, Berteaux D, Speakman JR (2005) Expenditure freeze: the metabolic response of small mammals to cold environments. Ecol Lett 8:1326–1333
Karasov WH (1981) Daily energy expenditure and cost of activity in a free-living mammal. Oecologia 51:253–259
Körtner G, Geiser F (2009) The key to winter survival: daily torpor in a small arid-zone marsupial. Naturwissenschaften. doi:10.1007/s00114-008-0492-7
Krol E, Speakman JR (1999) Isotope dilution spaces of mice injected simultaneously with deuterium, tritium and oxygen-18. J Exp Biol 202:2839–2849
Lahann P (2008) Habitat utilization of three sympatric Cheirogaleid lemur species in a littoral rain forest of southeastern Madagascar. Int J Primatol 29:117–134
Lahann P, Schmid J, Ganzhorn JU (2006) Geographic variation in life history traits of Microcebus murinus in Madagascar. Int J Primatol 27:983–999
Lifson N, McClintock R (1966) Theory of use of the turnover rates of body water for measuring energy and material balance. J Theor Biol 12:46–74
Lovegrove BG (2000a) Daily heterothermy in mammals: coping with unpredictable environments. In: Heldmaier G, Klingenspor M (eds) Life in the cold: 11th International Hibernation Symposium. Springer, Berlin, pp 29–40
Lovegrove BG (2000b) The zoogeography of mammalian basal metabolic rate. Am Nat 156(2):201–219
Lovegrove BG, Raman J, Perrin MR (2001) Daily torpor in elephant shrews (Macroscelidea: Elephantulus spp.) in response to food deprivation. J Comp Physiol B 171:11–21
Martin RD (1973) A review of the behaviour and ecology of the lesser mouse lemur (Microcebus murinus). In: Crook M (ed) Ecology and behaviour of primates. Acadmic, London, pp 1–68
McDevitt RM, Speakman JR (1994) Central limits to sustainable metabolic-rate have no role in cold-acclimation of the short-tailed field vole (Microtus-agrestis). Physiol Zool 67:1117–1139
McNab BK (1969) The economics of temperature regulation in neotropical bats. Comp Biochem Physiol A 31:227–268
McNab BK (1979) Climatic adaptation in the energetics of heteromyid rodents. Comp Biochem Physiol A 62:813–820
Mittermeier RA, Konstant WR, Hawkins AFA, Louis EE, Langrand O, Ratsimbazafy HJ, Rasoloarison RM, Ganzhorn JU, Rajaobelina S, Tattersall I, Meyers D (2006) Lemurs of Madagascar. Conservation International, Washington, DC
Nagy KA (1983) Doubly-labelled water: a guide to its use. UCLA Publications, UCLA, Los Angeles, USA
Nagy KA (1987) Field metabolic rate and food requirement scaling in mammals and birds. Ecol Monogr 57:111–128
Nagy KA, Bradshaw SD, Clay BT (1991) Field metabolic rate, water flux, and food requirements of short-nosed bandicoots, Isoodon obesulus (Marsupialia: Peramelidae). Aust J Zool 39:299–305
Ortmann S, Schmid J, Ganzhorn JU, Heldmaier G (1996) Body temperature and torpor in a Malagasy small primate, the mouse lemur. In: Geiser F, Hulbert AJ, Nicol SC (eds) Adaptations to the cold: The Tenth International Hibernation Symposium. University of New England Press, Armidale, pp 55–61
Ortmann S, Heldmaier G, Schmid J, Ganzhorn JU (1997) Spontaneous daily torpor in Malagasy mouse lemurs. Naturwissenschaften 84:28–32
Peinke DM, Brown CR (2003) Metabolism and thermoregulation in the springhare (Pedetes capensis). Comp Biochem Physiol B 173:347–353
Perret M, Aujard F, Vannier G (1998) Influence of daylength on metabolic rate and daily water loss in the male prosimian primate Microcebus murinus. Comp Biochem Physiol A 119:981–989
Radespiel U, Cepok S, Zietemann V, Zimmermann E (1998) Sex-specific usage patterns of sleeping sites in grey mouse lemurs (Microcebus murinus) in northwestern Madagascar. Am J Primatol 46:77–84
Radespiel U, Ehresmann P, Zimmermann E (2003) Species-specific usage of sleeping sites in two sympatric mouse lemur spcecies (Microcebus murinus and M. ravelobensis) in northwestern Madagascar. Am J Primatol 59:139–151
Ramanamanjato J-B, Ganzhorn JU (2001) Effects of forest fragmentation, introduced Rattus rattus and the role of exotic tree plantations and secondary vegetation for the conservation of an endemic rodent and a small lemur in littoral forests of southeastern Madagascar. Anim Conserv 4:175–183
Ricklefs RE, Konarzewski M, Daan S (1996) The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am Nat 147:1047–1971
Scantlebury M, Shanas U, Speakman JR, Kupshtein H, Afik D, Haim A (2003) Energetics and water economy of common spiny mice Acomys cahirinus from north- and south-facing slopes of a Mediterranean valley. Funct Ecol 17:178–185
Scantlebury M, Oosthuizen MK, Speakman JR, Jackson CR, Bennett NC (2005) Seasonal energetics of the Hottentot golden mole (Ambysomus hottentottus longiceps) at high altitude (1500 m). Physiol Behav 84:739–745
Schad J, Ganzhorn JU, Sommer S (2005) Parasite burden and constitution of major histocompatibility complex in the malagasy mouse lemur, Microcebus murinus. Evolution 59(2):439–450
Schmid J (1996) Oxygen consumption and torpor in mouse lemurs (Microcebus murinus and Microcebus myoxinus): preliminary results of a study in western Madagascar. In: Geiser F, Hulbert AJ, Nicol SC (eds) Adaptations to the cold: The Tenth Hibernation Symposium. University of New England Press, Armidale, pp 47–54
Schmid J (1998) Tree holes used for resting by gray mouse lemurs (Microcebus murinus) in Madagascar: insulation capacities and energetic consequences. Int J Primatol 19:797–809
Schmid J (2000) Daily torpor in the gray mouse lemur (Microcebus murinus) in Madagascar: energetical consequences and biological significance. Oecologia 123:175–183
Schmid J (2001) Daily torpor in free-ranging gray mouse lemurs (Microcebus murinus) in Madagascar. Int J Primatol 22(no. 6):1021–1031
Schmid J, Speakman JR (2000) Daily energy expenditure of the grey mouse lemur (Microcebus murinus): a small primate that uses torpor. J Comp Physiol B 170:633–641
Schmid J, Ganzhorn JU (2009) Optional strategies for reduced metabolism in gray mouse lemurs. Naturwissenschaften (in press)
Schmid J, Ruf T, Heldmaier G (2000) Metabolism and temperature regulation during daily torpor in the smallest primate, the pygmy mouse lemur (Microcebus myoxinus) in Madagascar. J Comp Physiol B 170:59–68
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment. University Press, Cambridge
Song X, Körtner G, Geiser F (1997) Thermal relations of metabolic rate reduction in a hibernating marsupial. Am Physiol Soc 42:R2097–R2104
Sorg J-P, Rohner U (1996) Climate and tree phenology of the dry deciduous forest of the Kirindy forest. In: Ganzhorn JU, Sorg J-P (eds) Ecology and economy of a tropical dry forest in Madagascar. Primate report. Erich Goltz GmbH, Germany, pp 57–80
Speakman JR (1993) How should we calculate CO2 production in doubly labelled water studies of animals? Funct Ecol 7:746–750
Speakman JR (1995) Estimation of precision in DLW studies using the two-point methodology. In: Speakman JR, Roberts SB (eds) Obesity research 3(Suppl. 1): recent advances in the doubly-labelled water technique, pp 31–41
Speakman JR (1997) Doubly labelled water. Theory and practise. Chapman and Hall, London
Speakman JR (1998) The history and theory of the doubly labeled water technique. Am J Clin Nutr 68:932S–938S
Speakman JR (2000) The cost of living: field metabolic rates of small mammals. Adv Ecol Res 30:177–297
Speakman JR, Krol E (2005) Validation of the doubly-labelled water method in a small mammal. Physiol Biochem Zool 78:650–667
Speakman JR, Lemen C (1999) Doubly labelled water analyses program. http://www.natureware.com/double.htm
Speakman JR, Racey PA (1988) Consequences of non steady-state CO2 production for accuracy of the doubly labeled water technique—the importance of recapture interval. Comp Biochem Physiol A 90:337–340
Speakman JR, Nagy KA, Masman D, Mook WG, Poppitt SD, Strathearn GE, Racey PA (1990) Interlaboratory comparison of different analytical techniques for the determination of O-18 abundance. Anal Chem 62:703–708
Speakman JR, Racey PA, Haim A, Webb PI, Ellison GTH, Skinner JD (1994) Interindividual and intraindividual variation in daily energy-expenditure of the pouched mouse (Saccostomus campestris). Funct Ecol 8:336–342
Thomas DW, Cloutier D (1992) Evaporative water loss by hibernating little brown bats, Myotis lucifugus. Physiol Zool 65:443–456
Van Trigt R, Kerstel ERT, Neubert REM, Meijer HAJ, McLean M, Visser GH (2002) Validation of the DLW method in Japanese quail at different water fluxes using laser and IRMS. J Appl Physiol 93:2147–2154
Vincelette M, Dumouchel J, Giroux J, Heriarivo R (2007) The Tolagnaro (Fort Dauphin) region: a brief overview of the geology, hydrology, and climatology. In: Alonso A (eds) Biodiversity, ecology and conservation of littoral ecosystems in southeastern Madagascar, Tolagnaro (Fort Dauphin). SI/MAB Series #11, Washington, DC, pp 9–18
Visser GH, Schekkerman H (1999) Validation of the doubly labeled water method in growing precocial birds: the importance of assumptions concerning evaporative water loss. Physiol Biochem Zool 72:740–750
Wang LCH (1989) Ecological, physiological, and biochemical aspects of torpor in mammals and birds. In: Wang LCH (ed) Advances in comparative and environmental physiology. Springer, Berlin, pp 361–393
Ward S, Scantlebury M, Krol E, Thomson PJ, Sparling C, Speakman JR (2000) Preparation of hydrogen from water by reduction with lithium aluminium hydride for the analysis of delta H-2 by isotope ratio mass spectrometry. Rapid Commun Mass Spectrom 14:450–453
Warnecke L, Turner JM, Geiser F (2008) Torpor and basking in a small arid zone marsupial. Naturwissenschaften 95:73–78
Warren RD, Crompton RH (1998) Diet, body size and the energy costs of locomotion in saltatory primates. Folia Primatol 69(Supplement 1):86–108
Westman W, Geiser F (2004) The effect of metabolic fuel availability on thermoregulation and torpor in a marsupial hibernator. J Comp Physiol B 174:49–57
Acknowledgements
We thank the Direction des Eaux et Forêts and the Commission Tripartite for their authorisation to carry out this work. The study has been conducted within the framework of biodiversity assessment studies of the littoral forest fragments initiated by QIT Madagascar Minerals (QMM). J.-B. Ramanamanjato, M. Vincelette and their environmental and conservation team of QMM as well as R. Ernest provided excellent support in the field. We are grateful to Paula Redman and Peter Thomson for technical assistance in the isotope analysis. We thank J. Fietz, J.U. Ganzhorn and three anonymous referees for very helpful comments on the manuscript. This paper is part of the Accord de Collaboration between the Université d’Anananarivo (Départements de Biologie Animale and d’Anthropologie et Biologie Evolutive), QMM and Hamburg University. Financial support from the German Research Foundation (SCHM 1391/2-1, 2-3, 2-4) is gratefully acknowledged. We declare that the experiments complied with the current laws on Madagascar (no. 101–DGDRF/SCB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmid, J., Speakman, J.R. Torpor and energetic consequences in free-ranging grey mouse lemurs (Microcebus murinus): a comparison of dry and wet forests. Naturwissenschaften 96, 609–620 (2009). https://doi.org/10.1007/s00114-009-0515-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-009-0515-z