Abstract
Hibernation is the most effective way to reduce thermoregulatory costs during periods of unfavourable environmental conditions. In preparation to hibernation, fat-storing hibernators accumulate large quantities of body fat, which increases their locomotor costs and also the risk of predation. As a consequence, there should be a strong selective pressure to restrict pre-hibernation fattening to a short-time period before the onset of hibernation. The edible dormouse (Glis glis) is characterized by having adapted its whole life history to the irregularly occurring mast-seeding pattern of the European beech (Fagus sylvaticus). Thus, the question arises how this small endotherm copes with huge differences in food availability between years. Therefore, we investigated body mass and thermal energetics of edible dormice during high and low food years. Our results demonstrate that during periods of low food availability, edible dormice enter an energy-saving mode with reduced body temperature (Tb) and resting metabolic rate (RMR), and high torpor frequencies. During irregularly occurring short events of high food availability in mast years, however, Tb was higher, torpor did not occur, and RMR was drastically elevated possibly due to an enlarged digestive tract and the heat increment of feeding associated with a dietary switch to high-quality food and an increase in the amount of food ingested. This physiological flexibility allows edible dormice to efficiently accumulate body fat reserves under extremely different situations of food availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endotherms such as mammals and birds allocate a large proportion of their energy into the maintenance of high body temperature (Tb). On one hand, a high Tb optimizes physiological processes, but on the other hand, it is energetically extremely costly to maintain, especially when it is cold. Small endotherms, in particular, loose a substantial amount of energy as heat over their relatively large body surface during periods of cold exposure (Kleiber 1947; Heldmaier and Neuweiler 2013). The physiologically most effective way to escape these thermoregulatory challenges is to suspend the maintenance of high Tb and to enter hibernation. During hibernation, virtually, all body functions including heart and metabolic rate are drastically declined and Tb may be reduced down to ambient temperature (Ta; Barnes 1989; Geiser et al. 1990; Ruf and Geiser 2015). Fat-storing hibernators such as dormice (Gliridae) and marmots completely cease feeding during the hibernation period and rely entirely on stored body fat accumulated during pre-hibernation fattening for energy metabolism (reviewed in Humphries et al. 2003).
Disadvantages associated with the accumulation of these large amounts of body fat are increased locomotor costs and also a higher predation risk due to reduced mobility (Trombulak 1989). Thus, there should be a strong selective pressure to restrict pre-hibernation fattening to a short-time period just before the onset of hibernation especially for arboreal mammals as they often climb from the ground into the canopy and use fine branches to escape from predators. In contrast, ground dwelling hibernators increase their body mass earlier during the active season and usually decrease above ground activity and foraging time before hibernation (Johns and Armitage 1979; Körtner and Geiser 1995; Long et al. 2005; Sheriff et al. 2013).
However, especially for species that rely on seeds of mast-seeding tree species, such as chipmunks and edible dormice (Wolff 1996; Schlund et al. 2002), food conditions drastically vary between years. Thus, these species are forced to accumulate sufficient fat reserves even when high-quality food is not available and have to flexibly adapt to ever changing environmental challenges. This can either be achieved by increasing the amount of food ingested and expanding the time spent foraging per day or a drastic reduction of energy expenditure.
The edible dormouse (Glis glis Linnaeus, 1766) is a small arboreal rodent characterized by an extraordinarily long hibernation period with a mean of 8 months (Bieber and Ruf 2009). Its whole life history is closely adapted to the irregularly occurring mast-seeding patterns of the European beech (Fagus sylvatica Linnaeus) and oaks (Quercus spec.; Fietz et al. 2005; Ruf et al. 2006). These tree species are dominating European Forests and do not necessarily mast in the same years (e.g., Sork et al. 1993; local forestry office in Nagold). During years of seed mast, high-quality food is plentiful for a few weeks and G. glis exploits this for reproduction and pre-hibernation fattening. However, high mast years are usually followed by at least 1 year of mast failure, during which trees fail to produce seeds. This means that this small endotherm must cope with extended periods of food scarcity (Hilton and Packham 2003) in forests dominated by one of these tree species. Previous studies have shown that during periods of low food availability, edible dormice remain sexually quiescent and may extend their hibernation period throughout summer (Bieber 1998; Schlund et al. 2002; Hoelzl et al. 2015), which seems to lead to higher survival rates, reflecting a trade-off between reproduction and survival (Ruf et al. 2006; Lebl et al. 2011). Thus, edible dormice flexibly adjust life-history tactics to local mast patterns and “sit tight” until environmental conditions are favourable for reproduction (Pilastro et al. 2003; Ruf et al. 2006). Up to now, it is still unclear, how edible dormice evaluate the status of mast at emergence from hibernation. Even though food supplementation experiments increased reproductive rate in females during a year of low seed masting (Lebl et al. 2010), it could not trigger reproduction in a year of mast failure (Fietz et al. 2009). Thus, energy supply at the beginning of the active season may promote reproduction, but does not seem to represent the trigger to induce reproductive activity.
During years of mast seeding, dormice are challenged to effectively accumulate body fat during short periods when food is plentiful. Previous studies have shown that during high mast years, edible dormice switch their diet to predominantly energy-rich tree seeds as soon as these become available in late summer, and while their assimilation rates remain constant, the amount of food ingested increases by about 20% (Fietz et al. 2005; Sailer and Fietz 2009; Juškaitis et al. 2015).
The aim of our study was to examine how small endotherms such as the edible dormouse respond physiologically and prepare for hibernation under extremely different food conditions. We hypothesized that extended periods of low food availability and short periods of extremely high food availability should be reflected in extreme fluctuations in body mass, metabolic and thermal physiology as well as behaviour of edible dormice.
Methods
Study animal
The nocturnal edible dormouse (G. glis; rodentia) is an arboreal small mammal with a mass of about 100 g. Its range mainly coincides with the deciduous forest zone in the western Palearctic, (for details, see Kryštufek 2010). In Central Europe, it occurs mainly in deciduous mixed forests dominated by European beech (Schlund et al. 1997; Fietz et al. 2005), but also in forests with a considerable proportion of other broad-leafed trees (e.g., oaks), which provide alternative food resources in years without beech seeding (Cornils et al. 2017). In central Europe, adults hibernate underground from mid-September until May (von Vietinghoff-Riesch 1960). Males emerge from hibernation about 2 weeks before the females and mating takes place shortly after females emerge at the end of June. Females give birth to one litter per year with about 5 (up to 11) pups that are weaned after 30 days (Kager and Fietz 2009). Due to their close adaptation to the irregular seed production of their main food tree species, virtually, all adult dormice belonging to one population can be assumed to be reproductively active at the same time during high mast years, whereas all individuals remain reproductively quiescent in years of mast failure (Schlund et al. 2002; Fietz et al. 2004). In this study, we restricted our analyses to data obtained on adult dormouse males, as in females, it is impossible to disentangle the effects of reproduction and food availability on body mass and energy consumption, because females gestate and lactate when food is abundant.
Study sites
Our study was conducted at five different study sites, located in SW Germany, within 73 km of each other (for a detailed description see Fietz et al. 2014). These study sites were in deciduous mixed forests dominated by European beech, but the forest at Beimerstetten also had a high proportion of sessile oak [Quercus petrea (Mattuschka) Lieblein]. In these study sites, nest boxes (Schwegler 3SV, Schorndorf, Germany) were mounted on trees 3 m above ground, 30 m apart, at the nodes of rectangular grids. Edible dormice use nest boxes frequently during the day, making them easily accessible.
Food availability
Within the federal state of Baden-Württemberg (Germany), tree seed production is monitored by the local forestry office in Nagold. In our study sites, 2005, 2008, and 2012 represent years of mast failure, as beeches did not produce any seeds, whereas 2006, 2009, 2013, and 2014 were mast years and beeches produced high numbers of seeds. The years 2007, 2010, and 2011 were not included into the analyses, as for these years, the data sets were not as comprehensive as in the other years. In contrast to the other study sites, Beimerstetten contains a high proportion of oak trees that produced seeds in 2012. Thus, for Beimerstetten, 2012 represented a high mast year and dormice were reproductive.
Capture–mark–recapture
At all study sites, nest boxes were monitored from the end of May until early October at 2-week intervals. Nest boxes were monitored between 2005 and 2014. Upon capture, dormice were individually marked using subcutaneously-implanted passive integrated transponders (Trovan, EURO I.D. Usling GmbH, Weilerswist, Germany). Mass was determined with a 300 g spring balance (Pesola, Baar, Switzerland; division: 2 g, accuracy: 99.7%) to the nearest g. Individuals were sexed and classified as yearlings or adults by the length of their tibia and by fur colour (Schlund 1997). For investigating seasonal variations in body mass in high and low mast years, we used data obtained on adult males between 2005 and 2014 (see “Food availability” section).
Ambient temperature measurements
We measured Ta at all study sites during 2012 and 2013 using temperature data loggers (iButton DS1922L-F5, Maxim Integrated Products, Inc., San Jose, USA) set to a resolution of 0.5 °C. We fixed one data logger to a tree trunk in each study site to continuously record Ta every 60 min throughout the entire study period. One temperature data logger was additionally attached to the outside of the nest box during metabolic measurements (see below) and programmed to sample Ta every 60 s. Ta loggers were not exposed to direct solar radiation.
Torpor frequencies
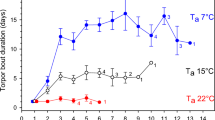
We analysed torpor frequencies during a low (2012) and a high (2013) mast year. Individuals were considered torpid if they felt cold to the touch and were impaired in their locomotor activity (Bieber and Ruf 2009; Smit et al. 2011; Dausmann 2014). In the analysis of torpor frequencies (see statistical analyses), we used the corresponding minimum Ta (Tmin) in the night before the observation as a predictor, as torpor bouts predominantly start during the second half of the night (Wilz and Heldmaier 2000).
Body temperature measurements
During 2013, we measured skin temperature (Tskin) in adult males in which we also measured metabolic rate (see below) with collar-mounted temperature data loggers [iButton; DS1922L-F5, mass 1.5 g; for details see Langer and Fietz (2014)] at a resolution of 0.5 °C every min. Results of previous studies (Langer and Fietz 2014) and the present study have demonstrated that in edible dormice, Tb measured subcutaneously (Tsub) was on average 2.5 °C higher than Tskin and that Tb measured intraperitoneally was on average 1.8 °C higher than Tsub (Bieber et al. 2017). Since the resolution of the iButtons is 0.5 °C, we corrected our measured Tskin by adding 4.5 °C to get an approximation of core body temperature (Tcore). These values were comparable to Tcore measured in individuals of the study of Bieber et al. (2017) during their resting phase. Henceforth, we call this calculated body temperature Tbc. Simultaneous measurements of Tskin and metabolic rate (MR) were conducted for 74 individuals in 76 respirometry trials. We programmed and attached the temperature data loggers in the morning just before starting MR measurements and took them off in the evening after measurements were terminated. The Tskin measurement interval was 60 s [for details, see Langer and Fietz (2014)]. To investigate the effect of Ta on Tb measurements obtained with collar-mounted temperature data loggers, we simultaneously measured Ta, Tskin, and Tsub in nine resting, adult male edible dormice on eight different days. Tskin was measured as described above and Tsub was measured every 5 min with temperature-sensitive transponders implanted subcutaneously in the dorsolateral region [for details, see Langer and Fietz (2014)].
Measurement of oxygen consumption
Oxygen consumption was measured in single non-torpid adult males during 2012 and 2013. While animals were in nest boxes (volume: 1.5 L), oxygen consumption was measured for at least 5 h between 11 am and 8 pm throughout the whole study period by open-flow respirometry, using portable gas analysers [OxBox, designed and constructed by Thomas Ruf and Thomas Paumann, Research Institute of Wildlife Ecology, Vienna; for a detailed description see Fietz et al. (2010); exemplary measurements in Fig. 1]. Flow rate was set to 100 l/h and gas analyzers were calibrated regularly with fresh air (20.95% O2) as well as calibration gas (20.1% O2, rest N2; Westfalen AG, Münster, Germany). Mass flowmeters (AWM5101, Honeywell, Morris Plains, USA) were calibrated against a certified calibration mass flow meter (Type 358-11, 0–2 L/min, Analyt-MTC, Müllheim, Germany) and volumes were corrected to STPD conditions. Measurement air was dried (Molecular sieve, 3 Å, Merck, Darmstadt, Germany) prior to flowmeters. O2 data were corrected for drift of the analyzer by automated switching to sample reference air at regular intervals. When the respiratory quotient is < 1, flow rate is slightly underestimated as more O2 is consumed than CO2 produced. We accounted for this by calculating MR with the following equation: VO2 = FD × (FIO2–FEO2)/[1 − FIO2 × (1 − 0.85)] (l/h; FD = dry flow, FIO2 = fractional concentration of O2 in the incoming airflow, FEO2 = fractional concentration of O2 in the outgoing airflow), assuming a respiratory quotient of 0.85 (Lighton 2008). Data were collected at 1 min intervals. Moving averages were calculated over 10 min intervals. The lowest moving average during each measurement was defined as RMR, and the corresponding Ta and Tskin measurements were averaged over the same 10 min. To make sure that animals were at rest, we measured oxygen consumption for at least 5 h without disturbance and excluded measurements from the analysis if the coefficient of variance of MR exceeded 20% during the respective time period. RMR generally specifies the rate of energy expenditure necessary to maintain the energy balance of resting endotherms (Heldmaier and Neuweiler 2013). As we were interested in the effect of Ta on RMR, our measurements were not restricted to Ta within the thermoneutral zone (TNZ).
Statistical analysis
Statistical analyses were performed in R (R Core Team 2011). To explain intraspecific variation in RMR and Tbc of G. glis, we used linear mixed effects models, function “lmer” from the lme4 package extended by the “lmerTest” package (Bates et al. 2015; Kuznetsova et al. 2015), using the Satterthwaite approximation for degrees of freedom. We were primarily interested in the effect of high-quality food on RMR. As ripe seeds are only available in the late season during high mast years (Fietz et al. 2005), we split the year on the 15th of August and included the factors “mast year” [two levels: high (2013) and low (2012)] and “season” (two levels: early and late) into the analyses. All measurements taken prior to the 15th of August were assigned to the early season and the ones taken afterwards to the late season. Note that high mast years perfectly coincide with years of high reproductive activity in edible dormice (Ruf et al. 2006). In the model for the dependent variable RMR based on data of both study years, we included the interaction of mast year and season. In addition, we controlled for the effects of mass, Ta, and animal ID nested in study site as random factors. In captive G. glis, the lower critical temperature of the TNZ was shown to be 22 °C (Heldmaier and Elvert 2004). Therefore, we controlled for the effects of Ta on RMR within and below the TNZ separately, using a broken stick regression analysis with the break point at 22 °C.
We calculated a generalized linear mixed effects model, with the function “glmer” (family = binomial, package lme4, using the Laplace approximation for degrees of freedom), to test for the effects of the predictors: mass, Tmin, mast year, season and animal ID nested in study site as random factor on the response variable “occurrence of torpor” (binomial: torpid or non-torpid) during 2012 and 2013. In the “lmer” models, we checked the residuals for deviations from the normal distribution (visual check of histograms and quantile–quantile–plots) and homogeneity of variance (visual check of residuals plotted against fitted values) and transformed (see tables) covariates and respective response variables where necessary. In “glmer” models, we checked for overdispersion. Furthermore, we tested for collinearity with the function “vif” (Fox and Weisberg 2011) and for validity and stability of our models (dffits, dfbetas, cook’s distances, and leverage). In cases where assumptions were violated, we tested whether a reduced model (excluding the violating cases) led to different conclusions. As this was never the case, we present the model results with all cases included. Data from Beimerstetten were only included in the models for the high mast year, because in this study site, food trees produced seeds in both years.
Estimates, standard errors (se), degrees of freedom (df), t- or z-values, and p values are taken from a regression analysis, function “summary”. Level of significance was set to p = 0.05.
To determine the effect of high and low mast years on seasonal mass variations in male edible dormice, we calculated “lmer”s containing the fixed factor “mast” [two levels: high (2006, 2009, 2013, and 2014) and low (2005, 2008, and 2012)], “mastpre” [the mast level of the previous year; two levels: high (2005, 2008, 2012, and 2014) and low (2006, 2009, and 2013)]; “tibia length” [as a proxy for body size (Schlund 1997)]; and the animal ID nested in the study site as random factor, separately for each month. Furthermore, to compare changes across the season, we calculated “lmer”s for the mast years separately with “month” and “tibia length” as fixed factors and animal ID nested in the study site as random factor and used a Tukey’s post-hoc test in case of multiple comparisons (R package “multcomp”; Hothorn et al. 2008).
To exclude the possibility that Tskin measured with a collar-mounted temperature, logger is mainly influenced by Ta and not by Tsub and to further evaluate their precision, we used the linear mixed effects model function “lme” of the R-package nlme (Pinheiro et al. 2011) to calculate regressions of Tbc against Tsub and Ta in resting individuals with animal ID as a random factor and fitted an autoregressive correlation structure (corAR1()) to correct for autocorrelation.
Results
Relationship between subcutaneous and skin temperature
Results of the linear mixed effects model (Tskin ~ Ta + Tsub) corrected for animal ID and autocorrelation revealed that Tskin was significantly correlated with Tsub (p < 0.001, estimate: 0.74; n = 473; Fig. 2) within a Ta range of 12–34 °C, whereas Ta did not directly affect measured Tskin values (p = 0.7). Thus, Tskin closely tracked variation in Tsub and was on average within 2.2 ± 0.8 °C of Tsub.
Relationship between skin temperature and subcutaneous temperature of adult male edible dormice. Tskin was strongly correlated with Tsub (spearmans rho = 0.95; p < 0.001). The insert shows the quotient of subcutaneous temperature and skin temperature measured at ambient temperatures ranging between 12 and 35 °C
Variations in body temperature
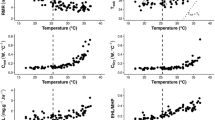
During a high mast year, Tbc in resting adult male G. glis ranged from 30 to 38 °C and was significantly reduced at lower Ta between 10 and 33 °C (Fig. 3a; Table 1). Tbc was significantly higher during the late season compared to the early season, but the effect of Ta on Tbc did not differ between those two seasons (interaction between Ta and season: F1,79 = 0.03, p = 0.87). In the early season, dormice resting within the TNZ lowered their Tbc below euthermic values (Fig. 3a) down to 32 °C, whereas dormice in the late season kept euthermic Tbc. Resting euthermic dormice have Tb of 35.6 ± 0.7 °C (Wilz 1997).
a Tskin measured in adult male edible dormice during the high mast year (2013, respiratory trials n = 76) plotted against ambient temperature (Ta). b Mass-specific metabolic rate (RMRbm) from the same animals plotted against Ta. c RMRbm plotted against Ta in adult male edible dormice in the low mast year (2012, respiratory trials n = 42). Circles represent the early season and filled circles represent the late season. The horizontal line in plot a indicates euthermia, the vertical line indicates the lower critical temperature of the TNZ and the regression lines are based on laboratory experiments by (Heldmaier and Elvert 2004). RMRbm (RMR/BM) was only used in this figure for visual purposes and was not included in the statistical models
Seasonal body mass variation in high and low mast years
High mast in the preceding year positively affected masses at emergence (F1,473.73 = 32.0, p < 0.001), but negatively in July (F1,492.48 = 18.3, p < 0.001). Later in the year, the effect of the mast during the preceding year was no longer detectable (August: F1,386.12 = 0.19, p = 0.664; September: F1,150.59 = 1.053, p = 0.307). Adult male edible dormice exhibited different seasonal body mass patterns during high and low mast years (Fig. 4). In low mast years, dormice emerged from hibernation with a higher mass than in high mast years (F1,474.25 = 6.1, p = 0.014) and lost about 9 g by July (t = − 5.7, p < 0.001). Between July and August mass remained constant (t = 0.6, p = 0.534) and increased subsequently by about 10 g during September (t = 3.47, p < 0.001). Starting with a lower mass in June, dormice in high mast years also lost about 10 g by July (t = − 10.1, p < 0.001). Between July and September mass increased by about 20 g (t = 22.549, p < 0.001). Male dormice had a lower mass in high mast years (July: F1,506.25 = 158.7, p < 0.001; August: F1,368.3 = 15.9, p < 0.001), until they caught up in September when mass of males in high mast years was not significantly different from males in years of mast failure (F1,131.61 = 0.07, p = 0.7881).
Variations in resting metabolic rate
There was a significant interaction between season and mast year for RMR (Table 2). During the low mast year, RMR did not show pronounced seasonal variations (Fig. 5), whereas RMR measured during the high mast year was significantly higher in the late season than in the early season. During the high mast year, the effect of Ta on RMR below the TNZ was not significant, whereas there was a negative effect of Ta on RMR within the TNZ (Table 2).
Residuals of a reduced model based on the high and low mast year (log(RMR) ~ sqrt(mass) + study site + Ta below TNZ + Ta within TNZ), missing the variables year, season and their interaction. Given are mean ± SD (low mast nearly season = 29, nlate season = 13; high mast nearly season = 43, nlate season = 21). Significance is based on the full model (Table 2)
Occurrence of torpor
Torpor frequency was significantly affected by Tmin, with more adult males using torpor during days following cooler nights. Furthermore, dormice were more likely to enter torpor in the low mast year and during the early season of the high mast year (Table 3). Mass had no effect on torpor use.
Discussion
Results of this study show distinct seasonal variations in RMR of adult male edible dormice during high mast years. We found relatively low RMRs at the beginning of the active season, whereas RMRs were extremely elevated as soon as high-quality food became available in the last weeks before hibernation started.
The gastrointestinal tract is the first organ system directly affected by changes in nutrient intake and displays the most rapid and extensive responses to nutrient deprivation, both in structure and in function (Carey 2005). Fat-storing hibernators, such as edible dormice, represent an extreme example of phenotypic flexibility in regard to digestive function, as they completely cease feeding during winter months, leading to significant atrophy of the intestinal mucosa during hibernation (Carey 2005). After emergence, size and activity of the gut and nutrient processing organs are regained, possibly induced by the resumption of food intake (Hume et al. 2002; Carey 2005). In addition, also in edible dormice, there is a significant increase in liver size throughout the active season (Bieber et al. 2011). In the alpine marmot (Marmota marmota Linnaeus, 1758), the mass of the small intestine has been shown to increase by about 260% from the end of hibernation to midsummer and similar values were found for 13-lined ground squirrels [Spermophilus tridecemlineatus Mitchill, 1821; (Hume et al. 2002; Carey 2005)]. In this study RMR values measured after emergence from hibernation were only about 50% of the value predicted for palearctic mesic rodents of comparable body size (according to Lovegrove 2000). Even though we did not investigate gut mass and activity in our study, we presume that in G. glis, these comparably low RMRs observed during periods of low food availability during the early season can in part be explained by digestive organs that remain reduced in activity and size after emergence from hibernation. However, as soon as high-quality food becomes abundant in late summer of a mast year, edible dormice switch their diet to predominantly energy-rich tree seeds (Fietz et al. 2005; Sailer and Fietz 2009; Juškaitis et al. 2015). In dormice fed on rodent chow assimilation, rates remained constant throughout the year, whereas the amount of food ingested increases by about 20% during pre-hibernation fattening (Sailer and Fietz 2009). These changes in foraging behaviour during mast years lead to an extreme body mass increase until the onset of hibernation and also positively affects body mass after emergence in June during the following year (Fig. 4). The finding, that dormice have lower body masses in July following a high mast year, can likely be explained by the fact, that high mast years are in general followed by a year of mast failure, when food is limited. However, at the first sight, it seems to be contradictory that even though dormice enter hibernation with the same body mass in years of high and low food availability, they emerge with a lower body mass after a year of mast failure. Several studies have shown that hibernation performance (e.g., torpor depth and length of euthermic periods), and therefore, energy consumption during hibernation depends not only on pre-hibernation body mass (Bieber et al. 2014), but also on the composition of the body fat (Geiser and Kenagy 1987; Ruf and Arnold 2008) and possibly also on preparatory mechanisms for the challenges of the forthcoming active period such as gonadal development. During hibernation, gonadal development as well as all other cell processes are basically restricted to the euthermic phases (Carey et al. 2003; Clarke and Fraser 2004) and are, therefore, associated with high thermoregulatory cost. These energetic costs needed for gonadal development might explain, why edible dormice emerge with comparatively low body masses at the beginning of reproductive years.
Concomitant with the dietary switch and increase of food intake in the late season of high mast years, RMR in adult male dormice increases dramatically, and was distinctly higher (167%) than expected for a rodent of comparable body size (according to Lovegrove 2000). As the alimentary tract and associated organs contribute a disproportionately large amount to energy consumption (Krebs 1950; Konarzewski and Diamond 1995; Cant et al. 1996) and respond rapidly to changes in food availability, the enlargement of the digestive system and its increase in activity can be assumed to contribute to high RMR values measured during periods of high food availability.
However, even though these changes might contribute significantly to RMR measured, they cannot fully explain extreme RMR increases observed in this study. In a previous study by Bieber et al. (2017) it could be shown that during mast years, active free-living edible dormice increase their maximum Tcore regularly and for up to 8 h above 40 °C starting 6 weeks prior to the onset of hibernation. This hyperthermia was explained by high levels of locomotor activity while foraging, which should be especially expensive in an arboreal species such as the edible dormouse (Bieber et al. 2017). Based on the present data and the facts that edible dormice switch their diet and also increase the amount of food ingested (Fietz et al. 2005; Sailer and Fietz 2009; Juškaitis et al. 2015), we assume that the heat increment of feeding, also called specific dynamic action (Rubner 1902; Maynard and Loosli 1969) adds substantially to measured RMRs and Tbc values, explaining their drastic increases observed in this study (Hume et al. 2002; Carey 2005; Konarzewski and Książek 2013). Reproductive activity in adult males, on the other hand, can be ruled out, as a cause for high RMR during the late season as dormice mate during July when RMR was still low. As already mentioned above in females, it is impossible to disentangle the effects of reproduction and food availability on RMR and Tb, because females always gestate and lactate when food is abundant. Nevertheless, we assume that comparable physiological changes should also occur in females.
During pre-hibernation fattening, we detected a high variability in RMR among individuals. This individual variability can be explained by different factors. First of all, the amount of food available within their home ranges and the food ingested likely differs among individuals and with it the associated heat increment of feeding. Second, individuals may differ in their immergence date and their rate of body fat accumulation and also in the size of their gastrointestinal tract. As different body components have different specific metabolic activities (Johnston et al. 2007), differences in body composition may add to the variance among individuals. In 13-lined ground squirrels, the mass of the small intestine was shown to decrease by about 50–75% shortly before the onset of hibernation (Carey 2005). These complex changes in preparation for the upcoming hibernation season in combination with individually differing immergence dates and hormonal changes (e.g., Kronfeld-Schor et al. 2000) likely explain observed RMR variability.
Edible dormice experience short periods of extremely high food availability during mast years; in between these irregular events, however, they are confronted with extended periods of low food availability. In contrast to strong seasonal variations in RMR observed during high mast years, RMR was relatively low and constant during a year of mast failure and below values expected for a rodent of comparable body size (Lovegrove 2000). Low RMR, Tbc, and elevated torpor frequencies suggest that adult male dormice enter an energy-saving mode during periods of low food availability. In addition, haematological investigations also revealed that the production of red blood cells is diminished during periods of low food availability and seems to represent part of this energy-saving mode (Havenstein et al. 2017). These physiological responses likely allow dormice to keep their body mass at a comparatively high level even when food is scarce.
Particularly at low Ta, the active reduction of Tbc represents an efficient physiological mechanism to reduce energy consumption (Morris 1968; Walton and Andrews 1981; Cygan 1985; Lyman 2013). Wilz and Heldmaier (2000) formerly showed that dormice may enter torpor already during their active period at night and our results show that G. glis uses torpor more frequently during periods of food shortage comparable to findings of Bieber et al. (2017). Although torpid individuals are limited in their locomotor ability and should, therefore, be more vulnerable to predation (Trombulak 1989), torpid individuals that retreat into well-protected tree holes should have a selective advantage. First, they are more likely to survive times of low food availability, as they can extend the period of fasting. Second, they can allocate more energy into their body fat reserves, by minimizing their energy expenditure. Third, owing to lower energy requirements the time spent foraging during the night can be shortened, which should decrease the risk of predation (Ruf and Heldmaier 1992; Stawski et al. 2015), one of the main causes of mortality in small mammals (Ims and Andreassen 2000; Bryant and Page 2005). In line with these assumptions, it has been shown that the use of torpor and prolonged hibernation of up to 11 months within sheltered sleeping holes and hibernacula reduces mortality in this species (Lebl et al. 2011; Bieber et al. 2014; Hoelzl et al. 2015) as well as in hibernators in general (Turbill et al. 2011). Thus, the higher proportion of torpid dormice during years of limited food availability and an earlier immergence into hibernation likely explains the reduced mortality rates measured previously (Ruf et al. 2006; Lebl et al. 2011).
Not only deep torpor, but even small reductions in the Tb–Ta differential reduce heat loss and, therefore, thermoregulatory costs. In our study, Tbc was affected by season and mast year and fine-tuned by the availability of internal energy resources, as individuals with a higher mass exhibited higher Tbc. Furthermore, reduced Tbc did not only occur during periods of fasting, but also during resting phases when high-quality food was abundant (i.e., late season during high mast year). However, Tbc was then generally higher than during periods of food restriction. Surprisingly, Tbc reduction also occurred in the early season at thermoneutrality when all body heat is derived from heat produced by basal metabolic rate and Tb is regulated by physical means, changing conductance by vasoconstriction and vasodilation in the periphery of the body. In this study, we cannot determine whether low Tbc was caused by the inhibition of MR and an active downregulation of Tcore. However, previous studies show that Djungarian hamsters (Ruf and Heldmaier 1992), small marsupials (Song et al. 1997), and also edible dormice enter torpor at thermoneutrality (Heldmaier and Elvert 2004), which can only be caused by an active inhibition of MR below the level of basal metabolic rate. These findings, together with the observed distinct effect of food availability on Tbc, suggest that adult male edible dormice may actively downregulate MR at thermoneutrality to reduce energy consumption in times of limited food supply and are able to accumulate fat reserves from less favourable food.
Conclusions
We demonstrate that during extended periods of fasting, male edible dormice enter an energy-saving mode with reduced Tbc, high torpor frequencies, and RMRs below values expected of a rodent of comparable body size. During irregularly occurring short events of high-quality food availability, however, Tbc and RMR were increased drastically possibly due to an enlarged digestive tract and the heat increment of feeding associated with a dietary switch and an increase in the amount and quality of food ingested. These effects have so far largely been ignored in studies on metabolic physiology and results of our study underline the importance of field studies, as the complex interactions of ever changing environmental conditions and behavioural and physiological responses can hardly be replicated in captive animals under laboratory conditions.
References
Barnes BM (1989) Freeze Avoidance in a mammal: body temperatures below 0 °C in an Arctic Hibernator. Science 244:1593–1595
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using {lme4}. J Stat Softw 67:1–48
Bieber C (1998) Population dynamics, sexual activity, and reproduction failure in the fat dormouse (Myoxus glis). J Zool 244:223–229. https://doi.org/10.1111/j.1469-7998.1998.tb00027.x
Bieber C, Ruf T (2009) Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwissenschaften 96:165–171. https://doi.org/10.1007/s00114-008-0471-z
Bieber C, Außerlechner K, Skerget C et al (2011) Seasonal changes in liver size in edible dormice (Glis glis): non-invasive measurements using ultrasonography. Eur J Wildl Res 57:657–662. https://doi.org/10.1007/s10344-010-0476-8
Bieber C, Lebl K, Stalder G et al (2014) Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct Ecol 28:167–177. https://doi.org/10.1111/1365-2435.12173
Bieber C, Cornils JS, Hoelzl F et al (2017) The costs of locomotor activity? Maximum body temperatures and the use of torpor during the active season in edible dormice. J Comp Physiol B. https://doi.org/10.1007/s00360-017-1080-y
Bryant AA, Page RE (2005) Timing and causes of mortality in the endangered Vancouver Island marmot (Marmota vancouverensis). Can J Zool 83:674–682. https://doi.org/10.1139/z05-055
Cant JP, McBride BW, Croom WJ (1996) The regulation of intestinal metabolism and its impact on whole animal energetics. J Anim Sci 74:2541. https://doi.org/10.2527/1996.74102541x
Carey HV (2005) Gastrointestinal responses to fasting in mammals: lessons from hibernators. Physiol Ecol Adapt Feed Vertebr Sci Enfield NH 229–254
Carey HV, Andrews MT, Martin SL (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83:1153–1181. https://doi.org/10.1152/physrev.00008.2003
Clarke A, Fraser KPP (2004) Why does metabolism scale with temperature? Funct Ecol 18:243–251. https://doi.org/10.1111/j.0269-8463.2004.00841.x
Cornils JS, Hoelzl F, Rotter B et al (2017) Edible dormice (Glis glis) avoid areas with a high density of their preferred food plant—the European beech. Front Zool 14:23. https://doi.org/10.1186/s12983-017-0206-0
Cygan T (1985) Seasonal changes in thermoregulation and maximum metabolism in the yellow-necked field mouse. Acta Theriol (Warsz) 30:115–130. https://doi.org/10.4098/AT.arch.85-4
Dausmann KH (2014) Flexible patterns in energy savings: heterothermy in primates. J Zool 292:101–111. https://doi.org/10.1111/jzo.12104
Fietz J, Schlund W, Dausmann KH et al (2004) Energetic constraints on sexual activity in the male edible dormouse (Glis glis). Oecologia 138:202–209. https://doi.org/10.1007/s00442-003-1423-0
Fietz J, Pflug M, Schlund W, Tataruch F (2005) Influences of the feeding ecology on body mass and possible implications for reproduction in the edible dormouse (Glis glis). J Comp Physiol [B] 175:45–55. https://doi.org/10.1007/s00360-004-0461-1
Fietz J, Kager T, Schauer S (2009) Is energy supply the trigger for reproductive activity in male edible dormice (Glis glis)? J Comp Physiol B 179:829–837. https://doi.org/10.1007/s00360-009-0364-2
Fietz J, Klose SM, Kalko EKV (2010) Behavioural and physiological consequences of male reproductive trade-offs in edible dormice (Glis glis). Naturwissenschaften 97:883–890. https://doi.org/10.1007/s00114-010-0704-9
Fietz J, Tomiuk J, Loeschcke V et al (2014) Genetic consequences of forest fragmentation for a highly specialized arboreal mammal—the edible dormouse. PLoS One 9:e88092. https://doi.org/10.1371/journal.pone.0088092
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks
Geiser F, Kenagy GJ (1987) Polyunsaturated lipid diet lengthens torpor and reduces body temperature in a hibernator. Am J Physiol Regul Integr Comp Physiol 252:R897–R901
Geiser F, Hiebert S, Kenagy GJ (1990) Torpor bout duration during the hibernation season of two sciurid rodents: interrelations with temperature and metabolism. Physiol Zool 63:489–503. https://doi.org/10.1086/physzool.63.3.30156224
Havenstein N, Langer F, Fietz J (2017) Life history written in blood: erythrocyte parameters in a small hibernator, the edible dormouse. J Comp Physiol B 1–13. https://doi.org/10.1007/s00360-017-1111-8
Heldmaier G, Elvert R (2004) How to enter torpor: thermodynamic and physiological mechanisms of metabolic depression. Life in the cold: evolution, mechanisms, adaptation, and application 12th international hibernation symposium, pp 185–198
Heldmaier G, Neuweiler G (2013) Vergleichende Tierphysiologie: Gerhard Heldmaier Vegetative Physiologie. Springer, Berlin
Hilton GM, Packham JR (2003) Variation in the masting of common beech (Fagus sylvatica L.) in northern Europe over two centuries (1800–2001). For Int J For Res 76:319–328. https://doi.org/10.1093/forestry/76.3.319
Hoelzl F, Bieber C, Cornils JS et al (2015) How to spend the summer? Free-living dormice (Glis glis) can hibernate for 11 months in non-reproductive years. J Comp Physiol B 185:931–939. https://doi.org/10.1007/s00360-015-0929-1
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biomed J 50:346–363. https://doi.org/10.1002/bimj.200810425
Hume I, Beiglböck C, Ruf T et al (2002) Seasonal changes in morphology and function of the gastrointestinal tract of free-living alpine marmots (Marmota marmota). J Comp Physiol [B] 172:197–207. https://doi.org/10.1007/s00360-001-0240-1
Humphries MM, Thomas DW, Kramer DL (2003) The role of energy availability in mammalian hibernation: a cost-benefit approach. Physiol Biochem Zool 76:165–179. https://doi.org/10.1086/367950
Ims RA, Andreassen HP (2000) Spatial synchronization of vole population dynamics by predatory birds. Nature 408:194–196. https://doi.org/10.1038/35041562
Johns DW, Armitage KB (1979) Behavioral ecology of alpine yellow-bellied marmots. Behav Ecol Sociobiol 5:133–157. https://doi.org/10.1007/BF00293302
Johnston SL, Souter DM, Erwin SS et al (2007) Associations between basal metabolic rate and reproductive performance in C57BL/6J mice. J Exp Biol 210:65–74. https://doi.org/10.1242/jeb.02625
Juškaitis R, Baltrūnaitė L, Augutė V (2015) Diet of the fat dormouse (Glis glis) on the northern periphery of its distributional range. Mammal Res 60:155–161. https://doi.org/10.1007/s13364-015-0213-5
Kager T, Fietz J (2009) Food availability in spring influences reproductive output in the seed-preying edible dormouse (Glis glis). Can J Zool 87:555–565. https://doi.org/10.1139/Z09-040
Kleiber M (1947) Body size and metabolic rate. Physiol Rev 27:511–541
Konarzewski M, Diamond J (1995) Evolution of basal metabolic rate and organ masses in laboratory mice. Evolution 49:1239–1248. https://doi.org/10.2307/2410448
Konarzewski M, Książek A (2013) Determinants of intra-specific variation in basal metabolic rate. J Comp Physiol [B] 183:27–41. https://doi.org/10.1007/s00360-012-0698-z
Körtner G, Geiser F (1995) Body temperature rhythms and activity in reproductive Antechinus (Marsupialia). Physiol Behav 58:31–36. https://doi.org/10.1016/0031-9384(94)00384-H
Krebs HA (1950) Body size and tissue respiration. Biochim Biophys Acta 4:249–269. https://doi.org/10.1016/0006-3002(50)90032-1
Kronfeld-Schor N, Richardson C, Silvia BA et al (2000) Dissociation of leptin secretion and adiposity during prehibernatory fattening in little brown bats. Am J Physiol Regul Integr Comp Physiol 279:R1277–R1281
Kryštufek B (2010) Glis glis (Rodentia: Gliridae). Mamm Species 42:195–206. https://doi.org/10.1644/865.1
Kuznetsova A, Brockhoff PB, Bojesen RH (2015) lmerTest: tests in linear mixed effects models. R package version 2.0–29
Langer F, Fietz J (2014) Ways to measure body temperature in the field. J Therm Biol 42:46–51. https://doi.org/10.1016/j.jtherbio.2014.03.002
Lebl K, Kürbisch K, Bieber C, Ruf T (2010) Energy or information? The role of seed availability for reproductive decisions in edible dormice. J Comp Physiol B 180:447–456. https://doi.org/10.1007/s00360-009-0425-6
Lebl K, Bieber C, Adamík P et al (2011) Survival rates in a small hibernator, the edible dormouse: a comparison across Europe. Ecography 34:683–692. https://doi.org/10.1111/j.1600-0587.2010.06691.x
Lighton JRB (2008) Measuring metabolic rates: a manual for scientists: a manual for scientists. Oxford University Press, USA
Long RA, Martin TJ, Barnes BM (2005) Body temperature and activity patterns in free-living arctic ground squirrels. J Mammal 86:314–322. https://doi.org/10.1644/BRG-224.1
Lovegrove BG (2000) The zoogeography of mammalian basal metabolic rate. Am Nat 156:201–219. https://doi.org/10.1086/303383
Lyman CP (2013) Hibernation and torpor in mammals and birds. Elsevier, Oxford
Maynard LA, Loosli JK (1969) Animal nutrition. McGraw-Hill, New York
Morris PA (1968) Apparent hypothermia in the wood mouse (Apodemus sylvaticus). J Zool 155:235–236. https://doi.org/10.1111/j.1469-7998.1968.tb03044.x
Pilastro A, Tavecchia G, Marin G (2003) Long living and reproduction skipping in the fat dormouse. Ecology 84:1784–1792. https://doi.org/10.1890/0012-9658(2003)084%5B1784:LLARSI%5D2.0.CO;2
Pinheiro J, Bates D, DebRoy S, R Development Core Team (2011) nlme: linear and nonlinear mixed effects models
R Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rubner M (1902) Die Gesetze des Energieverbrauchs bei der Ernährung. F. Deuticke, Leipzig
Ruf T, Arnold W (2008) Effects of polyunsaturated fatty acids on hibernation and torpor: a review and hypothesis. Am J Physiol Regul Integr Comp Physiol 294:R1044–R1052. https://doi.org/10.1152/ajpregu.00688.2007
Ruf T, Geiser F (2015) Daily torpor and hibernation in birds and mammals. Biol Rev 90:891–926. https://doi.org/10.1111/brv.12137
Ruf T, Heldmaier G (1992) The impact of daily torpor on energy requirements in the djungarian hamster, Phodopus sungorus. Physiol Zool 65:994–1010
Ruf T, Fietz J, Schlund W, Bieber C (2006) High survival in poor years: life history tactics adapted to mast seeding in the edible dormouse. Ecology 87:372–381. https://doi.org/10.1890/05-0672
Sailer MM, Fietz J (2009) Seasonal differences in the feeding ecology and behavior of male edible dormice (Glis glis). Mamm Biol Z Für Säugetierkd 74:114–124. https://doi.org/10.1016/j.mambio.2008.05.005
Schlund W (1997) Die Tibialänge als Maß für Körpergröße und als Hilfsmittel zur Altersbestimmung bei Siebenschläfern (Myoxus glis L.). Mamm Biol Z Für Säugetierkd:187–190
Schlund W, Scharfe F, Stauss MJ, Burkhardt JE (1997) Habitat fidelity and habitat utilization of an arboreal mammal (Myoxus glis) in two different forests. Mamm Biol Z Für Säugetierkd:158–171
Schlund W, Scharfe F, Ganzhorn JU (2002) Long-term comparison of food availability and reproduction in the edible dormouse (Glis glis). Mamm Biol Z Für Säugetierkd 67:219–232. https://doi.org/10.1078/1616-5047-00033
Sheriff MJ, Fridinger RW, Tøien Ø et al (2013) Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool 86:515–527. https://doi.org/10.1086/673092
Smit B, Boyles JG, Brigham RM, McKechnie AE (2011) Torpor in dark times: patterns of heterothermy are associated with the lunar cycle in a nocturnal bird. J Biol Rhythms 26:241–248. https://doi.org/10.1177/0748730411402632
Song X, Körtner G, Geiser F (1997) Thermal relations of metabolic rate reduction in a hibernating marsupial. Am J Physiol Regul Integr Comp Physiol 273:R2097–R2104
Sork VL, Bramble J, Sexton O (1993) Ecology of mast-fruiting in three species of North American deciduous oaks. Ecology 74:528–541. https://doi.org/10.2307/1939313
Stawski C, Körtner G, Nowack J, Geiser F (2015) The importance of mammalian torpor for survival in a post-fire landscape. Biol Lett 11:20150134. https://doi.org/10.1098/rsbl.2015.0134
Trombulak SC (1989) Running speed and body mass in belding’s ground squirrels. J Mammal 70:194–197. https://doi.org/10.2307/1381688
Turbill C, Bieber C, Ruf T (2011) Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc R Soc B 278:3355–3363. https://doi.org/10.1098/rspb.2011.0190
von Vietinghoff - Riesch A (1960) Der Siebenschlaefer (Glis glis L.). Fischer, Jena
Walton JB, Andrews JF (1981) Torpor induced by food deprivation in the Wood mouse Apodemus sylvaticus. J Zool 194:260–263. https://doi.org/10.1111/j.1469-7998.1981.tb05773.x
Wilz M (1997) Hibernation, Aestivation und täglicher Torpor beim Siebenschläfer (Glis glis L.). PhD Thesis, Philipps-Universität Marburg
Wilz M, Heldmaier G (2000) Comparison of hibernation, estivation and daily torpor in the edible dormouse, Glis glis. J Comp Physiol B 170:511–521. https://doi.org/10.1007/s003600000129
Wolff JO (1996) Population fluctuations of mast-eating rodents are correlated with production of acorns. J Mammal 77:850–856. https://doi.org/10.2307/1382690
Acknowledgements
We thank S. Bigalk, S. Block, J. Flügge, C. Franke, F. Hofmann K. Pilsl, L. Kastner, E. Keppner, J. Saar, and P. Schütz for support in the field. We further thank M. Tschapka for providing additional OxBoxes. James Turner, Thomas Ruf, and Claudia Bieber provided valuable comments on an earlier draft of this paper. The Forestry Office of Nagold kindly provided details on mast phenology. Financial support was provided by the Deutsche Forschungsgemeinschaft (FI 831/3-1; FI 831/5-1 and FI 831/6-1), the Margarete von Wrangell Programme, the German Wildlife Foundation and the Deutsche Bundesstiftung Umwelt all to JF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Our studies were conducted under license from the Nature Conservancy (Permit Numbers: 55-6/8852.15 and 55-6/8852.15-1) and the Committee on the Ethics of Animal Experiments of the Regional Commission of Tübingen (Permit Numbers: 892 and HOH 25/13).
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Langer, F., Havenstein, N. & Fietz, J. Flexibility is the key: metabolic and thermoregulatory behaviour in a small endotherm. J Comp Physiol B 188, 553–563 (2018). https://doi.org/10.1007/s00360-017-1140-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1140-3