Abstract
Over the past 3 years, gadolinium-based contrast agents have been linked to MRI signal changes in the brain, which have been found to be secondary to gadolinium deposition in the brain, particularly in the dentate nuclei and globus pallidus even in patients having an intact blood-brain barrier and a normal renal function. This tends to occur more in linear agents than with macrocyclic agents. Nonetheless, there has been no significant evidence that this has any clinical consequence. We reviewed the current evidence related to this new phenomenon and the precautionary approach taken by regulatory agencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of gadolinium-based contrast agents (GBCA) in humans [1, 2], dramatically expanded the diagnostic capabilities of magnetic resonance imaging (MRI) and 30 million doses of GBCA are administered annually in both clinical and research settings [3]. Initially, it was thought that the only risks associated with these agents would be allergic reactions and similar acute adverse events. The first case of nephrogenic systemic fibrosis (NSF) [4] was reported in 1997 and 9 years later this condition was eventually linked to gadolinium (Gd) exposure [5,6,7]. The incidence of NSF has been lowered dramatically by decreasing the dosage and limiting administration of these agents in high-risk patients. Over the past 3 years, evidence for deposition of these agents in the brain during clinical use has come to light: first on imaging as high-T1 signal intensity in the dentate nuclei (DN) (Fig. 1) and globus pallidus (GP) and subsequently the detection of Gd in both autopsy and biopsy tissue studies [8,9,10]. The question as to whether there is a clinical syndrome associated with Gd deposition remains open, although no definitive evidence has so far been published [11,12,13,14,15].
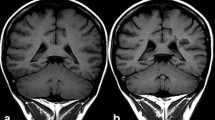
Unenhanced axial T1 spin echo image (1.5 T, 5 mm, TR: 608 TE:15) demonstrating high signal in both dentate nuclei (white arrows). This patient was being followed up for a pineoblastoma and had also undergone one radiotherapy session. He was imaged four times using a linear agent and once using a macrocyclic agent
Chemistry and Properties of Gadolinium Contrast Agents
Contrast agents in MRI increase the contrast between the cavity, vessel or organ in which they are present and the surrounding tissue. These are usually administered intravenously at a dose of 0.1 mmol Gd/kg body weight. The efficacy of GBCAs is determined by their pharmacokinetic and magnetic properties. Since they have no pharmacological activity, GBCAs typically demonstrate biexponential plasma kinetics, with distribution of the compound being followed by its elimination and a third phase of residual excretion. Since they are hydrophilic, they are usually eliminated via the renal route, although some also demonstrate hepatic elimination [16].
There are currently nine commercially available GBCAs, six which demonstrate extracellular fluid distribution while the remaining three agents are so-called organ-specific agents. The free Gd3+ ion is extremely toxic [17] mostly because of its ability to bind with calcium-ion channels, thus being quite harmful to neurons and monocytes [18]. By chelation with an organic ligand, toxicity is drastically reduced and water solubility increased [19]. Gadolinium contrast agents can be divided into two main groups based upon the structure of the ligand: linear or macrocyclic. Each group may be further subdivided into ionic or non-ionic. Macrocyclic ligands are derived from the tetraazacyclododecane ring system, providing a preorganized binding cavity offering tight binding to the Gd ion [20]. The linear ligands are derived from diethylenetriaminepentaacetic acid (DTPA) which wrap around the Gd3+ ion. They are however more flexible as they lack the conformational rigidity of a covalent ring structure [17,18,19].
The stability of these chelates has been described by two major parameters: thermodynamic stability and kinetic stability. Thermodynamic stability describes the favorability of the chelate under equilibrium conditions (usually reported as the conditional stability constant, which incorporates the effects of interfering equilibria, such as protonation of the ligand). In contrast, kinetic stability describes the rate of dissociation of the chelate, and therefore the rate at which equilibrium is reached [16, 18].
If the agent is eliminated substantially faster than it is dissociated, then the in vivo release of the Gd3+ ion would be negligible; however, if the elimination rate is slower, thermodynamic stability determines whether the agent is dissociated or not [20]. Macrocyclic agents show higher kinetic stability than linear agents by multiple orders of magnitude. Based on extrapolation from the acid dependent rate constant, the dissociation half-life for gadoterate has been estimated at 44 years at pH 7.0 [21].
The faster kinetics of linear agents (dissociation half-life estimated at 5–7 days at pH 7.4 for gadopentetate and gadodiamide) [22] place considerable importance on the thermodynamic stability. Although, the chelate is highly thermodynamically favored in a pure solution, in the physiological environment GBCAs are surrounded by various proteins and other ions, which can alter the equilibrium, either by anions (such as phosphate) or macromolecules forming favorable complexes with the Gd3+ itself, or by other metal cations forming stable complexes with the ligand [23]. This latter process is called transmetallation. Transmetalation by zinc has been long known to occur, based on the high concentration of free zinc ions in plasma, the observation of urinary zinc excretion following linear GBCA administration and changes in serum zinc concentration [24]. The released Gd3+ ions may then precipitate as insoluble compounds of phosphate and other anions [25]. Given the highly inert kinetics of the macrocyclic GBCAs, they are resistant to transmetalation [26]. The linear agents undergo variable transmetalation depending on thermodynamic stability (significantly greater for the ionic GBCA) and selectivity for Gd over other metals [20].
Evidence for Deposition in the Brain
Imaging Findings
The T1 hyperintensity of the DN in an unenhanced MRI scan was initially reported as potential grey matter damage in secondary progressive multiple sclerosis (MS) [27, 28] and was also found in patients who had received previous brain radiotherapy [29]. At the time of publication of these studies, Gd had not been considered as a possible confounder for these imaging findings, and instead was attributed to the conditions under study. In 2014, this imaging finding and high T1-signal intensity in the GP were found to be positively correlated with the number of administrations of GBCA [30] with none of the patients having a diagnosis of MS. Similar findings have been replicated in a number of retrospective imaging studies in both adults and children, drawn from a variety of populations and using multiple different agents. These imaging studies are summarized in Table 1. Overall, the majority of studies demonstrated an increase in the signal intensities of DN and GP with linear agents but not with macrocyclic agents and the increase is correlated with the number of doses. Patients with MS feature in multiple studies, and this group is subject to specific concerns over potential long-term effects, since this population is diagnosed at a young age and has a long life-expectancy [31].
Only two publications have reported more widespread imaging changes in patients who have received unusually numerous linear GBCA administrations. In patients who received >35 GBCA administrations, T1 hyperintensity has been found in other brain regions (substantia nigra, pulvinar, red nucleus, colliculi, superior cerebellar peduncle, caudate nucleus, thalamus and putamen) [32], and in the cortex of the pre-central and post-central gyri and around the calcarine sulcus [33] following at least 86 administrations (mostly linear).
More recently, quantitative MRI approaches have been applied using T1 relaxometry and quantitative-susceptibility mapping (QSM). The quantitative approach has shown a correlation between DN R1 relaxation and previous GBCA exposure [34]. As expected, QSM echoes the findings of T1 studies, namely that DN susceptibility is increased after administration of linear GBCAs [35]. Conversely, no signal alterations were found in patients who received large total doses of macrocyclic GBCAs (mainly gadobutrol) [36, 37]. Overall, retrospective studies in neurological pediatric patients replicate the findings observed in adults, namely that T1 hyperintensities in the brain are observed following administration of linear GBCAs [38,39,40] but not macrocyclic GBCAs [41, 42]; however, three imaging studies stand out as exceptions to reporting imaging changes with macrocyclic GBCAs. One study [43] claimed a change in DN signal intensity following use of macrocyclic agents, although the study had limitations in its design and did not completely rule out confounding factors (including prior exposure to linear agents) [44]. Later studies have not shown similar findings [45,46,47].
A single-center prospective study in non-neurological, oncological patients who received 0.05 mmol/kg of gadobenate rather than the 0.1 mmol/kg formulation of gadobenate or gadopentetate, showed no significant differences in the DNP and GPT signal intensity ratios [48] compared with unexposed controls. While it is true that these imaging findings are linked to the quantity of Gd being administered, the sensitivity of signal changes for the presence of deposited Gd remains unknown, and therefore this study does not exclude the presence of deposited Gd.
A retrospective analysis by Rossi Espagnet et al. demonstrated an increase in DNP and GPT signal ratios in pediatric patients exposed to macrocyclic GBCAs without a visible SI increase in the dentate nucleus or globus pallidus [49]. Subsequent correspondence has been critical [50], suggesting that the signal ratios claimed should have been associated with visible SI changes, and that this finding is difficult to explain as macromolecular-bound Gd, the high relaxivity species implicated as cause for this signal change, has been detected only following use of linear agents [51]. In response, the authors pointed out that not all of the published studies reported visible high SI despite an increase in DNP and GPT ratios [30] and an autopsy study [52] showed evidence of Gd deposition in the cerebellum without evidence of hyperintensity on MRI [53]. A repeat of the adult studies on children would give a clearer picture on an already controversial topic.
Human Tissue Studies
The use of inductively coupled plasma mass spectrometry (ICP-MS) combined with transmission electron microscopy and light microscopy, provided unambiguous evidence of Gd deposition. This has also permitted quantitation to the range 0.1–58.8 µg/g within the DN in one series [8], and 1.01 µg/g in another [52]. Subsequently, the higher spatial resolution techniques of scanning electron microscopy/energy dispersive X‑ray spectroscopy (SEM/EDS) and laser ablation ICP-MS (LA-ICP-MS) have provided further information. In biopsy specimens of tumors, the microscopic resolution of SEM/EDS showed Gd deposits in vascular areas, particularly walls of blood vessels and with calcification, with quantity related to number of exposures [9]. The LA-ICP-MS on autopsy specimens has localized Gd to the DN and throughout the cerebellar cortex [52]. Higher levels within the folia depths may be due to the presence of cerebellar microvascular end arteries [54]. Initially it was thought that this deposition occurred because of a disrupted blood-brain barrier (BBB) but autopsy studies using ICP-MS and TEM/EDS confirmed that this phenomenon was also quantitatively observed in patients at sites remote from intracranial pathology (e. g. tumors) with deposition in the endothelium, neuronal interstitium and cell nuclei [55]. Considering the agents’ pharmacodynamics and gadolinium’s association with nephrogenic systemic fibrosis (NSF) it was speculated that there is an association with renal failure; however, this finding was also present in patients with a normal renal function [56]. Moreover, cumulative doses were associated with the amount of Gd being deposited [8].
Animal Models
Repeated administrations of GBCAs, mostly with linear agents, have demonstrated T1 signal hyperintensity in deep cerebellar nuclei in animal models [57,58,59]. Histological analysis demonstrated no pathological alterations in examination of the H&E stained slides and using immunohistochemistry both in animal models [60] and in humans [55] although Gd deposits were noted in neuronal cells. Although different mechanisms have been proposed, the actual pathway of how Gd or GBCAs manage to cross the BBB is unknown [61].
Mechanisms of Deposition
Considering the differing stabilities of GBCAs, it is not surprising that deposition of Gd was mostly associated with linear agents [56, 62,63,64,65,66,67]. An autopsy study [56] has shown evidence of brain Gd deposition with macrocyclic agents although it was lower when compared to linear agents and the study had some confounding factors. The mechanism by which GBCAs enter the brain remains incompletely understood. Rat studies [68, 69] suggest that the first step is accumulation of the agent in the cerebrospinal fluid (CSF) presumably via the choroid plexus [70], followed by distribution to the brain. Human imaging studies provide additional evidence for CSF entry: in a study investigating BBB breakdown in stroke patients, progressive T1 changes in CSF were shown in the 30 min after administration of Gd [71]. Similar delayed CSF enhancement has been shown in a subsequent study using both linear and macrocyclic agents [72]. This latter study demonstrated CSF enhancement in healthy controls implying that GBCA entry into the CSF is physiological and not isolated to pathological states. The transfer of GBCA from CSF to the brain is currently thought to be via the “glymphatic” pathway. Originally described by Iliff et al. [73], the glymphatic system provides a mechanism of fluid exchange whereby CSF enters the brain parenchyma in peri-arterial channels, and circulates through the interstitial fluid of the brain, before exiting in peri-venular channels. Small molecules are readily transported by this system from the CSF into the brain, including GBCAs [74].
The pattern of brain deposition of Gd following high-dose exposure has been linked to the pattern of physiological iron deposition [33] on MRI. Similarly, in a recent review [14] this pattern was again noted with reference to detailed anatomical studies on brain iron. A rat study specifically investigating this hypothesis demonstrated that Gd concentration in brain regions following linear GBCA administration was strongly correlated with regional iron concentration (Fe-Gd correlation; gadodiamide: r = 0.9455, p < 0.0001; gadobenate: r = 0.7909, p < 0.01; gadoterate: r = 0.4455, p = 0.17) [75]. The authors proposed two hypotheses to explain this correlation: iron transmetalation or a shared entry pathway; however, work describing the glymphatic pathways shows wide distribution of Gd-DTPA (gadopentetate) in rat brain [74], which is at odds with the shared entry hypothesis. As the thermodynamic stability of various GBCA ligands are known be orders of magnitude higher for Fe3+ than for Gd3+ [76, 77], the iron transmetalation hypothesis is highly plausible.
Although the long-term effects of Gd exposure are unknown, research is being conducted to assess whether the phenomenon of Gd retention is potentially reversible. The possibility of slow excretion or washout of Gd was hinted at by the original report [30] which found an association between GBCA administration frequency and T1 signal hyperintensity. A later imaging study found a decrease in the high signal intensity of the DN when a linear GBCA is switched to a macrocyclic GBCA further supporting the existence of a washout effect [45]. This was subsequently demonstrated in a rat model in an industry-sponsored study [60], with a further rat study separating the retained Gd into three fractions: small water-soluble molecules, soluble macromolecules and water-insoluble forms, with evidence of continued excretion of the water-soluble forms between 3 and 24 days. Macromolecular and insoluble forms were found only after linear GBCA administration [51]. Based on theoretical knowledge from radionuclide decorporation treatments, another group have stated that using a chelator to remove Gd is theoretically possible [78].
Accumulation of Gd has also been demonstrated in organs other than the brain especially in bone and skin, including after macrocyclic agents, and with bone concentrations significantly higher than in brain [56]. Reports of delayed onset NSF following GBCA [79, 80] suggest that bone may act as a long-term reservoir of Gd which is subsequently released; however, in the absence of severe renal impairment the importance of this reservoir effect is unknown.
Clinical Effects
The main deep grey nuclei in the brain that are affected with Gd deposition are the DN and GP. The DN is recruited for motor functions, motor procedural learning, sensory functions and cognitive tasks [81]. Injury to the GP can lead to dystonia and parkinsonism [82]. So far there have been no studies in animals or humans that have demonstrated clear behavioral [57] or clinical changes (Parkinsonian symptoms) [83] secondary to Gd deposition in the brain, that is, there has been no definite evidence of harm; however, recently a retrospective study in patients with multiple sclerosis (MS) demonstrated that high T1 signal intensity in the GP and DN is associated with worse verbal fluency scores, although the authors also said that many other areas of the brain are affected in MS patients which may also have an influence on the neuropsychological test results [84].
In the last Consortium of Multiple Sclerosis Centers (CMSC) annual meeting it was established that Gd-enhanced MRI scans should be only reserved for specific cases and not be used for routine monitoring [85]. Even though a prospective single-center study had shown that cumulative doses of macrocyclic GBCA increases the detection of enhancing lesions in patients with clinical isolated syndromes or relapsing MS [86], the authors recommended against the routine use of this protocol because of the uncertainties associated with Gd deposition in the brain. Implications to practice may extend to other populations that require contrast-enhanced MRI scans for the monitoring of disease activity such as in patients suffering from Crohn’s disease [87].
One group has proposed the term “gadolinium storage condition” for the state of Gd deposition in brain, and the term “gadolinium deposition disease” for a symptomatic condition [88]. Based on a patient group self-reported symptom survey [89], they described variable symptoms including headache, bone and joint pain, clouded mentation and skin symptoms similar to NSF. Although this survey has major methodological limitations and does not address causality, it is the first attempt to describe a clinical syndrome that may occur after multiple Gd administrations.
Future Implications
Research into alternative contrast agents is currently a topic of interest. One research path has been the replacement of Gd3+ chelates with other transition metals chelates as T1 agents; a series of high relaxivity Mn2+ agents using novel ligands have been described, but stability is lower than existing GBCAs [90]. An alternative approach has been to exploit the very high thermodynamic stability of Fe3+ (several orders of magnitude higher than Gd3+) chelates with established ligands, and the use of higher doses to mitigate the lower relaxivity. [77]. A different approach has explored metal-free agents and demonstrated the potential of nitroxide-based, nano-structured polymers as T2 agents in a murine model [91].
Regulatory Responses
With regards to advice from regulatory bodies, in 2015 the U.S. Food and Drug Administration (FDA) had advised a review of the administration protocols of GBCAs so as to limit exposure while studies were being evaluated [92]. While these recommendations have remained unchanged, in the latest safety announcement they have asked for the creation of medication guides which every patient will be asked to read before being administered a GBCA [93].
Recently, the International Society of Magnetic Resonance in Medicine (ISMRM) Safety Committee also made similar recommendations [94] although they ignored the washout effect of chelated Gd [59] and state that there is no evidence that shows any harmful effects from deposition of chelated or unchelated Gd. Radbruch et al. have critiqued this recommendation and said that while it is true that there is no clinical evidence of any side-effects from either chelated or dechelated Gd deposition in the brain, the reason why Gd was chelated in the first place was to prevent its toxic effects in its free form [95]. In 2017 the European Medicines Agency (EMA) finalized its review on GBCAs and stated that although currently there is no evidence of any clinical side-effects, they advised the precautionary suspension of marketing authorisations for four linear agents: gadobenate dimeglumine, gadodiamide, gadopentetate dimeglumine, and gadoversetamide. Gadoxetic acid was not suspended because of its importance in liver MRI and a gadopentetic acid formulation for arthrograms was also not suspended because of its very low Gd concentration [96].
Considering the aforementioned evidence, the overall approach when administering GBCAs should be in line with what regulatory bodies [93, 94, 96] and associations of vulnerable patient groups such as the CMSC [85] have recommended.
The use of an appropriate level of caution is suggested: good clinical practice should already mean that Gd is only used when clinically indicated and should not be withheld where an appropriate indication exists.
In Europe, the EMA’s suspension of marketing authorization of several linear agents makes the clinical decision to change practice so as to use macrocyclic agents largely moot. Otherwise, the decision to change practice in the absence of robust evidence of harm is a difficult one. Nevertheless, there is evidence of pharmacokinetic differences between agents, and where all else is equal, it would seem reasonable to prefer an agent which shows less deposition than one which shows more.
Additionally, the use of patient information literature which explains the recent discovery of Gd deposition that it remains of uncertain significance, together with assurance that there is an appropriate indication for its use may also help avoid unnecessary anxiety. An explanation that GBCAs differ in their tendency to cause hyperintensities and detailed information about the prescribed GBCA have also been suggested [30].
Conclusion
There is enough evidence that GBCAs cross the BBB and deposit in the deep nuclei of the brain, especially after repeated exposures. While deposition happens in both linear and macrocyclic agents, it more likely to happen with linear agents, hence the recommendations by the regulatory authorities. There is currently no significant evidence of any biological or clinical effects. The GBCAs are an important and essential tool in the field of neuroradiology and the current challenge is actually quantifying the potential long-term risks in the light of their significant benefits. Further research is required into the mechanisms by which GBCAs enter and deposit Gd in the brain parenchyma. The potential long-term biological and clinical effects require ongoing surveillance in order to quantify the impact on patient care.
Abbreviations
- BBB:
-
Blood-brain barrier
- CMSC:
-
Consortium of Multiple Sclerosis Centers
- CSF:
-
Cerebrospinal fluid
- DN:
-
Dentate nucleus
- EMA:
-
European Medicines Agency
- FDA:
-
U.S. Food and Drug Administration
- Fe:
-
Iron
- GBCA:
-
Gadolinium-based contrast agent
- Gd:
-
Gadolinium
- GP:
-
Globus pallidus
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- LA-ICP-MS:
-
Laser ablation inductively coupled plasma mass spectrometry
- MRI:
-
Magnetic resonance imaging
- MS:
-
Multiple sclerosis
- NSF:
-
Nephrogenic systemic fibrosis
- QSM:
-
Quantitative-susceptibility mapping
- SEM/EDS:
-
Scanning electron microscopy/energy dispersive X‑ray spectroscopy
References
Laniado M, Weinmann HJ, Schörner W, Felix R, Speck U. First use of GdDTPA/dimeglumine in man. Physiol Chem Phys Med Nmr. 1984;16:157–65.
Runge VM, Clanton JA, Price AC, Herzer WA, Allen JH, Partain CL, James AE Jr. Dyke Award. Evaluation of contrast-enhanced MR imaging in a brain-abscess model. AJNR Am J Neuroradiol. 1985;6:139–47.
Runge VM. Safety of the gadolinium-based contrast agents for magnetic resonance imaging, focusing in part on their accumulation in the brain and especially the dentate nucleus. Invest Radiol. 2016;51:273–9.
Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000–1.
Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–8.
Marckmann P. An epidemic outbreak of nephrogenic systemic fibrosis in a Danish hospital. Eur J Radiol. 2008;66:187–90.
Bennett CL, Qureshi ZP, Sartor AO, Norris LB, Murday A, Xirasagar S, Thomsen HS. Gadolinium-induced nephrogenic systemic fibrosis: the rise and fall of an iatrogenic disease. Clin Kidney J. 2012;5:82–8.
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:772–82.
Xia D, Davis RL, Crawford JA, Abraham JL. Gadolinium released from MR contrast agents is deposited in brain tumors: in situdemonstration using scanning electron microscopy with energy dispersive X‑ray spectroscopy. Acta Radiol. 2010;51:1126–36.
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276:228–32.
Kanal E. Gadolinium based contrast agents (GBCA): safety overview after 3 decades of clinical experience. Magn Reson Imaging. 2016;34:1341–5.
Lenkinski RE. Gadolinium Retention and Deposition Revisited: How the Chemical Properties of Gadolinium-based Contrast Agents and the Use of Animal Models Inform Us about the Behavior of These Agents in the Human Brain. Radiology. 2017;285:721–4.
Tedeschi E, Caranci F, Giordano F, Angelini V, Cocozza S, Brunetti A. Gadolinium retention in the body: what we know and what we can do. Radiol Med. 2017;122:589–600.
Kanda T, Nakai Y, Hagiwara A, Oba H, Toyoda K, Furui S. Distribution and chemical forms of gadolinium in the brain: a review. Br J Radiol. 2017;90:20170115.
Olchowy C, Cebulski K, Łasecki M, Chaber R, Olchowy A, Kałwak K, Zaleska-Dorobisz U. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity—a systematic review. PLoS One. 2017;12:e171704.
Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–67.
Tweedle MF, Hagan JJ, Kumar K, Mantha S, Chang CA. Reaction of gadolinium chelates with endogenously available ions. Magn Reson Imaging. 1991;9:409–15.
Sherry AD, Caravan P, Lenkinski RE. Primer on gadolinium chemistry. J Magn Reson Imaging. 2009;30:1240–8.
Penfield JG, Reilly RF. What nephrologists need to know about gadolinium. Nat Rev Nephrol. 2007;3:654–68.
Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann HJ. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37°C. Invest Radiol. 2008;43:817–28.
Tóth É, Brücher E, Lázár I, Tóth I. Kinetics of formation and dissociation of lanthanide (III)-DOTA complexes. Inorganic chemistry. 1994;33:4070–6.
Sarka L, Burai L, Király R, Zékány L, Brücher E. Studies on the kinetic stabilities of the Gd(3+) complexes formed with the N‑mono(methylamide), N′-mono(methylamide) and N,N″-bis(methylamide) derivatives of diethylenetriamine-N,N,N′,N′,N′-pentaacetic acid. J Inorg Biochem. 2002;91:320–6.
Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–352.
Puttagunta NR, Gibby WA, Smith GT. Human in vivo comparative study of zinc and copper transmetallation after administration of magnetic resonance imaging contrast agents. Invest Radiol. 1996;31:739–42.
Gibby WA, Gibby KA, Gibby WA. Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol. 2004;39:138–42.
Laurent S, Elst LV, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol Imaging. 2006;1:128–37.
Roccatagliata L, Vuolo L, Bonzano L, Pichiecchio A, Mancardi GL. Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology. 2009;251:503–10.
Absinta M, Rocca MA, Filippi M. Dentate nucleus T1 hyperintensity in multiple sclerosis. AJNR Am J Neuroradiol. 2011;32:E120–1.
Kasahara S, Miki Y, Kanagaki M, Yamamoto A, Mori N, Sawada T, Taoka T, Okada T, Togashi K. Hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with a history of brain irradiation. Radiology. 2011;258:222–8.
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–41.
Leray E, Moreau T, Fromont A, Edan G. Epidemiology of multiple sclerosis. Rev Neurol (Paris). 2016;172:3–13.
Zhang Y, Cao Y, Shih GL, Hecht EM, Prince MR. Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology. 2017;282:516–25.
Khant ZA, Hirai T, Kadota Y, Masuda R, Yano T, Azuma M, Suzuki Y, Tashiro K. T1 shortening in the cerebral cortex after multiple administrations of gadolinium-based contrast agents. Magn Reson Med Sci. 2017;16:84–6.
Tedeschi E, Palma G, Canna A, Cocozza S, Russo C, Borrelli P, Lanzillo R, Angelini V, Postiglione E, Morra VB, Salvatore M, Brunetti A, Quarantelli M. In vivo dentate nucleus MRI relaxometry correlates with previous administration of gadolinium-based contrast agents. Eur Radiol. 2016;26:4577–84.
Hinoda T, Fushimi Y, Okada T, Arakawa Y, Liu C, Yamamoto A, Okada T, Yoshida K, Miyamoto S, Togashi K. Quantitative assessment of gadolinium deposition in dentate nucleus using quantitative susceptibility mapping. J Magn Reson Imaging. 2016;45:1352–8.
Tedeschi E, Cocozza S, Borrelli P, Ugga L, Morra VB, Palma G. Longitudinal assessment of dentate nuclei relaxometry during massive gadobutrol exposure. Magn Reson Med Sci. 2018;17:100–4.
RRadbruch A, Haase R, Kieslich PJ, Weberling LD, Kickingereder P, Wick W, Schlemmer HP, Bendszus M. No signal intensity increase in the dentate nucleus on unenhanced T1-weighted MR images after more than 20 serial injections of macrocyclic gadolinium-based contrast agents. Radiology. 2017;282:699–707.
Flood TF, Stence NV, Maloney JA, Mirsky DM. Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology. 2017;282:222–8.
Hu HH, Pokorney A, Towbin RB, Miller JH. Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol. 2016;46:1590–8.
Roberts DR, Chatterjee AR, Yazdani M, Marebwa B, Brown T, Collins H, Bolles G, Jenrette JM, Nietert PJ, Zhu X. Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol. 2016;37:2340–7.
Tibussek D, Rademacher C, Caspers J, Turowski B, Schaper J, Antoch G, Klee D. Gadolinium brain deposition after macrocyclic gadolinium administration: a pediatric case-control study. Radiology. 2017;285:223–30.
Radbruch A, Haase R, Kickingereder P, Bäumer P, Bickelhaupt S, Paech D, Wick W, Schlemmer HP, Seitz A, Bendszus M. Pediatric brain: no increased signal intensity in the dentate nucleus on unenhanced T1-weighted MR images after consecutive exposure to a macrocyclic gadolinium-based contrast agent. Radiology. 2017;283:828–36.
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol. 2016;26:807–15.
Agris J, Pietsch H, Balzer T. What evidence is there that gadobutrol causes increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W MRI in patients with RRMS? Eur Radiol. 2016;26:816–7.
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, Schlemmer HP, Bendszus M. Intraindividual analysis of signal intensity changes in the dentate nucleus after consecutive serial applications of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol. 2016;51:683–90.
Cao Y, Huang DQ, Shih G, Prince MR. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate fimeglumine versus gadobutrol. AJR Am J Roentgenol. 2016;206:414–9.
Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, Takeshita K, Furui S. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology. 2015;275:803–9.
Schneider GK, Stroeder J, Roditi G, Colosimo C, Armstrong P, Martucci M, Buecker A, Raczeck P. T1 signal measurements in pediatric brain: findings after multiple exposures to gadobenate dimeglumine for imaging of nonneurologic disease. AJNR Am J Neuroradiol. 2017;38:1799–806.
Rossi Espagnet MC, Bernardi B, Pasquini L, Figà-Talamanca L, Tomà P, Napolitano A. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol. 2017;47:1345–52.
Radbruch A, Quattrocchi CC. Interpreting signal-intensity ratios without visible T1 hyperintensities in clinical gadolinium retention studies. Pediatr Radiol. 2017;47:1688–9.
Frenzel T, Apte C, Jost G, Schöckel L, Lohrke J, Pietsch H. Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents: comparative study in rats. Invest Radiol. 2017;52:396–404.
Roberts DR, Welsh CA, LeBel DP, Davis WC. Distribution map of gadolinium deposition within the cerebellum following GBCA administration. Neurology. 2017;88:1206–8.
Rossi Espagnet MC, Tomà P, Napolitano A. Reply to Radbruch et al.: ‘interpreting signal-intensity ratios without visible T1 hyperintensities in clinical gadolinium retention studies’. Pediatr Radiol. 2017;47:1690–1.
Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F. The microvasculature of the human cerebellar meninges. Acta Neuropathol. 2002;104:608–14.
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Paolini MA, Murray DL, Williamson EE, Eckel LJ. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without Intracranial abnormalities. Radiology. 2017;285:546–54.
Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51:447–53.
Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idée JM, Ballet S, Corot C. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol. 2015;50:473–80.
Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H. Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Invest Radiol. 2016;51:83–9.
Robert P, Violas X, Grand S, Lehericy S, Idée JM, Ballet S, Corot C. Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol. 2016;51:73–82.
Smith AP, Marino M, Roberts J, Crowder JM, Castle J, Lowery L, Morton C, Hibberd MG, Evans PM. Clearance of gadolinium from the brain with no pathologic effect after repeated administration of gadodiamide in healthy rats: an analytical and histologic study. Radiology. 2017;282:743–51.
Prybylski JP, Maxwell E, Coste Sanchez C, Jay M. Gadolinium deposition in the brain: lessons learned from other metals known to cross the blood-brain barrier. Magn Reson Imaging. 2016;34:1366–72.
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, Semelka RC. High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology. 2015;276:836–44.
Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, Radbruch A. Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol. 2015;50:743–8.
Roberts DR, Holden KR. Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev. 2016;38:331–6.
Radbruch A. Are some agents less likely to deposit gadolinium in the brain? Magn Reson Imaging. 2016;34:1351–4.
Schlemm L, Chien C, Bellmann-Strobl J, Dörr J, Wuerfel J, Brandt AU, Paul F, Scheel M. Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler. 2017;23:963–72.
Kahn J, Posch H, Steffen IG, Geisel D, Bauknecht C, Liebig T, Denecke T. Is there long-term signal intensity increase in the central nervous system on T1-weighted images after MR imaging with the hepatospecific contrast agent Gadoxetic acid? A cross-sectional study in 91 patients. Radiology. 2017;282:708–16.
Takeda A, Akiyama T, Sawashita J, Okada S. Brain uptake of trace metals, zinc and manganese. Brain Res. 1994;640:341–4.
Aoki I, Wu YJ, Silva AC, Lynch RM, Koretsky AP. In vivo detection of neuroarchitecture in the rodent brain using manganese-enhanced MRI. Neuroimage. 2004;22:1046–59.
Jost G, Frenzel T, Lohrke J, Lenhard DC, Naganawa S, Pietsch H. Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue. Eur Radiol. 2017;27:2877–85.
Wardlaw JM, Farrall A, Armitage PA, Carpenter T, Chappell F, Doubal F, Chowdhury D, Cvoro V, Dennis MS. Changes in background blood-brain barrier integrity between lacunar and cortical ischemic stroke subtypes. Stroke. 2008;39:1327–32.
Naganawa S, Kawai H, Taoka T, Sone M. Improved HYDROPS: imaging of endolymphatic hydrops after intravenous administration of gadolinium. Magn Reson Med Sci. 2017;16:357–61.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111.
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–309.
Rasschaert M, Emerit A, Fretellier N, Factor C, Robert P, Idée JM, Corot C. Gadolinium retention, brain T1 hyperintensity, and endogenous metals: a comparative study of macrocyclic versus linear gadolinium chelates in renally sensitized rats. Invest Radiol. 2018 Jan 12. [Epub ahead of print]
Fretellier N, Poteau N, Factor C, Mayer JF, Medina C, Port M, Idée JM, Corot C. Analytical interference in serum iron determination reveals iron versus gadolinium transmetallation with linear gadolinium-based contrast agents. Invest Radiol. 2014;49:766–72.
Boehm-Sturm P, Haeckel A, Hauptmann R, Mueller S, Kuhl CK, Schellenberger EA. Low-molecular-weight iron chelates may be an alternative to gadolinium-based contrast agents for T1-weighted contrast-enhanced MR imaging. Radiology. 2018;286:537–46.
Prybylski JP, Semelka RC, Jay M. Can gadolinium be re-chelated in vivo? Considerations from decorporation therapy. Magn Reson Imaging. 2016;34:1391–3.
Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. Jama Dermatol. 2015;151:1117–20.
Thomson LK, Thomson PC, Kingsmore DB, Blessing K, Daly CD, Cowper SE, Roditi GH. Diagnosing nephrogenic systemic fibrosis in the post-FDA restriction era. J Magn Reson Imaging. 2015;41:1268–71.
Habas C. Functional imaging of the deep cerebellar nuclei: a review. Cerebellum. 2009;9:22–8.
Münchau A, Mathen D, Cox T, Quinn NP, Marsden CD, Bhatia KP. Unilateral lesions of the globus pallidus: report of four patients presenting with focal or segmental dystonia. J Neurol Neurosurg Psychiatr. 2000;69:494–8.
Welk B, McArthur E, Morrow SA, MacDonald P, Hayward J, Leung A, Lum A. Association between gadolinium contrast exposure and the risk of parkinsonism. JAMA. 2016;316:96–3.
Forslin Y, Shams S, Hashim F, Aspelin P, Bergendal G, Martola J, Fredrikson S, Kristoffersen-Wiberg M, Granberg T. Retention of gadolinium-based contrast agents in multiple sclerosis: retrospective analysis of an 18-year longitudinal study. AJNR Am J Neuroradiol. 2017;38:1311–6.
Helwick C. Brain gadolinium deposition varies with contrast agent in MS. Medscape. 2017. http://www.medscape.com/viewarticle/880649. Accessed 25 May 2017.
Rovira A, Auger C, Huerga E, Corral JF, Mitjana R, Sastre-Garriga J, Tintoré M, Montalban X. Cumulative dose of macrocyclic gadolinium-based contrast agent improves detection of enhancing lesions in patients with multiple sclerosis. AJNR Am J Neuroradiol. 2017;38:1486–93.
Savarino E, Chianca V, Bodini G, Albano D, Messina C, Tontini GE, Sconfienza LM7. Gadolinium accumulation after contrast-enhanced magnetic resonance imaging: which implications in patients with Crohn’s disease? Dig Liver Dis. 2017;49:728–30.
Semelka RC, Ramalho M, AlObaidy M, Ramalho J. Gadolinium in Humans: A Family of Disorders. AJR Am J Roentgenol. 2016;207:229–33.
Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M. Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol. 2016;37:1192–8.
Forgács A, Regueiro-Figueroa M, Barriada JL, Esteban-Gómez D, de Blas A, Rodríguez-Blas T, Botta M, Platas-Iglesias C. Mono-, bi-, and trinuclear bis-hydrated Mn(2+) complexes as potential MRI contrast agents. Inorg Chem. 2015;54:9576–87.
Nguyen HV, Chen Q, Paletta JT, Harvey P, Jiang Y, Zhang H, Boska MD, Ottaviani MF, Jasanoff A, Rajca A, Johnson JA. Nitroxide-based macromolecular contrast agents with unprecedented transverse relaxivity and stability for magnetic resonance imaging of tumors. Acs Cent Sci. 2017;3:800–11.
U.S. Food and Drug Administration. Center for Drug Evaluation and Research. FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging (MRI). 2015. http://www.fda.gov/Drugs/DrugSafety/ucm455386.htm#collapseOne. Accessed 21 Dec 2016.
U.S. Food and Drug Administration. FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. 2017. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM589442.pdf. Accessed 19 Dec 2017.
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16:564–70.
Radbruch A, Roberts DR, Clement O, Rovira A, Quattrocchi CC. Chelated or dechelated gadolinium deposition. Lancet Neurol. 2017;16:955.
European Medicines Agency. EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/gadolinium_contrast_agents_31/European_Commission_final_decision/WC500240575.pdf. Accessed 17 Nov 2017.
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014;49:685–90.
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, Schlemmer HP, Bendszus M. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images. Invest Radiol. 2015;50:805–10.
Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A, Zobel BB. Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol. 2015;50:470–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Pullicino, M. Radon, S. Biswas, M. Bhojak and K. Das declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Pullicino, R., Radon, M., Biswas, S. et al. A Review of the Current Evidence on Gadolinium Deposition in the Brain. Clin Neuroradiol 28, 159–169 (2018). https://doi.org/10.1007/s00062-018-0678-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-018-0678-0