Abstract
Background
The objective of this study was to compare the three most prominent systems for stereotactic radiosurgery in terms of dosimetric characteristics: the Cyberknife system, the Gamma Knife Perfexion and the Novalis system.
Methods
Ten patients treated for recurrent grade I meningioma after surgery using the Cyberknife system were identified; the Cyberknife contours were exported and comparative treatment plans were generated for the Novalis system and Gamma Knife Perfexion. Dosimetric values were compared with respect to coverage, conformity index (CI), gradient index (GI) and beam-on time (BOT).
Results
All three systems showed comparable results in terms of coverage. The Gamma Knife and the Cyberknife system showed significantly higher levels of conformity than the Novalis system (Cyberknife vs Novalis, p = 0.002; Gamma Knife vs Novalis, p = 0.002). The Gamma Knife showed significantly steeper gradients compared with the Novalis and the Cyberknife system (Gamma Knife vs Novalis, p = 0.014; Gamma Knife vs Cyberknife, p = 0.002) and significantly longer beam-on times than the other two systems (BOT = 66 ± 21.3 min, Gamma Knife vs Novalis, p = 0.002; Gamma Knife vs Cyberknife, p = 0.002).
Conclusions
The multiple focal entry systems (Gamma Knife and Cyberknife) achieve higher conformity than the Novalis system. The Gamma Knife delivers the steepest dose gradient of all examined systems. However, the Gamma Knife is known to require long beam-on times, and despite worse dose gradients, LINAC-based systems (Novalis and Cyberknife) offer image verification at the time of treatment delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lars Leksell defined radiosurgery as “a single high-dose fraction of radiation, stereotactically directed to an intracranial region of interest” [5]. A high ablative dose is applied to the lesion of interest; thus, a sharp dose fall-off is needed to spare the healthy surrounding tissue.
Meningiomas are the most common benign intracranial tumours, most of which are slow-growing grade I tumours [3]. Stereotactic radiotherapy (SRT) or stereotactic radiosurgery (SRS) is recommended after incomplete resection, or upon recurrence [8].
Three widely used systems for SRS are the Leksell Gamma Knife (Elekta, Stockholm, Sweden), the Cyberknife (Accuray, Sunnyvale, CA, USA) and the Novalis (BrainLAB, Feldkirchen, Germany). Each system uses different methods of dose delivery.
The Cyberknife is a robot-controlled 6-MV linear accelerator (LINAC) with non-isocentric cone beams [1]. Novalis is a linear accelerator with a micro-multileaf collimator. It uses isocentric, fixed intensity and modulated or dynamically shaped, arc beams to deliver intensity-modulated radiotherapy (DMLC IMRT) [2]. Both systems are equipped with dedicated image-guiding components, as well as robotic treatment tables. The Gamma Knife Perfexion uses multiple isocentres, created by numerous collimated beams of different sizes, coming from 192 individual cobalt-60 sources converging to a single point, called the isocenter [5].
Even though all three systems are used in well-defined small and mid-sized localised tumours in daily practice, only a few studies have presented a dosimetric comparison for the Cyberknife and Novalis system; until now, most of the available dosimetric data have evaluated Gamma Knife treatment in brain metastases. A dosimetric comparison was already performed for arteriovenous malformation and vestibular schwannoma, representing regular and irregular shapes, respectively [4]. Meningiomas are another benign lesion, which is a good candidate for SRS treatments. This study focuses on benign meningiomas, which are usually challenging targets, due to their irregular shape. In addition, dosimetric indices of radiosurgery plans for benign lesions are even more relevant with regard to long-term toxicity as patients with meningioma usually have a long life expectancy.
We identified patients who had been treated for histologically proven grade I meningioma using the Cyberknife system, and designed comparative treatment plans for them using the Novalis system and Gamma Knife Perfexion. Results were compared with respect to coverage, conformity, dose gradient and beam-on time.
Methods
The Cyberknife system
The CyberKnife VSI radiosurgery system (Accuray) combines a compact 6-MV linear accelerator (1,000 MU/min LINAC), mounted on a computer-controlled six-axis robotic manipulator, with a robotic treatment couch. The treatment table can move in three translational and three rotational directions. The system contains a high-resolution image-guided tracking system, consisting of a pair of orthogonally positioned X-ray sources and detectors that acquire images during treatment at given time intervals. The images are registered with previously generated projection images derived from the planning CT volume data set. The online-detected offsets from the patient’s planning position are used to reposition the LINAC automatically.
The therapeutic dose was prescribed to 70 % of the isodose line, which completely encircled the contrast-enhanced macroscopic tumour. This prescription mode allowed some dose heterogeneity in the tumour with the maximum dose set to 100 %. However, as a result of inherent physical properties of the radiosurgery system, the steepest dose gradient outside the target against the surrounding healthy tissue can be reached if the dose prescription lies around the 70 % isodose line.
The Gamma Knife Perfexion
The Leksell Gamma Knife Perfexion (Elekta) has been described previously [4] and is currently the newest version of the Gamma Knife, with a new beam geometry in comparison to previous models. However, the dose profile is comparable to the previous versions [6]. The system uses 192 cobalt-60 sources that are arranged in a cone section configuration, encompassing five rings. This arrangement results in a different source-to-focus distance for each ring. One large 12-cm-thick tungsten collimator array ring of three different sizes (4, 8 and 16 mm) is divided into eight sectors, each containing 72 collimators (24 collimators for each size). Moving 24 sources over the selected collimator set, modifies the beam size for each sector. Each sector containing 24 sources can be moved into five different positions: home position, 4, 8 or 16 mm collimator size position, or off position, providing blockage of all 24 sources. Each shot consists of gamma beams from the eight sectors, and each sector has 4-, 8- or 16-mm collimation or complete beam blocking. There are 3 options for treatment planning: (1) one collimator size for all eight sectors (equivalent to the classical approach); (2) composite shots where any of the eight sectors can have a 4-, 8- or 16-mm collimator or a completely blocked collimator, and (3) the use of dynamic shaping where certain sectors are automatically blocked to protect critical structures. Forward planning is applied for this system with multiple shots and different collimators if necessary to shape the prescription isodose (usually 50 %) to conform to the lesions.
Novalis system
The Novalis system (BrainLAB) is a LINAC with beam shaping capability, build-in micro-multileaf collimator, and image guidance. Employing the Novalis ExacTrac image-guided frameless system the patient can be controlled via imaging at any couch position using a frameless positioning array. To generate Novalis treatment plans, the DICOM structure sets were exported to the Novalis treatment planning system (iPlan image v. 4.1.1; BrainLAB). The dose was prescribed to a reference point at the isocentre of the GTV with the 70 % isodose line encompassing the target volume.
In our institution, Novalis planning is either performed using micro-multileaf intensity-modulated beams (DMLC IMRT) or by using Dynamic Conformal Arcs (DCA). The physicists in the planning team were free to choose either option for each patient in order to achieve the best but still realistic plan in terms of applicability and treatment time.
Patient population
Ten patients with meningioma, who had been treated using the Cyberknife system, were randomly selected from our pool of previously treated patients (Table 1). All patients suffered from a single, histologically proven grade I meningioma, which had recurred after prior surgery, with a mean and median volume of 6.26 and 6.21 cm3 respectively (ranging between 1.79 and 11.05 cm3). After delineation, the CT images as well as the contours represented by their related DICOM RadioTherapy Structure Sets were exported to the Gamma Knife planning system (GammaPlan v. 9.0; Elekta) and to the NTx planning system (iPlan dose v.4.5; BrainLAB); in this way the same contours were used for all three planning systems. All targets had been delineated by a dedicated neurosurgeon experienced in radiosurgery on the planning computed tomography (CT) scans and fused magnetic resonance imaging (MRI) scans using the Multiplan 4.5 (Accuray) planning workstation. Cyberknife and Novalis planning and optimisation was performed by Diana Pasemann, Gamma Knife planning and optimisation was performed by Thierry Gevaert. Both physicists have several years of experience in planning on the respective systems.
The mean prescription dose was 14.7 Gy, ranging between 14 and 15 Gy, the same prescription doses were used in all three planning systems.
Plan comparison
Target coverage, conformity index (CI), gradient index (GI) and beam-on time of the different plans were calculated.
Coverage is defined as the target volume (TV) covered by the prescription isodose volume (PIV).
The Paddick CI is defined as [9]:
where TVPIV is the TV covered by the PIV. Thus, in a perfect plan, CI would be equal to 1. A CI <1 can point to either over-treatment (thus impairment of the adjacent tissue) or under-treatment of the TV.
The GI is defined as [10]:
Where PIVhalf is the PIV at half the prescription isodose, and GI is 1.00 in a perfect plan. The lower the GI, the more adjacent tissue is harmed.
Statistical analysis
Statistics were performed using GraphPad Prism 6.02 (GraphPad Software, CA, USA). Coverage, CI and GI were compared using the Wilcoxon signed-rank test. A p value of less than 0.05 was considered as statistically significant.
Results
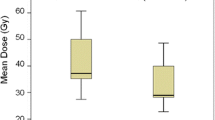
In Fig. 1, we summarise the coverage, beam-on time and the examined indices for the Gamma Knife Perfexion, Cyberknife and Novalis systems.
Dosimetric indices for meningiomas treated using the Cyberknife and the Novalis system (n = 10 per group, mean ± SD). a Coverage, b conformity index (CI), c gradient index (GI), d beam-on time; *p < 0.05, **p < 0.01 in the Wilcoxon signed rank test. In a perfect plan, the coverage is 100 %, the CI is 1 and the GI is 1
The Gamma Knife showed a high level of conformity (CI = 0.77 ± 0.06) and good coverage (coverage = 99.4 ± 1.29 %), as well as long beam-on times. The CI of Gamma Knife plans was significantly higher than the CI of the Novalis plans (Gamma Knife vs Novalis, p = 0.002). The median number of isocentres in the Gamma Knife plans was 26.5 ± 5. Outside the TV, the Gamma Knife showed low doses in the surrounding healthy tissues (GI = 2.71 ± 0.18, Gamma Knife vs Novalis, p = 0.014; Gamma Knife vs Cyberknife, p = 0.002). Beam-on times were significantly longer than with the Cyberknife and Novalis systems (BOT = 66 ± 21.3 min; Gamma Knife vs Novalis, p = 0.002; Gamma Knife vs Cyberknife, p = 0.002).
The Cyberknife system achieved a level of conformity (CI = 0.76 ± 0.07) and coverage (coverage = 99.3 ± 1.29) comparable to the Gamma Knife and higher than the Novalis system. Low doses in the surrounding healthy tissue, represented by the GI, were higher than in the Gamma Knife and comparable to the doses of the Novalis system (GI = 3.38 ± 0.06; Gamma Knife vs Cyberknife, p = 0.002).
The LINAC-based Novalis system uses micro-multileaf collimation for DMLC IMRT-based or DCA-based radiotherapy. The planning physicist was free to choose either technique to achieve the best results. In eight cases, the physicist chose DMLC IMRT (median amount of beams = 10 ± 1.39), in one case DCA was chosen (three arcs) and in another, a combination of both was chosen (three arcs and eight beams).
The Novalis system achieved good coverage (coverage = 99.3 ± 0.69), but a lower level of conformity than the Cyberknife and the Gamma Knife (CI = 0.66 ± 0.07; Cyberknife vs Novalis, p = 0.002; Gamma Knife vs Novalis, p = 0.002). The low doses in the surrounding healthy tissue were significantly higher than with the Gamma Knife and comparable to the doses of Cyberknife (GI = 3.51 ± 0.85; Gamma Knife vs Novalis, p = 0.014).
Discussion
The current study was performed to evaluate coverage, dose conformity and the GI as a surrogate for healthy tissue sparing around the target of three widely used stereotactic radiosurgery systems: the Gamma Knife, Cyberknife and Novalis systems. Until now, only one study has compared dosimetric values in all three systems [4].
It is obvious that it is impossible to find the perfect plan for a given patient, both for the Cyberknife and Novalis systems, as well as for the Gamma Knife, since there is a plethora of planning options and decisions depend on the goal defined by the responsible physicians and physicists.
In order to rule out the possibility of “tuned-up dose calculations” and to ensure an unbiased, and practice-driven comparison, planners for the different systems had to design the treatment plan within an acceptable time frame, comparable to clinical everyday planning in the different centres.
In radiosurgery, the total dose is usually applied in one session. Therefore, one of the major aims in radiosurgical planning is a high level of conformity and a steep dose gradient, in order to spare the surrounding healthy tissue, and organs at risk (OARs) in close proximity.
In this study, the Gamma Knife system showed the highest overall conformity and the steepest dose gradient with respect to the GI as surrogate parameter.
The Perfexion model is a redesigned Gamma Knife system, where the collimator arrangement is automated, based on eight robot-controlled sectors. The possibility to use the so-called composite shots with different collimator values, in different sectors, made the Gamma Knife Perfexion a multi-isocentre system with very conformal dose distribution at the cost of long beam-on times compared with the Novalis and the Cyberknife [4]. However, if not well optimised, the inflationary usage of composite shots can lead to cold or hot spots in the target, as well as to worse dose gradients [11].
The LINAC-based Novalis system uses a micro-multileaf collimation for DMLC IMRT-based or DCA-based radiotherapy.
The planning physicist was free to choose either technique in order to achieve the best result as long as planning time and estimated treatment time were comparable to everyday clinical practice. The Novalis system showed significantly worse conformity than the Cyberknife and the Gamma Knife, and a significantly flatter dose gradient than the Gamma Knife, which is in accordance with the findings of Gevaert et al. [4].
The Cyberknife system uses a different LINAC-based approach, where the lesion is irradiated with non-isocentric non-coplanar circular beams from up to 1,600 targeting angles.
We have shown that this approach of the Cyberknife system can achieve conformity comparable to the Gamma Knife and better than the Novalis system. This is in accordance with findings of Gevaert et al. [4], who showed better conformity for the Cyberknife system than the Novalis system in arterio-venous malformations and acoustic neuromas. One argument in favour of the Cyberknife, in comparison to the Gamma Knife, is that the beam-on time of the Cyberknife system is significantly shorter than that of the Gamma Knife [4]. However, total treatment time may also depend on other parameters related to image-verification and other installation procedures that are mandatory in the treatment process. Unlike the Gamma Knife, the Cyberknife can deliver verification images at the time of treatment. However, when performing invasive frame-based SRS procedures, real-time imaging might not be needed. It must also be mentioned that in the future the Gamma Knife Perfexion Plus will include a cone-beam CT system for frameless procedures. In this study the Gamma Knife Perfexion delivers steeper dose gradients and, therefore, may spare OARs in close proximity to the target better than the Cyberknife. There was no significant difference between the GI for the Cyberknife and the Novalis system. This is in agreement with Ma et al. [7], who showed comparable GIs for the Cyberknife and the Novalis system for intracranial lesions.
Conclusions
Our study showed that all evaluated systems—the Gamma Knife Perfexion, Cyberknife and Novalis systems—offer meningioma-treatment plans with excellent coverage.
The Cyberknife and Gamma Knife deliver plans with higher conformity than the Novalis system. While the Gamma Knife shows the steepest dose gradient, the Novalis and Cyberknife systems offer live imaging, and the radiosurgery can be performed in a shorter time. However, the impact of these plan quality parameters on the clinical outcome especially with regard to organs at risk needs further investigation and was not aim of this plan comparison study. In conclusion, the decision which treatment system would be more appropriate in a certain patient is case dependent.
References
Adler JR Jr, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL (1997) The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg 69:124–128
Chen JC, Girvigian M, Greathouse H, Miller M, Rahimian J (2004) Treatment of trigeminal neuralgia with linear accelerator radiosurgery: initial results. J Neurosurg 101(Suppl 3):346–350
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57:1088–1095, discussion 1088–1095
Gevaert T, Levivier M, Lacornerie T, Verellen D, Engels B, Reynaert N, Tournel K, Duchateau M, Reynders T, Depuydt T, Collen C, Lartigau E, De Ridder M (2013) Dosimetric comparison of different treatment modalities for stereotactic radiosurgery of arteriovenous malformations and acoustic neuromas. Radiother Oncol 106:192–197
Leksell L (1951) The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 102:316–319
Lindquist C, Paddick I (2007) The Leksell Gamma Knife Perfexion and comparisons with its predecessors. Neurosurgery 61:130–140, discussion 140–131
Ma L, Sahgal A, Descovich M, Cho YB, Chuang C, Huang K, Laperriere NJ, Shrieve DC, Larson DA (2010) Equivalence in dose fall-off for isocentric and nonisocentric intracranial treatment modalities and its impact on dose fractionation schemes. Int J Radiat Oncol Biol Phys 76:943–948
Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL (1985) Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 62:18–24
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 93(Suppl 3):219–222
Paddick I, Lippitz B (2006) A simple dose gradient measurement tool to complement the conformity index. J Neurosurg 105(Suppl):194–201
Petti PL, Larson DA, Kunwar S (2008) Use of hybrid shots in planning Perfexion Gamma Knife treatments for lesions close to critical structures. J Neurosurg 109(Suppl):34–40
Conflicts of interest
V. Budach and M. Kufeld received travel and speech grants from Accuray Inc.
The other authors confirm that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
This article is the latest in a series of papers comparing different devices for stereotactic radiosurgery. The obvious aim of such papers is to see which one is “best”, perhaps with the aim of making purchase of these machines more attractive, or indeed for patients or referring doctors to choose one or other department for treatment.
Most of these papers suffer from the weakness that there is no simple answer to this question: the different comparison parameters favour one or another technology. This leaves the reader somewhat uncertain how to interpret the findings.
It is a strength of this paper that they calculated the necessary beam-on-time, which is not often shown in previous papers. This is a relevant feature for the delivery of the treatment on the day. Of course it is only part of the whole process, as the planning time is also vastly different (according to the information available to me it is the fastest using GammaPlan, the software utilised for Gamma Knife treatments. There is recent evidence to show that the biological equivalent dose (BED) depends on the length of time the radiation treatment is delivered, because of the fast component of cell repair that is in operation even during the treatment. As this factor is so dramatically different in the three technologies, experience with one machine (most clinical data are published with the Gamma Knife), cannot be automatically utilised for the faster LINAC based machines.
Andras Kemeny
Sheffield, UK
Hopewell JW, Millar WT, Lindquist C (2012) Radiobiological principles: their application to γ knife therapy. Prog Neurol Surg 25:39-54
David Kaul and Harun Badakhshi contributed equally to this study.
Rights and permissions
About this article
Cite this article
Kaul, D., Badakhshi, H., Gevaert, T. et al. Dosimetric comparison of different treatment modalities for stereotactic radiosurgery of meningioma. Acta Neurochir 157, 559–564 (2015). https://doi.org/10.1007/s00701-014-2272-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2272-9