Abstract
Purpose
The purpose of the present study was to evaluate the clinical outcome of CT-guided high-dose-rate brachytherapy (CT-HDRBT) in patients with unresectable hepatocellular carcinoma (HCC).
Patients and methods
Over a 6-year period, 98 patients with 212 unresectable HCC underwent CT-HDRBT applying a 192Ir source at our institution. Magnetic resonance imaging (MRI) follow-up was performed 6 weeks after the intervention and then every 3 months. The primary endpoint was local tumor control (LTC); secondary endpoints included progression-free survival (PFS) and overall survival (OS).
Results
Patients were available for MRI evaluation for a mean follow-up of 23.1 months (range 4–64 months; median 20 months). Mean tumor diameter was 5 cm (range 1.8–12 cm). Eighteen of 212 (8.5 %) tumors showed local progression after a mean LTC of 21.1 months. In all, 67 patients (68.4 %) experienced distant tumor progression. The mean PFS was 15.2 months. Forty-six patients died during the follow-up period. Median OS was 29.2 months. Actuarial 1-, 2-, and 3-year OS rates were 80, 62, and 46 %, respectively.
Conclusion
CT-HDRBT is an effective therapy to attain local tumor control in patients with unresectable HCC. Prospective randomized studies comparing CT-HDRBT with the standard treatments like Radiofrequency ablation (RFA) and chemoembolization (TACE) are mandatory.
Zusammenfassung
Ziel
Zweck der vorliegenden Arbeit war die Analyse der klinischen Effektivität der CT-gesteuerten Hochdosis-Brachytherapie (CT-HDRBT) bei Patienten mit inoperablem hepatozellulären Karzinom (HCC).
Patienten und Methoden
Über einen Zeitraum von 6 Jahren, wurden an unserer Klinik 98 Patienten mit 212 inoperablen HCC mittels CT-HDRBT mit 192Ir behandelt. MRT-Verlaufskontrollen erfolgten 6 Wochen nach der Intervention und dann alle 3 Monate. Primärer Endpunkt der Studie war die lokale Tumorkontrolle (LTC); sekundäre Endpunkte waren das progressionsfreie Überleben (PFS) und Gesamtüberleben (OS).
Ergebnisse
Die mittlere Nachbeobachtungszeit betrug 23,1 Monate (Spanne 4–64 Monate, Median 20 Monate). Der mittlere Tumordurchmesser betrug 5 cm (Spanne 1,8–12 cm). Nach einer mittleren LTC von 21,1 Monaten zeigten 18 von 212 Tumoren (8,5 %) eine lokale Progression. Im weiteren Verlauf schritt die Tumorerkrankung bei 67 Patienten (68,4 %)in Form eines nichtlokalen Tumorprogress voran. Das mittlere PFS betrug 15,2 Monate. Während der Nachbeobachtungszeit verstarben 46 Patienten. Das mediane OS betrug 29,2 Monate. Die 1-Jahres-, 2-Jahres- und 3-Jahres-OS-Raten waren 80, 62 und 46 %.
Schlussfolgerung
Die CT-HDRBT ist eine effektive Therapie zur lokalen Kontrolle des Tumors bei Patienten mit inoperablem HCC. Vergleichende prospektive, randomisierte Studien gegenüber den Standardtherapien, wie Radiofrequenzablation (RFA) und Chemoembolisation (TACE) werden benötigt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and the second most frequent cause of cancer-related death worldwide [1]. According to the Barcelona Clinic Liver Cancer (BCLC) staging system, patients with ‘very early’ and ‘early’ stage HCC can benefit from curative treatments including resection, ablation, and transplantation and potentially achieve long-term cure [2]. Radiofrequency ablation (RFA) is the most commonly used ablative technique and, because of its high effectiveness (nearly 100 %) in small (≤ 3 cm) HCC it has been advocated as a first-line curative therapy for small HCC [3]. However, despite its high efficacy in small tumors, RFA is still limited by a number of tumor factors including size, location, and vascularization [4]. Furthermore, although an increasing proportion of patients are now diagnosed at an earlier stage, thanks to improved surveillance, most patients have more advanced HCC at the time of diagnosis, and curative treatment is not a viable option for them [1]. Current guidelines recommend transarterial chemoembolization (TACE) as the main treatment strategy for patients with unresectable large or multifocal HCC (BCLC B) without portal vein invasion or lymph node or systemic metastasis [3, 5, 6]. Despite extensive clinical use of TACE worldwide, in recent years some authors have reignited the debate about the scientific evidence behind the use of TACE, advocating the need for studies investigating the efficacy and safety of new, alternative treatment strategies for patients with unresectable HCC [7].
Computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) is a minimally invasive, radioablative technique that has shown promising results in the management of primary and secondary liver tumors, including tumors not amenable to thermal ablation [8]. The purpose of the present study was to evaluate the use of CT-HDRBT as an alternative therapy for patients with unresectable HCC.
Patients and methods
Study population
Data from all consecutive patients with unresectable HCC treated with CT-HDRBT at our institution between January 2007 and January 2013 were retrospectively reviewed. All patients were discussed by the institutional interdisciplinary tumor board, which considered potential alternative therapies and established the indication for CT-HDRBT. Our institutional inclusion criteria for CT-HDRBT include the following: (1) liver function status in Child–Pugh class A or B, (2) total serum bilirubin < 2 mg/dl, (3) platelet count > 50,000/nl, (4) prothrombin time (PT) > 50 %, and (5) partial thromboplastin time (PTT) < 50 s. If needed, hemostatic function was corrected (e.g., platelet concentrates) and ascites were drained before intervention. Exclusion criteria were (1) evidence of progressive extrahepatic disease and (2) more than five HCCs. No upper limit was placed upon (largest) tumor diameter. Written informed consent was obtained from each patient before the procedure. The institutional review board approved the present retrospective study.
Treatment planning and interventional technique
To evaluate technical feasibility, a contrast-enhanced magnetic resonance imaging (MRI) examination of the liver using gadoxetic acid (Gd-EOB-DTPA, Primovist®; Bayer, Berlin, Germany) was performed on the day before the procedure. Interventional technique and treatment delivery have been described in previous reports [9]. In brief, afterloading catheters (Primed™ʼ; Halberstadt Medizintechnik GmbH, Halberstadt, Germany) were inserted into the tumor by Seldinger’s technique under CT-fluoroscopy guidance. After catheter placement, a contrast-enhanced CT scan of the liver was acquired to confirm correct positioning of the catheter and to plan irradiation. Computer-based 3D treatment planning was performed on a dedicated workstation using the acquired data set and the Brachyvision™ afterloading planning software (Gammamed™; Varian, Palo Alto, CA, USA). Irradiations were performed as single-fraction irradiations in afterloading technique using an iridium-192 radiation source (VARIAN GammaMed Plus iX HDR; Varian, Palo Alto, CA, USA) with a nominal activity of 10 Ci. The minimum dose to cover the clinical target volume (CTV) was 20 Gy. Maximum doses above 50 Gy were allowed at the tumor center.
To prevent radiogenic complications the irradiation was so designed that the volume of the liver that was irradiated with ≥ 10 Gy did not exceed one third of the total liver volume. The maximum radiation dose at duodenal mucosa and hilar structures did not exceed 12 and 18 Gy per 1 ml (D1 ml), respectively. In case exposure of the gastric wall or duodenal mucosa exceeded the critical dose of 10 Gy per mm3 of the risk organ, proton pump inhibitors were prescribed (pantoprazole 40 mg) for 6 weeks. Secondary bleeding after removal of the brachytherapy catheter was prevented by insertion of a torpedo-shaped gelatin sponge (Gelfoam; Pfizer Inc., New York, NY, USA).
Follow-up after CT-HDRBT
Contrast-enhanced MRI using gadoxetic acid was performed before and 6 weeks after treatment and then at 3-month intervals to evaluate tumor response to CT-HDRBT. Complete tumor enclosure with maximum symmetric increase of the largest lesion diameter < 25 % compared with baseline or with absence of asymmetric lesion growth at any time during follow-up was defined as local tumor control (LTC) [10]. The absence of patient’s death, new or enlarging intrahepatic lesions, extrahepatic tumor progression, or local progression after CT-HDRBT was defined as progression-free survival (PFS).
Complications and toxicity
Complications of CT-HDRBT were defined according to the guidelines of the Society of Interventional Radiology (SIR) [11]. Liver toxicity after CT-HDRBT was defined according to the accepted definition of radiation-induced liver disease (RILD) and radioembolization-induced liver disease (REILD), characterized by the appearance of ascites accompanied by elevated alkaline phosphatase levels or by a serum total bilirubin level of 3 mg/dl or higher and ascites 1–2 months after CT-HDRBT in the absence of tumor progression or bile duct obstruction, respectively [12].
Statistical methods
Baseline characteristics were presented by using descriptive statistics. Local tumor control (LTC), progression-free survival (PFS), and overall survival (OS) probability were calculated by Kaplan–Meier analysis. Prognostic factors for OS were evaluated at the univariate level by the log-rank test. All tests were 2-sided and performed using SPSS (Superior Performance Software System Version 20, IBM North America, New York, NY, USA). A p value below 0.05 defined statistical significance. The potential prognostic factors analyzed were age (≤ 65 or > 65 years), etiologic cause of cirrhosis (hepatitis or alcohol), degree of liver dysfunction (Child–Pugh class A or B), diameter of the largest tumor (< 5 or ≥ 5 cm), disease pattern (uninodular or multinodular tumor) and minimal tumor enclosing dose (< 20 or ≥ 20 Gy).
Results
Patients and tumors
Over the 6-year interval, 98 patients underwent CT-HDRBT at our institution. The demographic data of the patients are summarized in Table 1. A total of 192 tumors were treated with a single CT-HDRBT session. Eleven very large tumors were treated in two sessions 6 weeks apart, with the CTV divided into two regions (inferior and superior). The number of catheters used varied with the tumor size and configuration. On average one catheter was used for every 48 ml of CTV. A maximum of five catheters were used in four very large HCC (> 8 cm). The mean minimum tumor-enclosing dose was 16.51 Gy [standard deviation (SD) 2.64]. The mean CTV was 62.19 ml (SD 80.78), and the mean coverage was 98.20 % (SD 4.23) of the CTV.
Follow-up and complications
The mean follow-up period for the 98 patients was 23.1 months (range 4–64 months, median 20 months). No major complications were recorded in the first 30 days after CT-HDRBT. None of the patients developed a RILD or REILD. One patient showed a discrete subcapsular liver hematoma at the routine postinterventional ultrasound examination. The hematoma resolved spontaneously with no need for additional therapy or prolonged hospital stay. One patient died 1 week after CT-HDRBT owing to massive basal ganglia hemorrhage.
Local tumor control and disease progression
During the follow-up period, a total of 18 of 212 (8.5 %) tumors treated with CT-HDRBT showed local tumor progression after a mean LTC of 21.1 months (Fig. 1). Six (33 %) of the 18 local tumor progressions were seen in patients with tumors greater than 5 cm in diameter; the remaining 12 (67 %) local tumor progressions occurred in patients with tumors less than 5 cm in diameter. Nine of the 18 local progressions were treated by repeated CT-HDRBT; all of these displayed persistent local control during the follow-up period. Of the remaining nine local tumor progressions, two were treated by TACE and three by radioembolization, while systemic chemotherapy was used in 4 patients who had local progression and concomitant systemic tumor progression. Figure 2 presents a representative course in a patient with persistent local tumor control. In all, 67 patients (68.4 %) experienced distant tumor progression in form of new or enlarging nonablated intrahepatic HCCs or extrahepatic tumor progression. The mean PFS was 15.2 months (range 1–64 months).
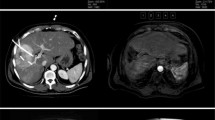
Example of hepatocellular carcinoma (HCC) shrinkage and persistent tumor control. Images are from a 73-year-old patient with histologically proven HCC and multiple comorbidities. a Preoperative axial Gd-EOB-DTPA-enhanced liver MRI shows an 11-cm, centrally located HCC. b The tumor was treated with two sessions of CT-guided high-dose-rate brachytherapy (CT-HDRBT) using a total of five catheters. c–f Follow-up Gd-EOB-DTPA-enhanced liver MRI taken 12 (c), 24 (d), 36 (e), and 60 (f) months after CT-HDRBT shows progressive shrinkage of the HCC with persistent local tumor control
Overall survival
Contact with 12 of 98 (12.2 %) patients was lost during follow-up (mean follow-up time, 14.8 months) and these patients were therefore excluded from survival analysis. Another 2 patients received CT-HDBRT to bridge the time to liver transplantation and were therefore also excluded (Fig. 3). The remaining 84 patients were included in the survival analysis; 41 patients (48.8 %) were alive at the time of last follow-up. The median OS was 29.2 months (Fig. 4). Actuarial 1-, 2-, and 3-year OS rates were 80, 62, and 46 %, respectively.
Histological investigation. Patient who underwent CT-guided high-dose-rate brachytherapy (CT-HDRBT) of two hepatocellular carcinoma (HCC) lesions 3 weeks (liver segment VI) and 7 months (liver segment VII) before liver transplantation (a, b). Histology of the tumor in liver segment VI 3 weeks after CT-HDRBT shows moderately differentiated HCC with no significant cellular changes (c). Histology of the tumor in liver segment VII, treated 7 months before liver transplantation shows total tumor cell necrosis (d)
Prognostic factors
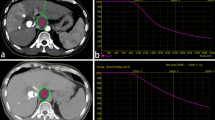
The influence of the various patient-, tumor- and therapy-related factors on OS is shown in Table 2. Survival was not affected by the patients’ age, gender, or disease pattern. The diameter of the largest lesion did not seem to influence OS after CT-HDRBT (Fig. 5). Patients with better liver function according to the Child–Pugh classification showed better OS, albeit not statistically significantly. Patients with viral hepatitis as cause of cirrhosis demonstrated a trend to better prognosis compared with patients who had alcoholic cirrhosis, and so did patients treated with a minimum tumor-enclosing dose ≥ 20 Gy compared with patients treated with a minimum tumor-enclosing dose < 20 Gy.
Overall survival by tumor diameter. Survival curves of patients with unresectable hepatocellular carcinoma (HCC) treated with percutaneous CT-guided high-dose-rate brachytherapy according to maximum tumor diameter. Survival of patients with HCC diameter ≤ 5 cm was not significantly better than that of patients with HCC > 5 cm in diameter
Discussion
TACE is recommended as the first-line noncurative therapy for patients with large or multifocal disease (BCLC B) [2]. Current recommendations for TACE as the standard of care for unresectable HCC are based on the demonstration of improved survival compared with best supportive care or suboptimal therapies in a meta-analysis of six randomized controlled trials published in the early 2000s [13]. However, only two of these six trials demonstrated a statistically significant improvement compared with conservative management [5, 6]. In the past decade, a variety of prospective and retrospective studies have been published, providing evidence in support of TACE for the management of patients with unresectable HCC. Reported mean OS rates range from 3.4–48 months (median 14 months), reflecting the large heterogeneity in treatment modalities and patient selection [7]. A recently published meta-analysis by Oliveri et al. [14], with low risk of selection bias, concluded that to date there is no firm evidence to support or refute TACE or TAE for patients with unresectable HCC.
Hence, although TACE remains the only recommended first-line treatment for patients with intermediate-stage HCC, the debate on its effectiveness in all BCLC B patients is still open, and lately a panel of experts expressed the need for studies investigating the efficacy and safety of new, alternative treatment strategies for patients with bulky and/or multifocal unresectable HCC [7].
To date, alternative therapies for patients with unresectable and unablatable HCC have comprised yttrium-90 radioembolization (RE) and stereotactic body radiation therapy (SBRT). RE has shown promising antitumor effects and an acceptable safety profile: Salem et al. [15] recently reported similar results for TACE and RE in a comparative study which included 245 patients with HCC who were treated with RE (N = 123) or TACE (N = 122). RE resulted in less toxicity than TACE. However, the wider use of RE is limited by the cost of this procedure.
In the past, radiotherapy for HCC was limited by its potential for causing radiation-induced liver disease (RILD) [16]. SBRT, which allows the delivery of high doses of radiation in a short time to well-defined tumor sites, has emerged as an alternative treatment for patients with liver tumors. Early results with SBRT and other newer techniques are promising in terms of local control and toxicity [16–22]. Recently, Bujold et al. [23] reported a local control rate of 87 % after 1 year and a median overall survival 17.0 months in 102 patients with locally advanced HCC unsuitable for standard locoregional therapies treated with SBRT (median prescription dose of 36 Gy in six fractions).
CT-HDRBT is an alternative radioablative technique that was introduced and implemented in clinical practice almost 20 years ago. Thanks to intratumoral application of the probe, precise 3D radiation planning, and the rapid drop in dose outside the target tissue, CT-HDRBT allows the application of a very high radiation dose to the target volume in a single fraction (> 50 Gy in the central tumor parts with high risk for hypoxic, radioresistant cell clones), while at the same time sparing sensitive organs and structures outside the target tumor [8].
In the present study, we reviewed our institution’s experience with CT-HDRBT in the management of patients with unresectable HCC. To the best of our knowledge, our cohort of 98 patients with 212 tumors is the largest to date in the CT-HDRBT literature. The local tumor progression rate of 8.5 % in our series is very satisfactory; especially in light of the fact that the mean tumor diameter was greater than 5 cm and that the majority of patients had multifocal disease. The fact that only 6 of 18 (33 %) local tumor progressions were seen in the 42 patients (43 % of the overall patient cohort) with tumors greater than 5 cm in diameter and the fact that tumor diameter was not shown to influence survival after CT-HDRBT clearly proves the effectiveness of CT-HDRBT in the treatment of HCC larger than 5 cm, as suggested in earlier work by various groups [24–26]. The reported median PFS (15.2 months) and OS (29.2 months) in our patients are also encouraging; they compare favorably with the results obtained with other liver-directed therapies including TACE and RE. In our study, we were not able to identify prognostic factors influencing OS after CT-HDRBT. Although patients in Child–Pugh class A, patients treated with a minimal tumor-enclosing dose of 20 Gy and patients with liver cirrhosis due to viral hepatitis demonstrated a positive trend compared with patients in Child–Pugh class B, patients treated with a minimal tumor-enclosing dose of less than 20 Gy and patients with alcoholic liver cirrhosis, the differences were not statistically significant. Furthermore, and more importantly, tumor size did not show any relevant effect upon survival, as shown clearly by the analysis in Figure 5.
No major complications were encountered in the study patient cohort. One patient died 1 week after CT-HDRBT because of massive basal ganglia hemorrhage. Although the hemorrhage occurred within the first 30 post-interventional days, we could not establish any degree of causality between the CT-HDRBT and the fatal bleeding. None of the patients treated developed a RILD or a REILD after CT-HDRBT. RILD is a frightening complication following liver irradiation. Severe RILD is reported to occur in up to 18.5 % of patients following stereotactic body radiotherapy (SBRT) and in up to 13.3 % of patients following radioembolization (RE) [12, 27]. Although 4 patients showed a transient elevation of liver enzymes after CT-HDRBT, no cases of RILD/REILD could be diagnosed in our cohort of patients. This appears probably due to the lower volume of healthy liver that is irradiated in CT-HDRBT. In fact, thanks to intratumoral application of the radiation source, to precise 3D-radiation planning, and to the rapid drop in dose outside the target volume, CT-HDRBT allows the application of very high radiation doses to the target volume while at the same time sparing sensitive organs, including healthy liver.
Our results are consistent with those reported by another group, which published the first study about the management of unresectable HCC by means of CT-HDRBT: Mohnike et al. [28] reported a mean PFS of 10.4 months and a median OS of 19.4 months, which were significantly higher compared with a historical control group in a retrospective matched-pair analysis. Several limitations to this study should be mentioned: it is a single-institutional retrospective study with a relatively small and inhomogeneous sample size. Owing to that fact, and to the lack of long-term follow-up, our results must be interpreted with caution.
Conclusion
To our knowledge this is the largest hitherto published study to examine patients with unresectable HCC treated with CT-HDRBT. It adduces evidence in favor of the use of CT-HDRBT as a safe and effective therapy for patients with unresectable HCC, and it suggests that CT-HDRBT can be considered a further alternative liver-directed therapy that complements existing options in a multimodality treatment regimen.
References
Maluccio M, Covey A (2012) Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin 62:394–399
Bolondi L, Burroughs A, Dufour JF et al (2012) Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 32:348–359
Forner A, Llovet JM, Bruix J (2010) Hepatocellular carcinoma. Lancet 379:1245–1255
Crocetti L, de Baere T, Lencioni R (2010) Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol 33:11–17
Llovet JM, Real MI, Montaña X et al (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359:1734–1739
Lo CM, Ngan H, Tso WK et al (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35:1164–1171
Raoul JL, Sangro B, Forner A et al (2011) Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 37:212–220
Ricke J, Wust P (2011) Computed tomography-guided brachytherapy for liver cancer. Semin Radiat Oncol 21:287–293
Ricke J, Wust P, Stohlmann A et al (2004) CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: phase I-II results of a novel technique. Int J Radiat Oncol Biol Phys 58:1496–1505
Ricke J, Thormann M, Ludewig M et al (2010) MR-guided liver tumor ablation employing open high-field 1.0 T MRI for image-guided brachytherapy. Eur Radiol 20:1985–1993
Goldberg SN, Grassi CJ, Cardella JF; For the Society of Interventional Radiology Technology Assessment Committee and the International Working Group on Image-guided Tumor Ablation (2009) Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 20:377–390
Gil-Alzugaray B, Chopitea A, Iňarrairaegui M et al (2013) Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 57:1078–1087
Llovet JM, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37:429–442
Oliveri RS, Wetterslev J, Gluud C (2011) Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev 16:CD004787
Salem R, Lewandowski RJ, Kulik L et al (2011) Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 140:497–507
Cárdenes HR (2009) Role of stereotactic body radiotherapy in the management of primary hepatocellular carcinoma. Rationale, technique and results. Clin Transl Oncol 11:276–283
Goodman KA, Wiegner EA, Maturen KE, Zhang Z et al (2010) Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 78:486–493
Méndez Romero A, Wunderink W, Hussain SM et al (2006) Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase i-ii study. Acta Oncol 45:831–837
Lee SU, Park JW, Kim TH et al (2014) Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol 190:806–814 [Epub ahead of print]
Wang PM, Hsu WC, Chung NN et al (2013) Radiotherapy with volumetric modulated arc therapy for hepatocellular carcinoma patients ineligible for surgery or ablative treatments. Strahlenther Onkol 189:301–307
Kim TH, Park JW, Kim YJ et al (2014) Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther Onkol 190:882–890 [Epub ahead of print]
Jereczek-Fossa BA, Bossi-Zanetti I, Mauro R et al (2013) CyberKnife robotic image-guided stereotactic radiotherapy for oligometastic cancer: a prospective evaluation of 95 patients/118 lesions. Strahlenther Onkol 189:448–455
Bujold A, Massey CA, Kim JJ et al (2013) Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 31:1631–1639
Ricke J, Wust P, Wieners G et al (2004) Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J Vasc Interv Radiol 15:1279–1286
Tselis N, Chatzikonstantinou G, Kolotas C et al (2012) Hypofractionated accelerated computed tomography-guided interstitial high-dose-rate brachytherapy for liver malignancies. Brachytherapy 11:507–514
Collettini F, Schnapauff D, Poellinger A et al (2012) Hepatocellular carcinoma: computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of large (5–7 cm) and very large (> 7 cm) tumours. Eur Radiol 22:1101–1109
Jung J, Yoon SM, Kim SY et al (2013) Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol 8:249
Mohnike K, Wieners G, Schwartz F et al (2010) Computed tomography-guided high-dose-rate brachytherapy in hepatocellular carcinoma: safety, efficacy, and effect on survival. Int J Radiat Oncol Biol Phys 78:172–179
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F. Collettini, N. Schreiber, D. Schnapauff, T. Denecke, P. Wust, E. Schott, B. Hamm, and B. Gebauerstate that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Rights and permissions
About this article
Cite this article
Collettini, F., Schreiber, N., Schnapauff, D. et al. CT-guided high-dose-rate brachytherapy of unresectable hepatocellular carcinoma. Strahlenther Onkol 191, 405–412 (2015). https://doi.org/10.1007/s00066-014-0781-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-014-0781-3