Abstract
Background and Aims
The aim of this single-center, open-label phase II study was to assess the efficacy of image-guided high-dose-rate (HDR) brachytherapy (iBT) compared with conventional transarterial embolization (cTACE) in unresectable hepatocellular carcinoma.
Methods
Seventy-seven patients were treated after randomization to iBT or cTACE, as single or repeated interventions. Crossover was allowed if clinically indicated. The primary endpoint was time to untreatable progression (TTUP). Eligibility criteria included a Child–Pugh score of ≤ 8 points, absence of portal vein thrombosis (PVT) at the affected liver lobe, and ≤ 4 lesions. Survival was analyzed by using the Cox proportional hazard model with stratification for Barcelona Clinic Liver Cancer (BCLC) stages.
Results
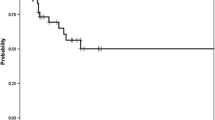
Twenty patients were classified as BCLC-A (iBT/cTACE 8/12), 35 as BCLC-B (16/19), and 22 as BCLC-C (13/9). The 1-, 2-, and 3-year TTUP probabilities for iBT compared with cTACE were 67.5% versus 55.2%, 56.0% versus 27.4%, and 29.5% versus 11.0%, respectively, with an adjusted hazard ratio (HR) of 0.49 (95% confidence interval 0.27–0.89; p = 0.019). The 1-, 2-, and 3-year TTPs for iBT versus cTACE were 56.0% versus 28.2%, 23.9% versus 6.3%, and 15.9% versus 6.3%, respectively, with an adjusted HR of 0.49 (0.29–0.85; p = 0.011). The 1-, 2-, and 3-year OS rates were 78.4% versus 67.7%, 62.0% versus 47.3%, and 36.7% versus 27.0%, respectively, with an adjusted HR of 0.62 (0.33–1.16; p = 0.136).
Conclusions
This explorative phase II trial showed a superior outcome of iBT compared with cTACE in hepatocellular carcinoma and supports proceeding to a phase III trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Europe, five-year survival rates of up to 51% have been shown for hepatocellular carcinoma (HCC) suitable for resection [1]. Unfortunately, 70–80% of patients are not candidates for resection, because of advanced cirrhosis, multiple lesions or diffuse tumor growth, and comorbidity. Liver transplantation is the only potentially curative option at present, with five-year post-transplantation survival rates of up to 70% for patients in early stages of disease [2]. The treatment of choice in the intermediate stage is transarterial chemoembolization (‘conventional TACE’ = cTACE; ‘drug-eluting beads TACE’ = DEB-TACE). In clinical practice, TACE is also applied in BCLC (Barcelona Clinic Liver Cancer stage)-A patients, often as an adjunct to radiofrequency ablation (RFA), and in BCLC-C patients, for whom sorafenib-only treatment is not considered appropriate [3,4,5,6]. However, effectiveness and feasibility of TACE are limited by factors such as advanced-stage cirrhosis, a hampered general condition and portal vein invasion. In ipsilateral complete portal vein thrombosis (PVT), TACE is known to be associated with a risk of ischemia and abscess formation. Thermal ablation is usually considered up to a tumor size of 3 cm. Beyond this limit, local recurrence rates increase [7, 8]. Some authors state the superiority of Gelaspon particles over Lipiodol for embolization purposes [9]. A recently invented method, CT (computed tomography)-guided interstitial HDR (high-dose-rate) brachytherapy (iBT), has successfully been used in various neoplasms of the liver and other sites [10,11,12,13,14,15,16,17,18]. As a unique feature, iBT is not restricted by tumor size or heat sink effect and PVT is not a contraindication [19,20,21,22].
A recent study encouraged us to address the clinical value of iBT as compared with standard treatment such as TACE in a future trial. A major intention of this explorative type II study was to investigate whether proceeding to phase III trial is supported [11].
Patients and Methods
Patient Population and Eligibility Criteria
Patient recruitment took place from October 2006 to September 2010. Patients with a diagnosis of HCC were randomized to receive either CT-guided HDR iBT or cTACE. Inclusion criteria were:
-
Diagnosis of HCC by histopathology or according to the criteria of the Consensus Conference of the European Association for the Study of Liver Disease
-
Unresectable HCC
-
Karnofsky Index > 70
-
Estimated life expectancy > 16 weeks
-
Adequate bone marrow function
-
Adequate contraception for female patients
-
Informed consent
Exclusion criteria were as follows:
-
PVT on the tumor side
-
Extrahepatic spread
-
Child C
-
Other untreated malignant diseases
-
General contraindication for chemotherapy
-
Active infectious disease
-
Neuropathy, platin-allergy
-
Pregnancy
All patients were rated unresectable and not eligible for radiofrequency ablation owing to lesion size and/or location.
All patients received a full clinical status evaluation at inclusion, comprising a physical examination, extensive laboratory assessments, whole-body computed tomography, and MRI of the liver (Fig. 1).
Complete remission of a single hepatocellular carcinoma (8.3 cm) of the right liver lobe. The patient refused surgery. Upper row: before treatment, arterial phase (left) and T2 FS (right), May 2006. Middle row: Left: catheter placement during treatment. Right: arterial phase, June 2014. Bottom row: left: T2 FS. Right: T1 WATS late contrast phase (GD-EOB-DTPA). Note the completely ablated segment with prolapsed intestinal loops
Study Design
The study represents an exploratory randomized phase II approach comparing two interventional treatment arms. The study was registered at clinicaltrials.gov (NCT00807300), and the study protocol conformed with the Declaration of Helsinki. The institutional review board approved the study, and informed consent was obtained from all patients. This explorative phase II study analyzes the efficacy and safety of iBT in comparison with cTACE and aims to generate a hypothesis for a potential phase III study. A high type I error of 20% was allowed to keep patient numbers reasonable, and the sample size was set to 80 including a dropout rate of 10% [23]. Owing to slower patient accrual the trial was closed with a lower patient number than anticipated. However, the minimum target sample size (without dropouts) was achieved.
Patients meeting the inclusion criteria were randomly assigned to first treatment either with cTACE (control arm) or with iBT (experimental arm). Simple randomization was performed allocating patients sequentially to treatment groups using shuffled sealed opaque envelopes containing equal numbers of identifiers for treatment A and treatment B. After untreatable progression had been reached, any further treatment decisions were left to the investigator’s judgment.
The primary endpoint was the time to untreatable progression (TTUP), defined as the time from the first treatment (either iBT or cTACE) to the time point when complete tumor ablation could not be repeated any further by applying the assigned method. The criteria for stopping the assigned treatment were as follows:
-
No radiological response at follow-up/local failure
-
Diffuse progression (> 3 new lesions)
-
Chronic hepatic decompensation, as defined by a Child–Pugh score deterioration of > 2 points
-
Clinical conditions other than hepatic decompensation, permanently precluding further treatment (e.g., performance status).
Technique-associated no-go criteria possibly occurring during follow-up, such as failure of Lipiodol to accumulate in the lesion, missing angiographic visibility, development of ipsilateral PVT, development of an arterioportal shunt visible by angiography (all cTACE), or contraindications to a percutaneous interstitial approach (only iBT, including severe coagulopathy and uncontrolled ascites), were not counted, in order to ensure that the criteria for TTUP were the same in the two groups. The corresponding time points were censored.
Secondary endpoints were time to progression (TTP) and overall survival (OS).
Interstitial HDR Brachytherapy (iBT)
The technique of CT-guided brachytherapy has been described in detail elsewhere [18, 19]. We performed irradiation employing the HDR brachytherapy technique based on a 10-Ci Iridium-192 source. Positioning of the brachytherapy catheters was performed by fluoroscopy CT. For analgosedation, fentanyl and midazolam were used according to individual requirement.
The target dose was defined as the minimum dose taken up by the visible tumor margin. We prescribed a minimum target dose of 15 Gy, based on the results of two pilot studies [20, 21].
Transarterial Chemoembolization (cTACE)
After puncture of the right or left femoral artery, an angiography of the celiac artery and superior mesenteric artery via a 4F catheter was performed. Parasitic feeders to HCC lesions were searched for with the same catheter. Chemoembolization was conducted in a supraselective manner with a 3F microcatheter, applying the drug/oil emulsion over the feeding arteries of the tumor only. Typically, 30–50 mg/m2 doxorubicin and cisplatin mixed with Lipiodol were administered. If the total tumor volume or tumor count could not be embolized in one session, the procedure was repeated after 6 weeks.
Assessments
Before therapy a physical examination, MRI and computed tomography (CT) scans, and laboratory tests were performed. These examinations were repeated every 3 months. Clinical evaluators (two experienced radiologists, consensus decision) were blinded to the chosen treatment.
Since the mRECIST criteria for tumor response had not been established for HCC in 2006, TTP was likewise assessed by following the recommendations of the European Association for the Study of the Liver (EASL) and American Association for the Study of the Liver (AASL) [24, 25].
Patients were censored at the time point of liver transplantation, liver resection, or crossover treatment. After untreatable progression (the primary endpoint TTUP) had been reached, any further treatment decisions were left to the investigator’s judgment.
Statistical Methods
The program suite IBM SPSS Statistics 22.0 and R version 3.1.3 (The R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Metric parameters are described using median and interquartile range (25th–75th percentile), and the Mann–Whitney U test was used for analyzing differences between unpaired groups. Categorical variables were analyzed by using contingency tables, the Chi-square test and Fisher’s exact test.
The observation period was 5 years. Patients were censored at crossover treatment, at loss to follow-up, and at the end of observation period. TTUP, TTP, and OS were estimated by the Kaplan–Meier (KM) method, and the Cox proportional hazard model was used to assess the association of TTUP, TTP, and OS with covariates. Parameters with p value ≤ 0.1 in univariate Cox regression were included in a multivariate Cox proportional hazard analysis. The multivariate model was optimized by using the Akaike information criterion with stepwise backward elimination. Analysis was performed on an intention-to-treat (ITT) basis (TTP, TTUP, and OS) and ‘as treated’ (safety). The Cox proportional hazard model was stratified for BCLC stages, as this parameter did not satisfy the proportional hazard assumption, which was assessed visually from log–log KM curves. Significance was assumed at a p value less than 0.05.
Results
Patients
In total, 392 patients were assessed for eligibility from October 2006 to September 2010. Of these patients 203 did not meet the inclusion criteria, 68 declined to participate, and 44 were excluded for other reasons. Of the remaining 77 patients 40 were randomly assigned to the cTACE group and 37 patients to the iBT group. Two patients allocated to receive cTACE were transferred to the iBT group for technical reasons. Thus, the per-protocol population comprised 38 patients in the cTACE group and 39 patients in the iBT group (Fig. 2).
Among the 77 enrolled patients (13 females and 64 males; mean age 68.5 years; range 43.4–82.7 years), in 34 patients, HCC was confirmed by biopsy (44%), whereas in 43 patients with cirrhosis, HCC was diagnosed on the basis of noninvasive criteria according to the EASL and AASL guidelines [24, 25]. Patient characteristics are summarized in Table 1.
Treatments and Follow-up
The number of treatments per patient was significantly lower in the iBT group (2.5 ± 1.6) compared with the cTACE group (4.0 ± 3.2, p = 0.039). Subsequent treatments after the end-of-study date are shown in Table 2.
In 8 of the 38 patients in the cTACE group, treatment had to be stopped for technique-related reasons such as AV shunts or ipsilateral PVT. Owing to missing visibility in CT or MRI, treatment had to be stopped in a patient with AFP recurrence in the iBT group. The difference was statistically significant (Chi-square test, p = 0.012).
During the 5-year observation period 52 patients died (iBT, n = 31; cTACE, n = 21) and 15 patients were censored because of crossover treatment after reaching untreatable progression (14 patients of the cTACE arm received iBT and 1 patient in the iBT arm received cTACE) at a mean follow-up time of 15.5 months (SD 9.3 months; range 3.1–38.5 months). Of the remaining 10 patients 6 were still alive at the end of the 5-year observation period and the mean follow-up time was 41.2 months (SD 24.6 months; range 4.9–60 months).
Survival
The 1-, 2-, and 3 year TTUP survival rates for the iBT compared with the cTACE group were 67.5% versus 55.2%, 56.0% versus 27.4%, and 29.5% versus 11.0%, respectively, with an HR of 0.52 (0.30–0.90; p = 0.021; Fig. 3A). Stratifying by BCLC stages revealed an HR of 0.92 (95% CI 0.31–2.72; p = 0.887) for BCLC-A, 0.38 (95% CI 0.16–0.87; p = 0.021) for BCLC-B, and 0.51 (95% CI 0.18–1.42; p = 0.195) for BCLC-C (Fig. 4). Further significant influencing factors were female gender (HR = 3.31; p = 0.001), AFP (unit: µg/ml; HR = 1.08; p = 0.038), Child–Pugh score B (HR = 3.91; p = 0.018), and pretherapeutic bilirubin > 19 µmol/l (HR = 2.03; p = 0.024). Near-significance was observed for lesion diameter > 5 cm (HR = 1.82; p = 0.057) and the number of lesions > 2 (HR = 1.77; p = 0.056). The multivariate Cox regression model included female gender (HR = 4.21, p < 0.001), iBT arm (HR = 0.49, p = 0.019), the number of lesions > 2 (HR = 1.80, p = 0.069), AFP (unit:µg/ml; HR = 1.13, p = 0.001), and Child–Pugh score B (HR = 3.81; p = 0.036).
The 1-, 2-, and 3-year TTP survival rates for iBT compared with cTACE were 56.0% versus 28.2%, 23.9% versus 6.3%, and 15.9% versus 6.3%, respectively, with a univariate HR of 0.49 (0.29–0.83; p = 0.008; Fig. 3B). Stratifying by BCLC stage revealed an HR of 0.68 (95% CI 0.26–1.77; p = 0.430) for BCLC-A, 0.46 (95% CI 0.21–1.00; p = 0.051) for BCLC-B, and 0.36 (95% CI 0.13–1.06; p = 0.063) for BCLC-C (Fig. 4). Further significant factors in univariate Cox regression were Child–Pugh score (HR = 3.33; p = 0.031) and pretherapeutic bilirubin > 19 µmol/l (HR = 1.86; p = 0.042). Near-significance was observed for age (unit: 10 years; HR = 0.74; p = 0.070), the number of lesions > 2 (HR = 1.73; p = 0.060), and AFP (unit: µg/mol; HR = 1.08; p = 0.058). The multivariate Cox regression model included age (unit: 10 years; HR = 0.78; p = 0.130), iBT (HR = 0.49, p = 0.011), AFP (unit: µg/mol; HR = 1.08; p = 0.063), and Child–Pugh score (HR = 3.12; p = 0.045).
The 1-, 2-, and 3-year overall survival rates in the iBT compared with the TACE group were 78.4% versus 67.7%, 62.0% versus 47.3%, and 36.7% versus 27.0%, respectively, with a univariate HR of 0.61 (0.34–1.09; p = 0.097; Fig. 3C). Stratifying by BCLC stage revealed an HR of 0.92 (95% CI 0.27–3.16; p = 0.890) for BCLC-A, 0.55 (95% CI 0.23–1.31; p = 0.179) for BCLC-B, and 0.52 (95% CI 0.18–1.46; p = 0.212) for BCLC-C. The univariate Cox regression model revealed female gender (HR = 2.88, p = 0.006), AFP (unit: µg/mol; HR = 1.12; p = 0.004), Child–Pugh score (HR = 6.19; p = 0.002), and pretherapeutic bilirubin > 19 µmol/l (HR = 3.33; p < 0.001) as significant factors and the number of lesions > 2 (HR = 1.68; p = 0.089) as a factor showing close significance. The multivariate Cox regression model for OS comprised female gender (HR = 3.46, p = 0.002), iBT (HR = 0.62; p = 0.136), the number of lesions > 2 (HR = 1.86; p = 0.061), AFP (unit: µg/mol; HR = 1.17; p < 0.001), and Child–Pugh score (HR = 5.76, p = 0.006; Table 3).
Safety (as Treated) and 30-day Mortality (as Treated)
For complications and 30-day mortality, see Table 4.
Discussion
The intention of this exploratory, randomized, phase II study was to assess the efficacy and safety of iBT in comparison with the standard treatment modality (cTACE) in order to decide whether a multicentric phase III study is justified.
The adjusted hazard ratio of 0.49, as observed both for the primary endpoint TTUP and for the secondary endpoint TTP, is convincing. The adjusted hazard ratio for OS was 0.62 for the entire study group, which also indicates a possible superiority of iBT compared with cTACE. A higher overall survival effect size was observed in patients with BCLC-B (HR = 0.55) and BCLC-C (HR = 0.52), whereas iBT showed no superiority in patients with BCLC-A (HR = 0.92).
In two reported randomized trials of TACE with positive outcome, repetitive TACE was found to have benefit in terms of OS [26, 27]. Consequently, recent TACE trials such as the TACE–sorafenib combination trial SPACE employed TTUP as a secondary endpoint [28]. Some of the conditions preventing TACE—such as the development of PVT or technical failure of TACE indicated by failed uptake of Lipiodol in hypoperfused tumors—do not influence the applicability or the therapeutic effect of iBT [26, 27]. However, these technique-inherent conditions did not apply in the final TTUP analysis.
Results of the PRECISION V study demonstrated better tolerability of DEB-TACE in comparison with conventional TACE [29]. However, since that study did not demonstrate any effect on survival or treatment duration by the choice of the TACE technique, we do not consider that any negative bias was introduced by the use of conventional, Lipiodol-based TACE in our trial (which had been designed before DEB-TACE was established).
In Europe, the recommended application criterion for TACE is BCLC-B with up to 7 points [30]. As outlined previously, favorable results of iBT in our study were evident in patients inside the established range of TACE indications (BCLC-B). In patients with BCLC-A the iBT treatment showed no clinically relevant effect compared with cTACE, with a hazard ratio of 0.83 for TTUP and 0.92 for overall survival. This may have been due to the relatively high efficacy and safety of cTACE in small tumors and small tumor numbers. In contrast, in patients with BCLC-B and BCLC-C a substantial benefit of iBT over cTACE can be assumed regarding the respective hazard ratios for TTUP, TTP, and OS. Assuming an effect size of 0.55, an event probability of 65%, an alpha error of 0.01, and the power to 90%, the sample size required for a phase III study would be 136 per group, respectively.
Because of the exploratory character of this study and the correspondingly small sample size, the confidence intervals of the observed hazard ratios are large and the level of significance was not reached for OS. However, it has to be emphasized that the type I error of a randomized phase II trial is typically high, in the range of 10–20%, to keep patient numbers reasonable, whereas the crucial parameter for a decision to proceed to a phase III trial is the observed effect size [23]. The stratification concerning BCLC stages was not planned prospectively, and thus, the subgroup analyses demonstrating a greater effect in BCLC-B/BCLC-C compared with BCLC-A have to be evaluated critically, especially on account of the small sample size in the subgroups. However, we consider that this stratification may be justified, as the proportional hazard assumption was not satisfied for BCLC stages.
Conclusion
This explorative phase II trial showed a superior outcome of iBT compared with cTACE in HCC, notably in patients with BCLC-B/ BCLC-C and supports proceeding to a phase III trial.
Abbreviations
- AASL:
-
American Association for the Study of the Liver
- BCLC:
-
Barcelona Clinic Liver Cancer (staging system)
- CI:
-
Confidence interval
- CLIP:
-
Cancer of the Liver Italian Program
- CT:
-
Computed tomography
- cTACE:
-
Conventional transarterial chemoembolization
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- DEB-TACE:
-
Drug-eluting beads transarterial chemoembolization
- EASL:
-
European Association for the Study of the Liver
- HCC:
-
Hepatocellular carcinoma
- HDR:
-
High dose rate
- HR:
-
Hazard ratio
- iBT:
-
Interstitial brachytherapy
- OS:
-
Overall survival
- PVT:
-
Portal vein thrombosis
- RFA:
-
Radiofrequency ablation
- SBRT:
-
Stereotactic body radiotherapy
- TTP:
-
Time to progression
- TTUP:
-
Time to untreatable progression
References
Jaeck D, Bachellier P, Oussoultzoglou E, Weber JC, Wolf P. Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: an overview. Liver Transpl. 2004;10:S58–63.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14.
Cheng BQ, Jia CQ, Liu CT, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669–77.
Helmberger T, Dogan S, Straub G, et al. Liver resection or combined chemoembolization and radiofrequency ablation improve survival in patients with hepatocellular carcinoma. Digestion. 2007;75:104–12.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42.
Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol. 2009;72:505–16.
Cucchetti A, Piscaglia F, Cescon M, Ercolani G, Pinna AD. Systematic review of surgical resection vs radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2013;19:4106–18.
Cucchetti A, Piscaglia F, Cescon M, et al. An explorative data-analysis to support the choice between hepatic resection and radiofrequency ablation in the treatment of hepatocellular carcinoma. Dig Liver Dis. 2014;46:257–63.
Takayasu K, Arii S, Ikai I, et al. Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. AJR Am J Roentgenol. 2010;194:830–7.
Collettini F, Schnapauff D, Poellinger A, et al. Hepatocellular carcinoma: computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of large (5–7 cm) and very large (> 7 cm) tumours. Eur Radiol. 2012;22:1101–9.
Mohnike K, Wieners G, Schwartz F, et al. Computed tomography-guided high-dose-rate brachytherapy in hepatocellular carcinoma: safety, efficacy, and effect on survival. Int J Radiat Oncol Biol Phys. 2010;78:172–9.
Ricke J, Mohnike K, Pech M, et al. Local response and impact on survival after local ablation of liver metastases from colorectal carcinoma by computed tomography-guided high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2010;78:479–85.
Ricke J, Thormann M, Ludewig M, et al. MR-guided liver tumor ablation employing open high-field 1.0T MRI for image-guided brachytherapy. Eur Radiol. 2010;20:1985–93.
Ricke J, Wust P, Stohlmann A, et al. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: phase I–II results of a novel technique. Int J Radiat Oncol Biol Phys. 2004;58:1496–505.
Ricke J, Wust P, Wieners G, et al. Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J Vasc Interv Radiol. 2004;15:1279–86.
Tselis N, Chatzikonstantinou G, Kolotas C, Milickovic N, Baltas D, Zamboglou N. Computed tomography-guided interstitial high dose rate brachytherapy for centrally located liver tumours: a single institution study. Eur Radiol. 2013;23:2264–70.
Mohnike K, Neumann K, Hass P, et al. Radioablation of adrenal gland malignomas with interstitial high-dose-rate brachytherapy: efficacy and outcome. Strahlenther Onkol. 2017;193:612–9.
Mohnike K, Wolf S, Damm R, et al. Radioablation of liver malignancies with interstitial high-dose-rate brachytherapy: complications and risk factors. Strahlenther Onkol. 2016;192:288–96.
Hata M, Tokuuye K, Sugahara S, et al. Proton beam therapy for hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2005;104:794–801.
Lee SU, Park JW, Kim TH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014;190:806–14.
Sugahara S, Nakayama H, Fukuda K, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782–8.
Mohnike K, Sauerland H, Seidensticker M, et al. Haemorrhagic complications and symptomatic venous thromboembolism in interventional tumour ablations: the impact of peri-interventional thrombosis prophylaxis. Cardiovasc Intervent Radiol. 2016;39:1716–21.
Cannistra SA. Phase II trials in journal of clinical oncology. J Clin Oncol. 2009;27:3073–6.
Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the study of the liver. J Hepatol. 2001;35:421–30.
Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36.
Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9.
Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71.
Lencioni R. Chemoembolization in patients with hepatocellular carcinoma. Liver Cancer. 2012;1:41–50.
Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52.
Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–35.
Funding
This study was funded exclusively by the University of Magdeburg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Financial Support
This work was funded exclusively by the University of Magdeburg.
Ethical Considerations
The study was conducted in accordance with the protocol, the ethical principles that have their origin in the Declaration of Helsinki, and ICH-GCP. The study protocol and all study-related documentation were approved by all relevant authorities (Ethics Committee of the Medical Faculty, University of Magdeburg, 44/06).
Rights and permissions
About this article
Cite this article
Mohnike, K., Steffen, I.G., Seidensticker, M. et al. Radioablation by Image-Guided (HDR) Brachytherapy and Transarterial Chemoembolization in Hepatocellular Carcinoma: A Randomized Phase II Trial. Cardiovasc Intervent Radiol 42, 239–249 (2019). https://doi.org/10.1007/s00270-018-2127-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-2127-5