Abstract
Purpose
Hepatocellular carcinoma (HCC) in early stages benefits from local ablative treatments such as radiofrequency ablation (RFA) or transarterial chemoembolization (TACE). In this context, radiotherapy (RT) has shown promising results but has not been thoroughly evaluated. Magnetic resonance-guided RT (MRgRT) may represent a paradigm shifting improvement in stereotactic body radiotherapy (SBRT) for liver tumors.
Methods
We retrospectively evaluated HCC patients treated on a hybrid low-tesla MRgRT unit. A total biologically effective dose (BED) > 100 Gy was delivered in 5 consecutive fractions, respecting the appropriate organs-at-risk constraints. Hybrid MR scans were used for treatment planning and cine MR was used for delivery gating. Patients were followed up for toxicity and treatment–response assessment.
Results
Ten patients were enrolled, with a total of 12 lesions. All the lesions were irradiated with no interruptions. Six patients had already performed previous local therapies. Median follow-up after SBRT was 6.5 months (1–25). Two cases of acute toxicity were reported (G ≤ 2 according to CTCAE v4.0). At the time of the analysis, 90% of the population presented local control. Child–Pugh before and after treatment remained unchanged in all but one patient.
Conclusion
MRgRT is a feasible and safe option showing favorable toxicity profile for HCC treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the sixth-most diagnosed cancer and the fourth-most common cause of cancer-related death worldwide. Death rates increased by 43% between 2000 and 2016 in the United States, while a growing trend in Southern Europe and East Asia has been recently reported (Bertuccio et al. 2017).
Hepatocellular carcinoma (HCC) accounts for the large majority of primary liver cancers and usually occurs in patients affected by underlying chronic liver diseases. Its incidence increases with advancing age and with significant differences in its peak in the different geographical areas (El-Serag 2012).
Nevertheless, promising progresses have been made in the diagnosis and treatment of HCC in the last years.
Several systems have been developed for the classification and staging of HCC, considering disease characteristics, liver function, and patient performance status. The Barcelona Clinic Liver Cancer (BCLC) stage provides a prognostic prediction and treatment indication for each of the five identified stages and supports physicians in clinical decision-making (Kinoshita et al. 2015).
Patients belonging to the “very early” or “early” stages, particularly when not eligible for surgery, can benefit from ablative therapies, such as radiofrequency ablation (RFA) but also microwave ablation (MWA), percutaneous ethanol injection (PEI), and transarterial chemoembolization (TACE) (Murray and Dawson 2017).
The use of radiation therapy (RT) was historically limited due to the risk of radiation-induced liver disease (RILD) and the available evidence is scarce, so that the role of RT in HCC treatment is still far to be thoroughly explored and understood (European Association for the Study of the Liver 2018; Marrero et al. 2018).
In this context, RT treatment planning and delivery techniques have substantially improved in the last years; it is now possible to deliver high doses to well-visualized target volumes, monitoring their motion with confidence thanks to dedicated on-board imaging systems (i.e., stereotactic body radiation therapy—SBRT—with appropriate image guided radiotherapy—IGRT—solutions) and reducing the amount of unnecessarily irradiated normal liver tissue.
SBRT performances for HCC treatment have been prospectively and retrospectively evaluated, both in exclusive setting or coupled with other ablative procedures (e.g., TACE or RFA), showing good results in terms of local control and safety profile also in the cirrhotic liver (Culleton et al. 2014; Lasley et al. 2015; Wahl et al. 2016). Moreover, high-conformal high-dose rate (HDR) brachytherapy can also be considered an alternative in HCC patients with 0-A BCLC stage, having shown efficacy and favorable toxicity profile in several studies (Vogel et al. 2018; Hass et al. 2019).
The recent developments in the field of RT have opened new perspectives for the irradiation of liver volumes, significantly improving treatments’ quality and safety (Murray and Dawson 2017).
One of the most significant technical advancements is represented by MR-Linacs, RT hybrid delivery units that couple low- or high-tesla (0.35 or 1.5) on-board MR scanners with standard linear accelerators.
Compared to the current patients’ positioning imaging reference standard, represented by on-board cone beam computed tomography (CBCT), on-board MR imaging provides better anatomic target definition thanks to the higher soft tissue contrast and imaging characteristics.
Furthermore, the availability of continuous 2D cine MR images provides an accurate and reliable motion management solution, without the need for external markers or implanted fiducials, normally required in traditional irradiation techniques (Noel et al. 2015; Hunt et al. 2018).
Primary aim of this study is to assess the safety and feasibility of MR-guided Radiotherapy (MRgRT) for the treatment of HCC lesions.
Materials and methods
Patient selection
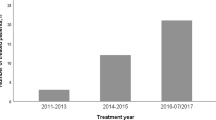
Patients affected by HCC and treated from February 2018 to July 2020 on a low-T MRgRT unit (MRIdian, ViewRay Inc, Mountain View, CA, USA) were retrospectively included in this study.
All patients underwent pathological diagnosis of the lesion and staging clinical imaging with dynamic multidetector computed tomography (MDCT) and/or MRI.
Disease was staged using BCLC and Child–Pugh classifications and all the patients were tested for viral hepatitis.
The therapeutic workflow was agreed after the discussion in the dedicated hepatobiliary malignancies multidisciplinary tumor board.
No restrictions about previous HCC ablative treatments (i.e., TACE, PEI, and RFA) have been applied for selecting the patients.
Poor performance status (ECOG ≥ 3) or clinical contraindication to MRI was instead considered absolute exclusion criteria (Boldrini et al. 2020).
All the patients were evaluated by the attending radiation oncologist to exclude the presence of absolute contraindications to RT and specific informed consent to MRgRT treatment was contextually obtained.
Acute and late toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 scale. Acute toxicity was evaluated during RT treatment and up to six months after the end of RT treatment. Late toxicity was evaluated six months after the end of RT treatment, during scheduled follow-up visits.
Treatment preparation
All the patients performed a 0.35 T MRI simulation on the MRIdian system.
Patients were immobilized in supine position with both arms above their head using dedicated immobilization device (Fluxboard™, MacroMedics, The Netherlands) in the most comfortable position. MRI coils were placed under and over the abdomen of the patient (see Fig. 1).
To increase the reproducibility of the treatment, patients were asked to fast for at least 4 h before simulation and treatment delivery.
Twenty-five seconds of true fast imaging (TRUFI) MR scans in free breathing (FB) and in breath-hold inspiration (BHI) conditions were acquired to verify inspiration breath-hold compliance and patient’s correct positioning. In case of inadequate BHI, patients were evaluated for FB treatment.
A cine MRI was acquired on sagittal plane to monitor target and organs’-at-risk (OARs) motion and to quantitatively assess patients’ compliance to BHI and its reproducibility.
No contrast agents have been administered to the patients for on-board MRI acquisition.
Following the evaluation of lesion’s visibility on the positioning MRI and the recorded motion, the attending radiation oncologist selected the delivery gating protocols deemed as most appropriate, using either the lesion itself or its indirect surrogates (i.e., surrounding large vascular structures, cystic lesions or whole liver) as gating target volumes (Boldrini et al. 2018; Massaccesi et al. 2019).
In this phase, treatment delivery parameters are also defined in terms of the region of interest percentage (ROI%) and relative boundary values to personalize the target gating approach.
The boundary determines the maximum allowed intra-fraction motion value of the target volume, while the ROI% is the maximum percentage value of the considered target structure allowed to be outside the boundary during treatment delivery. If the target structure exceeds the boundary for a value larger than the foreseen ROI%, treatment delivery is automatically stopped by the system. Figure 2 shows the relation between target volumes and boundaries.
To complete the simulation steps, a standard planning CT was also acquired in BHI on a helical CT scanner (GE HiSpeed DX/i Spiral, Boston, MA, USA) within 30 min since the acquisition of the MRI simulation, to guarantee anatomical consistency.
CT slice thickness was 1.25 mm and no intravenous contrast agent was administered.
This CT scan was then co-registered with the simulation MR using deformable registration algorithms, to obtain the electron density data required for dose calculation.
Treatment planning
The MR simulation scan was chosen as primary image, while planning CT and other diagnostic imaging (i.e., staging MRI) were used for the definition of the Gross Tumor Volume (GTV).
The target volume and organs at risk (i.e., healthy liver, inferior vena cava, chest wall, ribs, duodenum, small bowel, colon, kidneys, stomach, and spinal cord) were contoured by a radiation oncologist with specific expertise in the treatment of hepato-biliary malignancies.
The clinical target volume (CTV) was considered equal to GTV and the corresponding planning target volume (PTV) was obtained with an isotropic expansion of 3–5 mm from GTV, depending on the radiological characteristics of the lesions and on clinical judgment, to account for intra-fractional uncertainties (Cusumano et al. 2018).
A SBRT treatment was planned with a total prescribed dose resulting in biologically effective dose (BED) > 100 Gy in 5 fractions, considering a tumor alpha/beta of 10 (van Leeuwen et al. 2018).
The AAPM Task Group 101 dose constraints for SBRT treatments were applied to OARs (Benedict et al. 2010).
Furthermore, as suggested by some published experiences (Velec et al. 2017; Rosenberg et al. 2019), the mean dose to the liver was precautiously kept between 13 and 15 Gy and a volume inferior to 700 cc of healthy liver had to receive less than 15 Gy (Schefter et al. 2005; Baumann et al. 2018).
These planning objectives were preferable but not considered hard constraints, balancing between appropriate target coverage and OARs irradiation.
Treatment was prescribed to the PTV and planning was carried out using the stand-alone MRIdian treatment-planning system (ViewRay Inc., Mountain View, CA, USA).
Dose calculation was performed using a Monte Carlo computation algorithm considering the presence of the magnetic field since the elaboration of the fluence map and setting 2 mm as grid calculation size.
Step-and-shoot IMRT plans with 7–16 beams per plan were calculated, including the influence of the 0.35 T magnetic field on the dose calculation.
Treatment delivery
The delivery was carried out according to the parameters that were set during the simulation phase. First of all, a 25-s FB MR scan was acquired for patient alignment and then co-registered to the planning MR scan based on rigid co-registration on the target lesion.
After the set-up correction, a second scan was acquired in FB or BHI conditions, depending on the breathing characteristics of the simulation phase for a single patient.
After the last alignment adjustments of the patient, the most appropriate sagittal plane for gating was selected and the treatment parameters defined during simulation step (ROI% and boundary) were applied for online daily cine-MR monitoring.
Patients had the opportunity to actively participate in the treatment through the use of visual feedback system which displayed the online cine-MRI of their treatment. In this way, patients successfully contributed to the gating treatment by keeping the target within the boundary thanks to guided breaths.
In addition to the visual feedback, patients were also coached by the attending Radiation Therapy Technologist staff in the treatment room to optimize target positioning.
Follow-up
Patient follow-up was performed every 3–6 months. Clinical history, laboratory tests, and the required imaging (contrast enhanced CT or MRI) were collected during dedicated visits.
The follow-up diagnostic imaging was reviewed in comparison to the treatment plan images, to assess response or possible toxicity onset. Local response to treatment and impact of local therapy at systemic level were assessed according to mRECIST criteria (Llovet and Lencioni 2020).
Complete, partial response or stable disease were considered as tumor local control (LC).
Data describing acute toxicity, treatment tolerability, overall survival, progression-free survival, and local control were reported for all the enrolled patients.
Results
Ten (10) patients affected by HCC were retrospectively evaluated in this study.
Patients’ median age was 81.5 (70–87) years, ECOG performance status was ≤ 2, BCLC stage was A in 2 and C in 8 patients presenting portal vein invasion; while Child–Pugh disease stage was A in 9 and B in 1.
In 70% of cases, an anamnestic correlation of HCC with HCV infection was observed, while in the remaining 30% of cases, the cause was non-alcoholic steatohepatitis (NASH).
Six patients (60%) had performed previous local therapies (i.e., PEI,TACE,RFA,MWA) with a median of 2.5 (2–3) prior received procedures.
Of these, one patient was previously treated with MWA, TACE, and SBRT on a previous HCC nodule, different from the target considered for this analysis.
One patient (10%) received Sorafenib sequentially to local therapy, four patients (40%) had received no previous treatment and no patient underwent surgery.
A total of 12 HCC nodules were evaluated. The median number of lesions per patient was one (1–3); two patients presented two nodules each and one presented two synchronous lesions. The median lesion size was 18.5 mm (9–50 mm) in maximum diameter and nodules were distributed all over the hepatic parenchyma, as shown in Table 1. The median PTV size was 22.6 cc (3.2–116.9 cc). Patients were treated with a median dose of 50 (50–55) Gy delivered in 5 consecutive fractions, reaching a calculated BED10 > 100 Gy in all the cases.
For 7 nodules, a Dmean prescription was chosen, while the remaining 5 were prescribed to 80% isodose.
Target volumes were residual disease or de novo recurrence after focal therapies in 50% of the cases and nodules of new diagnosis in the remaining cases.
Patients’ compliance to gating was evaluated, on a case by case basis, during simulation phases; BHI-gated delivery was chosen in 80% of patients, while FB was preferred in the remaining 20%, due to poor compliance to breath hold.
All treatment plans have been approved in accordance with the planning objectives and dose limits recommended by AAPM Task Group 101 (Benedict et al. 2010).
Treatments were delivered in 5 patients using a MRIdian Tri-cobalt system 0.35 T from February 2018 to May 2019, while the remaining ones with a MRIdian 6 MV hybrid MR-Linac, due to local system upgrade.
The RT treatment was completed without interruptions and was overall well tolerated by all patients.
Acute toxicity onset was observed in two patients. One patient complained of mild gastrointestinal symptoms (nausea G1) and another patient reported fatigue G2 during treatment, subsequently developing ascites G2, three months after the end of RT.
The median follow-up was 6.5 months (1–25).
At the time of this analysis, 90% of patients reported local control of disease with 2 patients having complete response (CR), 3 stable disease (SD), and 4 partial response (PR), according to the aforementioned mRECIST criteria.
Only one patient underwent local failure with concomitant generalized progression of liver disease at a seven-month follow-up.
Patients’ Child–Pugh score persisted unchanged in all the cases except for one patient, who progressed from score A to B and presented ascites of grade G2 after three months from the end of RT treatment, although with stable liver disease.
This patient presented 2 lesions (in liver segments I and IV), which were simultaneously irradiated with a dose prescription of 50 Gy in 5 fractions to the mean dose and a resulting total PTV volume of 116 cc.
The mean liver dose was 17.94 Gy and the D700 cc was 15.3 Gy, with a V21Gy of 468.16 cc, thus fully respecting the constraints of AAPM Task Group 101.
Two patients are deceased at the time of analysis. Not surprisingly, they were the patients with the longest history of underlying liver disease (death occurred 87 and 69 months after diagnosis, respectively).
Discussion
The role of RT in the treatment of HCC has significantly changed over the years and the current recommendations are oriented toward the use of focal therapies in patients who are not candidate for surgery or as part of more complex patient's therapeutic paths (Murray and Dawson 2017).
Early experiences have described RT as a resource in palliative setting alone, with low-survival rates, generally burdened also by the underlying poor general clinical conditions of the irradiated patients. The recent evolution of radiotherapy delivery technologies has led to the introduction of SBRT in clinical practice, allowing the delivery of high doses with ablative intent, reducing the unnecessary irradiation of the surrounding organs at risk and preventing toxicity.
RFA, TACE, and SBRT are to-date considered as valid therapeutic options in early BCLC class patients. More specifically, RFA is recognized as a curative option for patients with < 3 HCC lesions, while TACE is generally reserved for patients who are not candidates for RFA whose lesions do not invade major vessels.
Comparison data between RFA and SBRT have reported discordant 2- and 3-year OS and toxicity rates (Wahl et al. 2016; Hara et al. 2019), with significantly lower 3-year LC rate in the SBRT patient cohort (5.3% vs 12.9%) (Hara et al. 2019).
SBRT has also been successfully associated with TACE, showing an improvement in LC and DFS when compared to TACE alone, but at the price of increased gastrointestinal toxicity, thrombocytopenia, and fever, despite generally easily manageable and successfully treated (Huo and Eslick 2015).
Moon et al. (2018) showed that prior liver-directed therapies did not affect LC or survival, also with no impact on toxicity. SBRT appeared therefore to be safe and effective even in the setting of prior ablative therapies o as bridge to transplantation option.
Dose levels also play a significant role in treatment efficacy and a total dose of at least 45 Gy in 3 fractions is suggested, as lower dose levels have been correlated with local failures (Murray and Dawson 2017).
Bujold et al. (2013) and Jang et al. (2013) have demonstrated that increased RT doses are associated with improved LC and patient survival in primary HCC patients was irradiated with conventional SBRT techniques. Two-year LC rates of 90% and 95% for 48 Gy in 3 fractions and 45 Gy in 3 fractions have been reported, respectively (Louis et al. 2010; Andolino et al. 2011).
Based on the TCP model, a dose of 54.8 Gy in 3 fractions produces 2-year LC with a probability of 90% (Jang et al. 2013). The need for dose escalation studies, supported by innovative imaging solutions for appropriate target identification and adequate motion management strategies has also been suggested by Xi et al. (2013) and Kong and colleagues (Kong et al. 2017), who have demonstrated the feasibility of high-dose SBRT with encouraging results on survival outcomes.
Besides dose escalation, an interesting area of further development is the use of concomitant drugs together with SBRT treatments, aiming to additive effects, even if the association of RT with Sorafenib has historically shown discordant results in different trials, mainly due to liver toxicity (Chen et al. 2014, p. 2).
Table 2 summarizes the studies published in the last 10 years on the application of SBRT for the management of HCC, highlighting the used technological solutions and dose levels.
As clearly reported in the table, many of these studies show large heterogeneity in terms of clinical conditions (e.g., previous treatments, comorbidities) and sample dimensions. Furthermore, at the time of this study, no randomized trials extensively compared SBRT with other local therapies for the treatment of HCC.
Nevertheless, the local control, survival, and toxicity rates reported by SBRT are very promising.
In particular, different experiences reported that LC is associated with lesions size, number, and received dose (Kang et al. 2012; Scorsetti et al. 2015), while survival outcomes are affected by the patient's general clinical conditions and liver function, as defined by the Child–Pugh status and cirrhosis severity (Huertas et al. 2015; Gerum et al. 2018).
The OS and LC results of our experience overlap with those published in literature, with promising toxicity rates.
The use of MRgRT allows to maximize SBRT objectives, taking advantage of all the opportunities offered by MR guidance and this advantage may have been translated in a particularly favorable and safe delivery setting.
The improved soft tissue contrast resolution offered by on-board MRI allows better target definition and may lead to clinically significant reductions in treatment volume margins.
Furthermore, the use of on-board cine-MRI allows direct visualization of tumor motion, ensuring accurate delivery and effective OARs sparing which represents a significant dose limiting factor with standard RT technologies.
The observed LC and OS rates are therefore excellent and potentially equivalent to RFA rates, although a direct comparison is unfortunately still not feasible due to the large inhomogeneity of the cohorts in literature.
As for the first specific MRgRT evidences, Rosenberg et al. (2019) analyzed 26 patients with HCC and metastatic liver lesions, reporting 7.7% acute G3 toxicity, while in the cohort treated by Feldman et al. (2019), which included 29 patients of which 26 HCC patients, the maximum acute reported toxicity was G2.
MRgRT has been well tolerated also in our cohort of patients, with no significant acute toxicity or evidence of RILD and only 20% of patients reporting mild toxicity symptoms, which were easily managed (max G2).
Online MRgRT adaptation strategies, that proved to be particularly efficient in the upper abdomen, pave the way to near future paradigm shifting dose escalation strategies aiming to optimize target coverage and ensure normal tissues preservation, especially in those patients who have had previous local treatments and suffer from underlying liver comorbidities that may hamper its function (Bohoudi et al. 2017; Boldrini et al. 2019; Placidi et al. 2020).
Online adaptive applications may therefore further enhance MRgRT treatment quality, as suggested in a first phase I prospective study investigating the potential of MR-guided online adaptive radiotherapy for upper GI malignancies (Henke et al. 2018).
Conclusions
Our experience confirms the safety and feasibility of MRgRT for the treatment of HCC nodules with favorable toxicity profile and may be of help as background for new dose escalating studies, taking full advantage of the significant innovations introduced by this technology in the field of clinical radiation oncology.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Andolino DL, Johnson CS, Maluccio M et al (2011) Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncology*Biology*Physics 81:e447–e453. https://doi.org/10.1016/j.ijrobp.2011.04.011
Bae SH, Kim M-S, Cho CK et al (2013) Feasibility and efficacy of stereotactic ablative radiotherapy for Barcelona Clinic Liver Cancer-C stage hepatocellular carcinoma. J Korean Med Sci 28:213–219. https://doi.org/10.3346/jkms.2013.28.2.213
Baumann BC, Wei J, Plastaras JP et al (2018) Stereotactic body radiation therapy (SBRT) for hepatocellular carcinoma: high rates of local control with low toxicity. Am J Clin Oncol 41:1118–1124. https://doi.org/10.1097/COC.0000000000000435
Benedict SH, Yenice KM, Followill D et al (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 37:4078–4101. https://doi.org/10.1118/1.3438081
Bertuccio P, Turati F, Carioli G et al (2017) Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 67:302–309. https://doi.org/10.1016/j.jhep.2017.03.011
Bibault J-E, Dewas S, Vautravers-Dewas C et al (2013) Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS ONE 8:e77472. https://doi.org/10.1371/journal.pone.0077472
Bohoudi O, Bruynzeel AME, Senan S et al (2017) Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol 125:439–444. https://doi.org/10.1016/j.radonc.2017.07.028
Boldrini L, Cellini F, Manfrida S et al (2018) Use of indirect target gating in magnetic resonance-guided liver stereotactic body radiotherapy: case report of an oligometastatic patient. Cureus. https://doi.org/10.7759/cureus.2292
Boldrini L, Colloca GF, Villani E et al (2020) Magnetic resonance–guided radiotherapy feasibility in elderly cancer patients: proposal of the MASTER scoring system. Tumori J. https://doi.org/10.1177/0300891620920709
Boldrini L, Cusumano D, Cellini F et al (2019) Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: state of the art, pearls and pitfalls. Radiat Oncol. https://doi.org/10.1186/s13014-019-1275-3
Bujold A, Massey C, Kim J, et al (2013) Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. In: Journal of clinical oncology : official journal of the American Society of Clinical Oncology. https://pubmed.ncbi.nlm.nih.gov/23547075/. Accessed 16 Sep 2020
Cárdenes HR, Price TR, Perkins SM et al (2010) Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 12:218–225. https://doi.org/10.1007/s12094-010-0492-x
Chen S-W, Lin L-C, Kuo Y-C et al (2014) Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncology*Biology*Physics 88:1041–1047. https://doi.org/10.1016/j.ijrobp.2014.01.017
Culleton S, Jiang H, Haddad CR et al (2014) Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol 111:412–417. https://doi.org/10.1016/j.radonc.2014.05.002
Cusumano D, Dhont J, Boldrini L et al (2018) Predicting tumour motion during the whole radiotherapy treatment: a systematic approach for thoracic and abdominal lesions based on real time MR. Radiother Oncol 129:456–462. https://doi.org/10.1016/j.radonc.2018.07.025
Dewas S, Bibault J-E, Mirabel X et al (2012) Prognostic factors affecting local control of hepatic tumors treated by stereotactic body radiation therapy. Radiat Oncol 7:166. https://doi.org/10.1186/1748-717X-7-166
El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142:1264-1273.e1. https://doi.org/10.1053/j.gastro.2011.12.061
European Association for the Study of the Liver (2018) Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Feldman AM, Modh A, Glide-Hurst C et al (2019) Real-time magnetic resonance-guided liver stereotactic body radiation therapy: an institutional report using a magnetic resonance-linac system. Cureus. https://doi.org/10.7759/cureus.5774
Gerum S, Heinz C, Belka C et al (2018) Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease. Radiat Oncol 13:100. https://doi.org/10.1186/s13014-018-1048-4
Goodman KA, Wiegner EA, Maturen KE et al (2010) Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 78:486–493. https://doi.org/10.1016/j.ijrobp.2009.08.020
Hara K, Takeda A, Tsurugai Y et al (2019) Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology 69:2533–2545. https://doi.org/10.1002/hep.30591
Hass P, Mohnike K, Kropf S et al (2019) Comparative analysis between interstitial brachytherapy and stereotactic body irradiation for local ablation in liver malignancies. Brachytherapy 18:823–828. https://doi.org/10.1016/j.brachy.2019.08.003
Henke L, Kashani R, Robinson C et al (2018) Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol 126:519–526. https://doi.org/10.1016/j.radonc.2017.11.032
Huang W-Y, Jen Y-M, Lee M-S et al (2012) Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 84:355–361. https://doi.org/10.1016/j.ijrobp.2011.11.058
Huertas A, Baumann A-S, Saunier-Kubs F et al (2015) Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol 115:211–216. https://doi.org/10.1016/j.radonc.2015.04.006
Hunt A, Hansen VN, Oelfke U et al (2018) Adaptive radiotherapy enabled by mri guidance. Clin Oncol (R Coll Radiol) 30:711–719. https://doi.org/10.1016/j.clon.2018.08.001
Huo YR, Eslick GD (2015) Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol 1:756–765. https://doi.org/10.1001/jamaoncol.2015.2189
Jang WI, Kim M-S, Bae SH et al (2013) High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol 8:250. https://doi.org/10.1186/1748-717X-8-250
Kang J-K, Kim M-S, Cho CK et al (2012) Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 118:5424–5431. https://doi.org/10.1002/cncr.27533
Kimura T, Aikata H, Takahashi S et al (2015) Stereotactic body radiotherapy for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Hepatol Res 45:378–386. https://doi.org/10.1111/hepr.12359
Kinoshita A, Onoda H, Fushiya N et al (2015) Staging systems for hepatocellular carcinoma: current status and future perspectives. World J Hepatol 7:406–424. https://doi.org/10.4254/wjh.v7.i3.406
Kong X, Dong Y, Wu J et al (2017) High-biologically effective dose palliative radiotherapy for a tumor thrombus might improve the long-term prognosis of hepatocellular carcinoma: a retrospective study. Radiat Oncol 12:92. https://doi.org/10.1186/s13014-017-0831-y
Kwon JH, Bae SH, Kim JY et al (2010) Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer 10:475. https://doi.org/10.1186/1471-2407-10-475
Lasley FD, Mannina EM, Johnson CS et al (2015) Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1–2 trial of stereotactic body radiation therapy. Pract Radiat Oncol 5:e443–e449. https://doi.org/10.1016/j.prro.2015.02.007
Liu E, Stenmark MH, Schipper MJ et al (2013) Stereotactic body radiation therapy for primary and metastatic liver tumors. Translat Oncol 6:442. https://doi.org/10.1593/tlo.12448
Llovet JM, Lencioni R (2020) mRECIST for HCC: performance and novel refinements. J Hepatol 72:288–306. https://doi.org/10.1016/j.jhep.2019.09.026
Louis C, Dewas S, Mirabel X et al (2010) Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat 9:479–487. https://doi.org/10.1177/153303461000900506
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology 68:723–750. https://doi.org/10.1002/hep.29913
Massaccesi M, Cusumano D, Boldrini L et al (2019) A new frontier of image guidance: organs at risk avoidance with mri-guided respiratory-gated intensity modulated radiotherapy: technical note and report of a case. J Appl Clin Med Phys 20:194–198. https://doi.org/10.1002/acm2.12575
Moon DH, Wang AZ, Tepper JE (2018) A prospective study of the safety and efficacy of liver stereotactic body radiotherapy in patients with and without prior liver-directed therapy. Radiother Oncol 126:527–533. https://doi.org/10.1016/j.radonc.2018.01.004
Murray LJ, Dawson LA (2017) Advances in stereotactic body radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol 27:247–255. https://doi.org/10.1016/j.semradonc.2017.02.002
Noel CE, Parikh PJ, Spencer CR et al (2015) Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol 54:1474–1482. https://doi.org/10.3109/0284186X.2015.1062541
Placidi L, Romano A, Chiloiro G et al (2020) On-line adaptive MR guided radiotherapy for locally advanced pancreatic cancer: clinical and dosimetric considerations. Tech Innovat Patient Support Radiat Oncol 15:15–21. https://doi.org/10.1016/j.tipsro.2020.06.001
Rosenberg SA, Henke LE, Shaverdian N et al (2019) A Multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Adv Radiat Oncol 4:142–149. https://doi.org/10.1016/j.adro.2018.08.005
Sanuki N, Takeda A, Oku Y et al (2014) Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncologica 53:399–404. https://doi.org/10.3109/0284186X.2013.820342
Schefter TE, Kavanagh BD, Timmerman RD et al (2005) A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 62:1371–1378. https://doi.org/10.1016/j.ijrobp.2005.01.002
Scorsetti M, Comito T, Cozzi L et al (2015) The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol 141:1301–1309. https://doi.org/10.1007/s00432-015-1929-y
Seo YS, Kim M-S, Yoo SY et al (2010) Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol 102:209–214. https://doi.org/10.1002/jso.21593
Su T-S, Liang P, Lu H-Z et al (2016) Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol 113:181–187. https://doi.org/10.1002/jso.24128
Takeda A, Sanuki N, Eriguchi T et al (2014) Stereotactic ablative body radiotherapy for previously untreated solitary hepatocellular carcinoma. J Gastroenterol Hepatol 29:372–379. https://doi.org/10.1111/jgh.12350
van Leeuwen CM, Oei AL, Crezee J et al (2018) The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol 13:96. https://doi.org/10.1186/s13014-018-1040-z
Velec M, Haddad CR, Craig T et al (2017) Predictors of liver toxicity following stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 97:939–946. https://doi.org/10.1016/j.ijrobp.2017.01.221
Vogel A, Cervantes A, Chau I et al (2018) Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:238–255. https://doi.org/10.1093/annonc/mdy308
Wahl DR, Stenmark MH, Tao Y et al (2016) Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol 34:452–459. https://doi.org/10.1200/JCO.2015.61.4925
Weiner AA, Olsen J, Ma D et al (2016) Stereotactic body radiotherapy for primary hepatic malignancies: report of a phase I/II institutional study. Radiother Oncol 121:79–85. https://doi.org/10.1016/j.radonc.2016.07.020
Xi M, Zhang L, Zhao L et al (2013) Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS ONE 8:e63864. https://doi.org/10.1371/journal.pone.0063864
Yamashita H, Onishi H, Murakami N et al (2015) Survival outcomes after stereotactic body radiotherapy for 79 Japanese patients with hepatocellular carcinoma. J Radiat Res 56:561–567. https://doi.org/10.1093/jrr/rru130
Yoon SM, Lim Y-S, Park MJ et al (2013) Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS ONE 8:e79854. https://doi.org/10.1371/journal.pone.0079854
Common Terminology Criteria for Adverse Events (CTCAE). 80
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization, LB, and AR; methodology, GCM, VV; data analysis, FC, DC; data curation, SM, LP, and LI; writing—original draft preparation, AR and SM; writing—review and editing, LB, GCM, FC; visualization, SM, GC; supervision, MAG, GCM, and VV. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Luca Boldrini and Dr. Davide Cusumano have active research agreements with ViewRay Inc and received speaker honoraria for scientific presentations. Prof. Vincenzo Valentini has received departmental research grants from Varian Medical Systems, ViewRay Inc., Elekta, Merck-Serono, Roche.
Ethical approval
This research study was conducted retrospectively in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boldrini, L., Romano, A., Mariani, S. et al. MRI-guided stereotactic radiation therapy for hepatocellular carcinoma: a feasible and safe innovative treatment approach. J Cancer Res Clin Oncol 147, 2057–2068 (2021). https://doi.org/10.1007/s00432-020-03480-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03480-8