Abstract
Gadolinium-based contrast agents (GBCA), widely used in Magnetic Resonance Imaging (MRI) for almost 30 years, were recently shown to be deposited in the brain and to induce persistent T1 shortening in deep gray matter structures in subjects with normal renal function. The aim of the present study is to summarize the evidence derived from the rapidly growing scientific literature on Gadolinium retention in the brain and in the rest of the body. To this end, the original articles that described imaging and pathology findings in humans and animals exposed to GBCA were reviewed. The main aspects that emerged were the different effects of linear and macrocyclic GBCA on brain MRI appearance, the evidence of Gadolinium tissue retention in multiple organs, and the debated issue of the possible clinical consequences. Although no adverse health effects have been documented so far, updated information about GBCA build-up in the body is necessary for health professionals, also in view of the increasing concern in the general population. To date, our knowledge about the mechanisms of Gadolinium tissue deposition and, above all, its long-term consequences is still largely incomplete. However, while official guidelines are eagerly awaited, some advices may already be given, to help our radiological daily practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gadolinium-based contrast agents (GBCA) are chemical compounds used in magnetic resonance imaging (MRI) to exploit their paramagnetic properties, i.e., their capability to regionally alter the MRI signal of the biological compartment in which they accumulate.
Gadolinium (Gd) is a paramagnetic lanthanide heavy metal, that, in its free ionic form (Gd3+), can compete with Ca2+ and become toxic in biological systems [1]; therefore, it must be chelated to an organic ligand. Commercially available GBCA contain Gd chelated in different forms, are usually administered intravenously, and have been used in over 100 million patients in the last 29 years [2] and in roughly 30–45% of all clinical MR studies today [3]. GBCA are credited with an excellent safety profile, as very few, and mostly mild, acute adverse reactions have been reported, despite the large and prolonged use (0.08–0.12%) [4, 5].
Once administered, GBCA are eliminated from the body through the urinary, and, to a lesser extent, biliary system. In subjects with normal renal function, they are usually cleared from the blood in about 1.5 h, and completely recovered from the urine in 7 days (>90% in the first 12 h) [6].
GBCA can be divided into linear and macrocyclic types, the latter being considered more stable. Indeed, macrocyclic GBCA form cage-like structures with Gd3+ enclosed in the cavity of the complex and tend to have lower dissociation constants [7]. The higher the dissociation constant, the more likely free Gd can be released into the circulation and tissues [8].
Between 2006 and 2009 a safety issue emerged for GBCA, as Nephrogenic Systemic Fibrosis (NSF), a subacute/chronic disease associated with significant morbidity, was described and put in relation to previous administration of some linear GBCA in patients with renal dysfunction [3]. However, once a careful evaluation of the renal glomerular filtration rate (GFR) has been imposed as a pre-requisite to perform a contrast-enhanced (CE) MRI scan, the incidence of new NSF cases has almost disappeared [9].
Since 2014, a new safety concern regarding the use of GBCA has spread over the scientific community: the evidence, and the possible consequences, of long-term retention of GBCA in the brain after multiple CE-MRI in subjects with normal renal function. In fact, there are consistent and ever-growing imaging and histopathologic findings of Gd accumulation in individuals with normal GFR who had received, even years earlier, multiple GBCA administrations.
In this review, we focused on original articles published in peer-reviewed journals in the last 3 years, aiming to: (I) summarize the latest evidence deriving from human and animal studies on Gd retention in the body, (II) evaluate the methodological aspects of the imaging findings reported so far and (III) critically address the issue of the possible clinical consequences of the existing data. Finally, some suggestions on the effects of this increased knowledge on our radiological daily practice are presented.
Gadolinium retention in the brain: imaging findings
Most CE-MRI brain acquisitions exploit the property of GBCA to shorten the T1 relaxation time of living tissues after extravasation in the interstitial space. In the central nervous system (CNS), this was initially believed to happen almost exclusively in areas with altered blood–brain barrier (BBB). However, it is now clear that intravenously injected Gd can slowly pass an intact BBB, with mechanisms possibly involving transmetallation, specific metal transporters, or even a pathway through the CSF, perivascular spaces and the glymphatic system [3, 10]. In T1-weighted (T1w) images, contrast-enhancing lesions appear hyperintense respect to the surrounding, unenhanced brain. This signal change usually persists up to 30 min, although sporadic extended persistence has been reported [11].
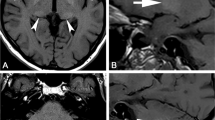
In the past 3 years, several papers reported the presence of spontaneous high signal intensity (SI) in unhenanced T1w images of the brain, mainly localized in deep gray matter structures such as dentate nuclei (DN) and globus pallidus (GP), in patients with normal renal function, all with a history of prior exposure to multiple GBCA administrations (Fig. 1).
Change in dentate nuclei signal intensity after multiple CE-MRI with linear Gadolinium-based contrast agents. Unenhanced coronal SE T1-weighted images in a patient with relapsing–remitting multiple sclerosis at diagnosis (a) and in a follow-up study 6 years later (b), after 6 injections of Magnevist®. The cerebellar dentate nuclei, initially isointense to the surrounding brain, show homogeneous bilateral and symmetrical T1w-hyperintensity at the follow-up scan. Also note worsening of the supratentorial demyelinating lesions, leading to increased axonal loss
The pioneering publication was a retrospective study in 19 brain tumor patients, who underwent at least 6 examinations with linear GBCA (gadopentate dimeglumine, Magnevist® and/or gadodiamide, Omniscan®), compared with 16 patients who received at least 6 unenhanced MRI [12]. In that study, only patients exposed to GBCA showed T1 shortening of deep gray matter nuclei, with an increase in the DN-to-pons (DNP) and GP-to-thalamus (GPT) SI ratios significantly correlated with the administered dose.
Afterwards, higher DNP SI after repeated administrations of Omniscan® has been described in relapsing–remitting multiple sclerosis (RR-MS) and meningioma patients, even after <6 GBCA administrations, with a dose–response relationship [13, 14].
Later, DN T1 shortening was associated with prior repeated exposure to Magnevist®, but not to the nonionic macrocyclic Gadoteridol (ProHance®) [15]. This important difference between linear and macrocyclic GBCAs was confirmed by other studies that assessed DN and GP SI in patients undergoing multiple MRI scans with Magnevist® or Gadobenate Meglumine (MultiHance®) vs those receiving Gadoterate Dimeglumine (Dotarem®) or Gadobutrol (Gadovist®); again, a dose-dependent T1 shortening effect was observed only for linear GBCAs [16–20]. This different behavior of the two GBCA classes supports the hypothesis that the observed T1 shortening is related to dissociation of the Gd ion from its chelating ligand molecule [21]. Table 1 lists the main features of the GBCA approved for CNS imaging along with the corresponding findings reported in imaging and pathology studies in humans and animals with a normal renal function.
Since 2014, some conflicting findings have also been reported. For example, multiple injections of MultiHance® were associated with significantly lower DN/middle cerebellar peduncle and GPT T1w-SI ratios compared to patients who received (also) Omniscan® [22, 23]. Conversely, in another study, MultiHance® induced increased DNP T1w-SI ratio [20]. Possible explanations for this discrepancy may include differences in the amount of Gd given or in the image analysis. As for Gadovist®, which has high kinetic stability coupled to a relatively low thermodynamic stability [8, 24], one report claiming that increased DNP and GPT SI ratios were present in MS patients who received >4 injections [25] has been heavily criticized, and its findings were not replicated in other subsequent studies that analyzed SI changes after administration of this GBCA in humans [18, 19, 26–28] and animals [29].
The DN consistently represented the major site of increased SI in the brain after multiple linear GBCA administrations in several studies [12, 15, 19, 26, 28, 30–36]. The reason for such susceptibility may be related to the fact that DN are preferential sites of accumulation of metallic ions and calcium [37, 38], as well as to their proximity to the choroid plexus of the fourth ventricle, which is known to sequester toxic heavy metals and metalloid ions [39]. One may thus speculate that a transport mechanism mediates the preferential accumulation of Gd in some brain regions, using the blood/CSF barrier as a passageway toward the interstitium [40].
The intriguing observation that, by increasing the administered GBCA volume, Gd accumulation becomes evident in other brain sites involved in the deposition of minerals and metallic ions also supports this hypothesis [41]. In fact, in 13 patients who received at least 35 doses of linear GBCA, a significant T1w hyperintensity was evident not only in DN and GP, but also in the substantia nigra, posterior thalamus, red nucleus, colliculi, superior cerebellar peduncle and caudate nucleus [36]. Probably, higher doses can saturate the most common sites of deposits, resulting in a more complex Gd distribution [10]. In a patient who had undergone >80 CE-MRI with mixed (linear and macrocyclic) GBCA, T1w hyperintensity was observed not only in DN and basal ganglia, but also in the cortex around the central and calcarine fissures [42]. Conversely, no evidence of significant T1 shortening was observed, both using “conventional” SI [16, 43] and relaxometry [44] methods, even after massive cumulative doses of macrocyclic GBCA. However, it should be remembered that the T1 shortening detectable by MRI does not by any means linearly reflect the actual amount of Gd deposited in the brain, as, on one hand, some Gd may be present in a “magnetically inert” (i.e., insoluble) form, and, on the other hand, tiny amounts of Gd, likely without biologic effect, may induce striking MRI evidence.
Imaging of gadolinium retention in the brain: methodological considerations
A better understanding of the Gd-related MRI changes in the brain requires the analysis of the imaging techniques used.
Most studies on SI changes evaluated T1w-hyperintensity on Spin Echo (SE) sequences, with a slice thickness of 4–5 mm, and compared DN SI to normal-appearing areas at the same level of the DN, such as pons or middle cerebellar peduncle. Similarly, studies analyzing GP SI changes have mainly used the thalamus as reference ROI. Although some Gd deposition is present also in these reference regions [45], this approach consistently proved that DN and GP SI increases with the increasing number of linear, but not macrocyclic, GBCA administrations. Some authors also used CSF as a reference ROI [17, 20]; however, GBCAs have been shown to pass, at least temporarily, into CSF [29, 46] and, as previously mentioned, CSF may actually represent a pathway to reach deep GM structures.
It is currently agreed that SE and Gradient Echo (GrE)-T1w sequences cannot be used interchangeably for evaluating SI, with some authors even preferring the latter for qualitative analysis [47]. Tanaka et al. [48] showed that post-contrast SE-T1 sequences could be used instead of unhenanced SE-T1, if the latter is unavailable. Furthermore, no study has compared the effect of different field strengths on these SI measurements [49]. However, serial measurements should obviously be performed using the same sequence and the same field strength, possibly on the same MR scanner.
To obviate some of the limitations related to qualitative parameters like SI, quantitative approaches for measuring GBCA-induced T1 shortening have been proposed. In 74 RR-MS patients exposed to different GBCA subtypes, Tedeschi et al. [50], using a validated relaxometry method [51, 52], assessed the R1 (1/T1) and R2* (1/T2*) relaxation rates, i.e., quantitative MRI metrics intrinsically related to tissue microstructure and not affected by the entangled contrasts of the SI images, or by acquisition-related confounding factors [53]. It was thus demonstrated that DN T1 shortening in patients exposed to Magnevist® is linked only to the number of previous GBCA administrations and not to R2*changes (and therefore possible iron build-up), nor to MS-related factors such as disease severity or duration. Using another relaxometry approach, other authors showed that global and regional T1 and T2 values correlate with the number and volume of prior Magnevist® injections in different gray matter structures [54].
Recently, Quantitative Susceptibility Mapping (QSM) has been used to evaluate DN susceptibility changes associated with Gd retention, showing significantly higher DN susceptibility values in patients exposed to GBCA compared to subjects with no history of GBCA administration [55]. In this study, the 5 patients of the GBCA group who received only macrocyclic agents showed QSM values close to those of the non-GBCA group.
Further, other clinical conditions such as Fahr disease, pseudohypoparathiroidism, post-radiation therapy changes, Wilson Disease, Hepatic encephalopathy, etc., may cause T1-hyperintensity in deep gray matter [56], somewhat resembling Gd deposition (Fig. 2). Quantitative techniques such as QSM were shown useful for differentiating accumulation of paramagnetic metals from calcifications [57].
Examples of increased T1-wighted signal intensity in the dentate nuclei due to different clinical conditions. Axial unenhanced SE-T1 (a, c, e, g, i, k) and GE-T2* (b, d, f, h, j, l) images at the level of dentate nuclei (upper row) and basal ganglia (lower row) in a patient with Fahr’s syndrome (a–d), in a patient with neurodegeneration with brain iron accumulation (e–h), and in a patient with hypoparathyroidism (i–l), respectively. In all cases, the dentate nuclei display increased signal intensity (less prominent in e), associated with: T2*-w hypointense signal, due to symmetrical bilateral calcifications of the basal ganglia (c, d), T2*-w mild hypointense signal in the basal ganglia due to homogeneous iron deposition (g, h), and diffuse cerebral atrophy and strong signal reduction on T2*-w in the basal ganglia (k–l), with the T1 shortening effect dominating peripherally (i–k)
Finally, regardless of the imaging technique [58], other sources of variability should be taken into account. These include the time interval between GBCA administration and MRI acquisition, patient’s characteristics such as age, type of disease, concurrent therapies [49], and the possible interactions between different classes of GBCA. For example, the DN T1w-hyperintensity due to multiple Magnevist® injection was apparently reduced when patients were subsequently given macrocyclic GBCAs, potentially indicating a washout effect or precipitation of Gd [19].
Gadolinium retention in the brain: histopathologic reports in humans and in animal models
Important information on tissue Gd deposition has also been provided by pathology studies. The first description of Gd retention in the brain of subjects without severe renal failure was a report on 30 biopsies and surgical resections of patients with brain tumors, and was firstly related to the loss of integrity of the BBB. This result provided indirect evidence of transmetallation and release of dechelated Gd in vivo, defined a different stability of GBCA (as Gd deposition was significantly higher in patients that received Omniscan® than in those exposed to Multihance®) and showed that Gd accumulation was related to the number of GBCA administrations [59]. Later, Gd deposition has been assessed using inductively coupled plasma mass spectrometry (ICP-MS) in post-mortem brain samples of subjects exposed to different linear GBCA [45, 60, 61].
In 13 autopsy subjects exposed in life to >4 administrations of Omniscan®, McDonald et al. detected 0.1–58.8 μg of Gd per gram of tissue in DN, GP, thalamus, and pons, with a significant correlation between dose and SI at MRI. Gd was deposited not only within the endothelial wall, but also in the neural tissue, confirming that Gd can pass through the BBB. Notably, no signs of neuronal damage in the involved brain tissue were observed [45].
In 5 autopsy subjects with an history of >2 administrations of Magnevist® and Omniscan®, a significant Gd concentration was observed in DN, GP, cerebellar white matter, frontal lobe cortex, and frontal lobe white matter, highest in DN and GP. Interestingly, no Gd deposition was found in the non-GBCA-exposed control group. Finally, no abnormal macroscopic changes were detected in the analyzed regions [60].
More recently, the autopsy specimens from 9 patients (five receiving 1–11 Prohance® injections, two receiving 1–2 Gadovist® injections, one receiving 1 dose of Multihance®, and one receiving 10 Gadoxetate® injections) were studied by ICP-MS [61]. Variable Gd deposition was found in all subjects in DN, GP, putamen, caudate nucleus, white matter and pons, with higher levels in DN and GP. Comparing these data with those of Mc Donald et al. [45], Gd deposition after Prohance® was lower than after Omniscan®. However, the population sample for the other GBCA tested in this study was insufficient for a formal statistical comparison. Moreover, some confounding factors could not be excluded by the authors, thus limiting the conclusions that can be drawn.
Several animal studies have also been conducted to evaluate Gd deposition after repeated administration of different GBCA using MRI and/or pathology metrics.
In healthy rats, Robert et al. [62, 63] evaluated the SI in the deep cerebellar nuclei (DCN) and the concentration of Gd, through ICP-MS, after repeated administrations of different GBCA (Omniscan®, Multihance®, and Magnevist®) compared to Dotarem® and saline. Both studies indicate that multiple injections of linear GBCA were associated with progressive and significant T1w-hyperintensity in DCN (highest after Omniscan®), and with Gd deposition in the cerebellum, while no effects (either histologic or at MRI) were observed after Dotarem® or saline administration.
In a similar rat model, Jost et al. [29] compared Omniscan®, Multihance® and Magnevist® (linear) with Dotarem® or Gadovist® (macrocyclic) and with saline. Rats that received macrocyclic GBCAs did not show an increased SI in the DCN or GP. In contrast, DCN/Pons SI ratio was increased after the administration of linear GBCAs, most pronounced after Omniscan®, followed by Multihance®.
Further studies with ICP-MS have shown that, by increasing the Gd load from linear GBCA (by either incrementing the dose or reducing the renal clearance), Gd deposition is present not only in the DCN, but also in cerebral cortex, subcortical brain, brainstem, olfactory bulbs and pons, [40, 62, 64]. However, it was recently shown that, even after 12 mmol/kg of Omniscan®, no histopathologic changes were observed in the rat brain, and only 0.00011% of the injected dose was retained at 20 weeks [65].
Gadolinium retention beyond the brain
Bone
Bone has long been known to be a preferential site of Gd deposition [66], and likely serves as a reservoir of Gd in the body [67]. Nevertheless, that was not common knowledge in the radiological community, likely because of the lack of association with signal abnormalities at MRI. It has been estimated that approximately 0.25–1% of the injected Gd may be released from the contrast agent and deposited in the bones, even in patients with normal renal function [68].
In particular, in the resected femoral heads of patients who underwent total hip arthroplasty 3–8 days after CE-MRI with Omniscan® or ProHance®, the amount of Gd deposited in bone was 2.5–4 times higher in subjects who received the former GBCA [69, 70]. This difference was not replicated in another study, where resected femoral head bone samples up to 8 years after Omniscan® or ProHance® exposure showed high concentrations of Gd, especially in the trabecular bone [68]. Recently, high levels of bone Gd deposition after macrocyclic GBCA administration were measured by ICP-MS in few decedents with a normal GFR. Therefore, bone measures of Gd accumulation have been proposed as an indirect method to indicate approximate levels of Gd in brain [61].
Skin and other sites
Most of the information about Gd deposition in the skin comes from studies in patients with NSF, who showed increased Gd content both in affected and unaffected skin [71–73]. The first evidence of NSF was reported in 2006, showing skin fibrosis resulting from abnormal proliferation of fibroblasts and collagen in patients with severe renal impairment after GBCA administration [74]. It soon became clear that, in patients with renal failure, the highest risk of NSF was associated with previous administration of linear GBCA with an incomplete ring [75]. In an autopsy case of a patient who died of NSF, deposition of insoluble Gd-phosphate was observed in skin, liver, lungs, intestinal wall, kidney, skeletal muscles and cerebellum [76]. However, while several studies suggest that impaired Gd clearance leads to tissue accumulation of dissociated Gd and promotes NSF development, only a tiny minority of patients with severe renal disease exposed to linear GBCA developed NSF [3].
More relevant for our purposes is the evidence of detectable Gd concentration also in the skin of subjects with normal GFR exposed to GBCA [73]. In a brain tumor patient with normal renal function who received 61 injections of mostly linear GBCA, arm and leg biopsies of deep skin layers were performed because of severe generalized joint contractures. High levels of Gd were deposited in the skin of this subject, associated with signs of inflammation (as depicted by an increased numbers of fibrocytes or macrophages, as well as increased CD34 immuno-reactivity in subcutaneous adipose tissue), in the absence of local skin alterations [77].
Using ICP-MS, Murata et al. detected a variable amount of Gd deposition in the skin (as well as brain and bone) in a series of autopsied patients with normal renal function who had received macrocyclic or linear GBCA. Gd concentration was lower in skin tissue after Prohance® than after Multihance® [61]. Therefore, in contrast with previous knowledge [78], even in patients with normal renal function, in vivo clinical exposure to GBCA results in Gd accumulation into different body tissues such as skin, bone matrix or brain. Moreover, Gd retention increases with repeated GBCA exposure. Data from rat studies, which have long shown Gd accumulation in skin, liver, spleen, bone and brain [79, 80], recently confirmed the marked difference in tissue deposition between linear and macrocyclic GBCA [81]. However, the final form in which Gd is deposited in the tissues is still uncertain and little is known about the levels of Gd required to induce tissue structural changes and to achieve clinical significance in humans [47].
Clinical concerns from gadolinium retention
After the almost complete disappearance of NSF by limiting or avoiding the use of GBCA in subjects with advanced renal failure and employing more stable GBCAs, the only unconfounded clinical events associated with GBCA administration were sporadic allergic reactions [3].
Following the reports of Gd retention in the brain in 2014, a variety of symptoms arising shortly after the administration of GBCA were described in patients with normal renal function. In some of these patients, the persistence of Gd was demonstrated by its elevated concentrations in urine, hair, or in the saphena vein [82, 83]. This presumed disease process, observed in subjects with normal (or borderline) renal function who develop symptoms unexplained by other preexistent or subsequent diseases, has been named “Gadolinium Deposition Disease” (GDD) [83, 84].
Alleged GDD symptoms include tightness or excruciating pain of the arms and legs (like sharp pins and needles, cutting, or burning), typically in a distal distribution (like being fitted with extremely tight “glove-and-sock”), but also in the central torso or generalized in location. Bone pain and persistent headache with clouded mentation (“brain fog”) were also commonly reported [83, 84]. These symptoms usually appear from hours up to 2 months after the last CE-MRI (mostly within 1 month), with persistent pain in the extremities [83]. However, it should be noted that the clinical picture of the presumed Gd toxicity in these subjects was collected by one single research center, using an online anonymous survey where 42 patients self-reported their symptoms, without a control group. Thus, this approach is heavily exposed to selection bias, as also stated by the authors themselves [83, 85].
In some patients, subcutaneous soft-tissue thickening (“Gadolinium-associated plaques”) may be observed. It appears spongy or rubbery, in contrast with the stiffness and redness typical of NSF lesions. Moreover, tendons and ligaments in a comparable distribution may also be thickened and painful [84]. In 2 patients without NSF exposed to Omniscan®, sclerotic bodies (eosinophilic, collagenous, round or ovoid bodies, thought to be pathognomonic for NSF) were found at histopathologic examination of the skin [86].
The symptoms described might be considered as a toxic effect of Gd, resembling the development of NSF. According to a Team of Patient Advocates, which timely entered in the field, “the reported physical symptoms of Gd deposition disease are similar but not identical, and lesser in severity, to those observed in NSF” [84, 87]. However, the causal relationship between GBCA and chronic effects is not fully established, and only hypothesized [84, 88].
The reason why only a small percentage of patients develop symptoms after GBCA administration is unclear, as with NSF. An hypothesis is that less stable GBCA are more likely associated with symptoms, similarly to NSF, with other host factors, such as genetic susceptibility and/or adaptive immune response, likely playing a relevant role in determining the development of GDD [84]. However, these clinical findings were surprisingly reported even after one single administration of all GBCA, excluding Dotarem® [82, 83]. Thus, a well-conducted, prospective evaluation of the real clinical incidence and pathogenesis of the presumed GDD is strongly warranted, especially due to the high impact of this topic not only in the scientific community, but also in non-specialized media.
Gd neurotoxicity has been rarely described in early cases of presumed Gd-induced encephalopathy, in patients with renal dysfunction and other significant comorbidities [89–92]. It is actually unknown if the Gd retention in the brain has a clinical correlate, or leads to adverse neurological effects. So far, no significant association between Gd exposure and the development of Parkinsonism or other movement disorders has been demonstrated [93, 94]. None of the imaging studies on Gd-induced T1w shortening reported neurological symptoms related to GBCA administration, and no signs of tissue damage were observed in human or animal pathology studies. However, due to the description of nonspecific symptoms, such as pain or cognitive changes, after Gd exposure [47], further research is warranted to assess the long-term impact on public health and safety of deposition of Gd in the brain.
The US Food and Drug Administration (FDA) and the National Institutes of Health (NIH) have recommended careful consideration about the indication of GBCA, by “limiting GBCA use to clinical circumstances in which the additional information provided by the contrast is necessary” [95], and suggested the preferential use of macrocyclic agents [96]. In January 2017, the European Medicines Agency (EMA) has announced that its Pharmacovigilance Risk Assessment Committee (PRAC) will continue to evaluate the risk of Gd deposition, and that, once PRAC’s recommendations will be issued, the agency’s Committee for Medicinal Products for Human Use will adopt a definite position. The European Commission will then complete the review process by adopting a legally binding decision applicable in all European Union member states [97]. Regarding the presumed GDD, the Drug Safety Communication of the US-FDA declared that, despite patient’s self-reports, to date there are no discernable clinical features reasonably linked to GBCA administration [95].
Thus, several clinical questions remain open [98]: does Gd retention affect the function of the tissues where it is deposited and lead to clinical consequences? Does GDD exist and, eventually, is it dose- or GBCA- dependent? Is the Gd deposition in the brain one manifestation of a more complex Gd deposition syndrome that may also encompass NSF? What is the role of immune system components and genetics in determining different symptoms?
For all these reasons, a systematic scientific approach is necessary to this delicate matter, which has manifold medical and legal implications [93]. Until additional information is obtained, radiologists and clinicians should work together to monitor the development of NSF-like disease or toxicity symptoms allegedly related to GBCA administration in patients with normal renal function, without however scaremongering patients undergoing a CE-MRI scan. In such cases, a 24-h urine testing may be useful for confirming the presence of Gd > 30 days after the most recent GBCA administration [84].
Conclusions: what we can do
Some final considerations and practical suggestions may be summarized from this review of the available literature about Gd retention, while waiting for official guidelines and consensus statements developed by major national/international scientific radiological societies or from an international strategy of cooperation, such as the recently established International Gadolinium Retention Evaluation Consortium, that involves several worldwide scientists [98].
First, in patients referred for a CE-MRI, we should try to obtain all information on possible previous GBCA administrations and evaluate the need of contrast administration even more critically than ever, especially in pediatric cases. In this respect, the idea of creating and updating an individual GBCA administration passport is warranted [98].
Second, the preferential use of macrocyclic GBCAs, due to their higher stability over the linear types, is recommended. This is especially true in children and in subjects for whom multiple studies are anticipated (e.g., patients with Crohn disease or MS) [84]. However, if macrocyclic GBCAs are unavailable and a CE-MRI is clinically indicated, we believe that linear GBCA may be administered, since all GBCAs provide essential radiological information with exceedingly positive risk/benefit ratio for the diagnostic challenge of individual patients.
Third, the presence of T1w hyperintensity in the DN (or in any other brain region) should always be described in our reports, and may prompt careful questioning of the patient about the history of prior Gd administration. However, interpretation errors should be avoided [99], and other possible causes of spontaneous T1w hyperintensity should always be considered (Fig. 2).
Fourth, it should be borne in our mind that, despite some alleged symptoms (as discussed above), at the time of this writing there is no known disease associated with Gd deposition in the brain, and several millions of patients with normal renal function have received GBCA without incurring in any related health problems. Therefore, we should not deny the patients a sure benefit in fear of a possible harm.
As radiologists we are given a delicate role in providing our patients balanced information on a largely unknown situation. A truly informed consent must be obtained from the patient or parent before GBCA administration, clearly explaining them the potential risk that Gd may be deposited in their body, with still unknown (but possibly unremarkable) clinical consequences. On the other hand, we should make clear that the diagnostic accuracy of many MR exams might be reduced if GBCA is not administered, with direct effects on the clinical management of the patients.
References
Sherry AD, Caravan P, Lenkinski RE (2009) Primer on gadolinium chemistry. J Magn Reson Imaging 30(6):1240–1248. doi:10.1002/jmri.21966
Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M (2012) MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging 36(5):1060–1071. doi:10.1002/jmri.23725
Kanal E (2016) Gadolinium based contrast agents (GBCA): safety overview after 3 decades of clinical experience. Magn Reson Imaging 34(10):1341–1345. doi:10.1016/j.mri.2016.08.017
Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH, Cho SH (2012) Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 264(2):414–422. doi:10.1148/radiol.12112025
Bruder O, Schneider S, Pilz G, van Rossum AC, Schwitter J, Nothnagel D, Lombardi M, Buss S, Wagner A, Petersen S, Greulich S, Jensen C, Nagel E, Sechtem U, Mahrholdt H (2015) 2015 Update on acute adverse reactions to gadolinium based contrast agents in cardiovascular MR. Large multi-national and multi-ethnical population experience with 37788 patients from the EuroCMR registry. J Cardiovasc Magn Reson 17:58. doi:10.1186/s12968-015-0168-3
Aime S, Caravan P (2009) Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 30(6):1259–1267. doi:10.1002/jmri.21969
Rogosnitzky M, Branch S (2016) Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 29(3):365–376. doi:10.1007/s10534-016-9931-7
Port M, Idee JM, Medina C, Robic C, Sabatou M, Corot C (2008) Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals 21(4):469–490. doi:10.1007/s10534-008-9135-x
Thomsen HS, Morcos SK, Almen T, Bellin MF, Bertolotto M, Bongartz G, Clement O, Leander P, Heinz-Peer G, Reimer P, Stacul F, van der Molen A, Webb JA (2013) Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR contrast medium safety committee guidelines. Eur Radiol 23(2):307–318. doi:10.1007/s00330-012-2597-9
Kanda T, Nakai Y, Oba H, Toyoda K, Kitajima K, Furui S (2016) Gadolinium deposition in the brain. Magn Reson Imaging 34(10):1346–1350. doi:10.1016/j.mri.2016.08.024
Tien RD, Brasch RC, Jackson DE, Dillon WP (1989) Cerebral Erdheim-Chester disease: persistent enhancement with Gd-DTPA on MR images. Radiology 172(3):791–792. doi:10.1148/radiology.172.3.2772189
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270(3):834–841. doi:10.1148/radiol.13131669
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 49(10):685–690. doi:10.1097/RLI.0000000000000072
Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A, Zobel BB (2015) Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol 50(7):470–472. doi:10.1097/RLI.0000000000000154
Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, Takeshita K, Furui S (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275(3):803–809. doi:10.1148/radiol.14140364
Radbruch A, Haase R, Kieslich PJ, Weberling LD, Kickingereder P, Wick W, Schlemmer HP, Bendszus M (2017) No signal intensity increase in the dentate nucleus on unenhanced T1-weighted MR images after more than 20 serial injections of macrocyclic gadolinium-based contrast agents. Radiology 282(3):699–707. doi:10.1148/radiol.2016162241
Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P, Heiland S, Wick W, Schlemmer HP, Bendszus M (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275(3):783–791. doi:10.1148/radiol.2015150337
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, Schlemmer HP, Bendszus M (2015) High-Signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Investig Radiol 50(12):805–810. doi:10.1097/RLI.0000000000000227
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, Schlemmer HP, Bendszus M (2016) Intraindividual analysis of signal intensity changes in the dentate nucleus after consecutive serial applications of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol 51(11):683–690. doi:10.1097/RLI.0000000000000308
Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, Radbruch A (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol 50(11):743–748. doi:10.1097/RLI.0000000000000206
Kanal E, Tweedle MF (2015) Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 275(3):630–634. doi:10.1148/radiol.2015150805
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, Semelka RC (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR Images: evaluation of two linear gadolinium-based contrast agents. Radiology 276(3):836–844. doi:10.1148/radiol.2015150872
Ramalho J, Semelka RC, AlObaidy M, Ramalho M, Nunes RH, Castillo M (2016) Signal intensity change on unenhanced T1-weighted images in dentate nucleus following gadobenate dimeglumine in patients with and without previous multiple administrations of gadodiamide. Eur Radiol 26(11):4080–4088. doi:10.1007/s00330-016-4269-7
Huckle JE, Altun E, Jay M, Semelka RC (2016) Gadolinium deposition in humans: when did we learn that gadolinium was deposited in vivo? Invest Radiol 51(4):236–240. doi:10.1097/RLI.0000000000000228
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S (2016) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1 W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 26(3):807–815. doi:10.1007/s00330-015-3879-9
Cao Y, Huang DQ, Shih G, Prince MR (2016) Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 206(2):414–419. doi:10.2214/AJR.15.15327
Kromrey ML, Liedtke KR, Ittermann T, Langner S, Kirsch M, Weitschies W, Kuhn JP (2017) Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol 27(2):772–777. doi:10.1007/s00330-016-4418-z
Schlemm L, Chien C, Bellmann-Strobl J, Dorr J, Wuerfel J, Brandt AU, Paul F, Scheel M (2016) Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler. doi:10.1177/1352458516670738
Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H (2016) Signal increase on unenhanced T1-weighted images in the rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Invest Radiol 51(2):83–89. doi:10.1097/RLI.0000000000000242
Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM (2015) Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol 36(10):1859–1865. doi:10.3174/ajnr.A4378
Bae S, Lee HJ, Han K, Park YW, Choi YS, Ahn SS, Kim J, Lee SK (2017) Gadolinium deposition in the brain: association with various GBCAs using a generalized additive model. Eur Radiol. doi:10.1007/s00330-016-4724-5
Flood TF, Stence NV, Maloney JA, Mirsky DM (2017) Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology 282(1):222–228. doi:10.1148/radiol.2016160356
Hu HH, Pokorney A, Towbin RB, Miller JH (2016) Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol 46(11):1590–1598. doi:10.1007/s00247-016-3646-3
Oner AY, Barutcu B, Aykol S, Tali ET (2016) Intrathecal contrast-enhanced magnetic resonance imaging-related brain signal changes: residual gadolinium deposition? Invest Radiol. doi:10.1097/RLI.0000000000000327
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38(3):331–336. doi:10.1016/j.braindev.2015.08.009
Zhang Y, Cao Y, Shih GL, Hecht EM, Prince MR (2017) Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology 282(2):516–525. doi:10.1148/radiol.2016152864
Popescu BF, Robinson CA, Rajput A, Rajput AH, Harder SL, Nichol H (2009) Iron, copper, and zinc distribution of the cerebellum. Cerebellum 8(2):74–79. doi:10.1007/s12311-008-0091-3
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13(10):1045–1060. doi:10.1016/S1474-4422(14)70117-6
Zheng W (2001) Toxicology of choroid plexus: special reference to metal-induced neurotoxicities. Microsc Res Tech 52(1):89–103. doi:10.1002/1097-0029(20010101)52:1<89:AID-JEMT11>3.0.CO;2-2
Rasschaert M, Idee JM, Robert P, Fretellier N, Vives V, Violas X, Ballet S, Corot C (2016) Moderate renal failure accentuates T1 signal enhancement in the deep cerebellar nuclei of gadodiamide-treated rats. Invest Radiol. doi:10.1097/RLI.0000000000000339
Kanda T, Oba H, Toyoda K, Kitajima K, Furui S (2016) Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol 34(1):3–9. doi:10.1007/s11604-015-0503-5
Khant ZA, Hirai T, Kadota Y, Masuda R, Yano T, Azuma M, Suzuki Y, Tashiro K (2017) T1 shortening in the cerebral cortex after multiple administrations of gadolinium-based contrast agents. Magn Reson Med Sci 16(1):84–86. doi:10.2463/mrms.mp.2016-0054
Eisele P, Alonso A, Szabo K, Ebert A, Ong M, Schoenberg SO, Gass A (2016) Lack of increased signal intensity in the dentate nucleus after repeated administration of a macrocyclic contrast agent in multiple sclerosis: an observational study. Medicine (Baltimore) 95(39):e4624. doi:10.1097/MD.0000000000004624
Tedeschi E, Cocozza S, Borrelli P, Ugga L, Morra VB, Palma G (2017) Longitudinal assessment of Dentate Nucleus relaxometry during massive exposure to gadobutrol. Magn Reson Med Sci. doi:10.2463/mrms.cr.2016-0137
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275(3):772–782. doi:10.1148/radiol.15150025
Jost G, Frenzel T, Lohrke J, Lenhard DC, Naganawa S, Pietsch H (2016) Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue. Eur Radiol. doi:10.1007/s00330-016-4654-2
Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M (2016) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 37(7):1192–1198. doi:10.3174/ajnr.A4615
Tanaka M, Nakahara K, Kinoshita M (2016) Increased signal intensity in the dentate nucleus of patients with multiple sclerosis in comparison with neuromyelitis optica spectrum disorder after multiple doses of gadolinium contrast. Eur Neurol 75(3–4):195–198. doi:10.1159/000445431
Ramalho J, Ramalho M, AlObaidy M, Semelka RC (2016) Technical aspects of MRI signal change quantification after gadolinium-based contrast agent’s administration. Magn Reson Imaging 34(10):1355–1358. doi:10.1016/j.mri.2016.09.004
Tedeschi E, Palma G, Canna A, Cocozza S, Russo C, Borrelli P, Lanzillo R, Angelini V, Postiglione E, Morra VB, Salvatore M, Brunetti A, Quarantelli M (2016) In vivo dentate nucleus MRI relaxometry correlates with previous administration of Gadolinium-based contrast agents. Eur Radiol 26(12):4577–4584. doi:10.1007/s00330-016-4245-2
Palma G, Tedeschi E, Borrelli P, Cocozza S, Russo C, Liu S, Ye Y, Comerci M, Alfano B, Salvatore M, Haacke EM, Mancini M (2015) A novel multiparametric approach to 3d quantitative mri of the brain. PLoS One 10(8):e0134963. doi:10.1371/journal.pone.0134963
Borrelli P, Palma G, Tedeschi E, Cocozza S, Comerci M, Alfano B, Haacke EM, Salvatore M (2015) Improving signal-to-noise ratio in susceptibility weighted imaging: a novel multicomponent non-local approach. PLoS One 10(6):e0126835. doi:10.1371/journal.pone.0126835
Cheng HL, Stikov N, Ghugre NR, Wright GA (2012) Practical medical applications of quantitative MR relaxometry. J Magn Reson Imaging 36(4):805–824. doi:10.1002/jmri.23718
Kuno H, Jara H, Buch K, Qureshi MM, Chapman MN, Sakai O (2017) Global and regional brain assessment with quantitative mr imaging in patients with prior exposure to linear gadolinium-based contrast Agents. Radiology 283:195–204. doi:10.1148/radiol.2016160674
Hinoda T, Fushimi Y, Okada T, Arakawa Y, Liu C, Yamamoto A, Yoshida K, Miyamoto S, Togashi K (2016) Quantitative assessment of gadolinium deposition in dentate nucleus using quantitative susceptibility mapping. J Magn Reson Imaging. doi:10.1002/jmri.25490
Ginat DT, Meyers SP (2012) Intracranial lesions with high signal intensity on T1-weighted MR images: differential diagnosis. Radiographics 32(2):499–516. doi:10.1148/rg.322105761
Chen W, Zhu W, Kovanlikaya I, Kovanlikaya A, Liu T, Wang S, Salustri C, Wang Y (2014) Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping. Radiology 270(2):496–505. doi:10.1148/radiol.13122640
Cocozza S, Russo C, Pontillo G, Ugga L, Macera A, Cervo A, De Liso M, Di Paolo N, Ginocchio MI, Giordano F, Leone G, Rusconi G, Stanzione A, Briganti F, Quarantelli M, Caranci F, D’Amico A, Elefante A, Tedeschi E, Brunetti A (2016) Is advanced neuroimaging for neuroradiologists? A systematic review of the scientific literature of the last decade. Neuroradiology 58(12):1233–1239. doi:10.1007/s00234-016-1761-3
Xia D, Davis RL, Crawford JA, Abraham JL (2010) Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiol 51(10):1126–1136. doi:10.3109/02841851.2010.515614
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276(1):228–232. doi:10.1148/radiol.2015142690
Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol 51(7):447–453. doi:10.1097/RLI.0000000000000252
Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idee JM, Ballet S, Corot C (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol 50(8):473–480. doi:10.1097/RLI.0000000000000181
Robert P, Violas X, Grand S, Lehericy S, Idee JM, Ballet S, Corot C (2016) Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol 51(2):73–82. doi:10.1097/RLI.0000000000000241
Kartamihardja AA, Nakajima T, Kameo S, Koyama H, Tsushima Y (2016) Distribution and clearance of retained gadolinium in the brain: differences between linear and macrocyclic gadolinium based contrast agents in a mouse model. Br J Radiol 89(1066):20160509. doi:10.1259/bjr.20160509
Smith AP, Marino M, Roberts J, Crowder JM, Castle J, Lowery L, Morton C, Hibberd MG, Evans PM (2016) Clearance of gadolinium from the brain with no pathologic effect after repeated administration of gadodiamide in healthy rats: an analytical and histologic study. Radiology. doi:10.1148/radiol.2016160905
Tweedle MF, Wedeking P, Kumar K (1995) Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Invest Radiol 30(6):372–380
Hirano S, Suzuki KT (1996) Exposure, metabolism, and toxicity of rare earths and related compounds. Environ Health Perspect 104(Suppl 1):85–95
Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, Ellen Campbell M, Hauschka PV, Hannigan RE (2009) Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics 1(6):479–488. doi:10.1039/b905145g
Gibby WA, Gibby KA (2004) Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Investig Radiol 39(3):138–142
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol 41(3):272–278. doi:10.1097/01.rli.0000186569.32408.95
Abraham JL, Thakral C, Skov L, Rossen K, Marckmann P (2008) Dermal inorganic gadolinium concentrations: evidence for in vivo transmetallation and long-term persistence in nephrogenic systemic fibrosis. Br J Dermatol 158(2):273–280. doi:10.1111/j.1365-2133.2007.08335.x
Birka M, Wentker KS, Lusmoller E, Arheilger B, Wehe CA, Sperling M, Stadler R, Karst U (2015) Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis. Anal Chem 87(6):3321–3328. doi:10.1021/ac504488k
Christensen KN, Lee CU, Hanley MM, Leung N, Moyer TP, Pittelkow MR (2011) Quantification of gadolinium in fresh skin and serum samples from patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 64(1):91–96. doi:10.1016/j.jaad.2009.12.044
Grobner T (2006) Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21(4):1104–1108. doi:10.1093/ndt/gfk062
Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17(9):2359–2362. doi:10.1681/ASN.2006060601
Sanyal S, Marckmann P, Scherer S, Abraham JL (2011) Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis–an autopsy-based review. Nephrol Dial Transplant 26(11):3616–3626. doi:10.1093/ndt/gfr085
Roberts DR, Lindhorst SM, Welsh CT, Maravilla KR, Herring MN, Braun KA, Thiers BH, Davis WC (2016) High levels of gadolinium deposition in the skin of a patient with normal renal function. Invest Radiol 51(5):280–289. doi:10.1097/RLI.0000000000000266
Boyd AS, Sanyal S, Abraham JL (2008) Gadolinium is not deposited in the skin of patients with normal renal function after exposure to gadolinium-based contrast agents. J Am Acad Dermatol 59(2):356–358. doi:10.1016/j.jaad.2008.01.025
Bussi S, Fouillet X, Morisetti A (2007) Toxicological assessment of gadolinium release from contrast media. Exp Toxicol Pathol 58(5):323–330. doi:10.1016/j.etp.2006.09.003
Sieber MA, Lengsfeld P, Frenzel T, Golfier S, Schmitt-Willich H, Siegmund F, Walter J, Weinmann HJ, Pietsch H (2008) Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 18(10):2164–2173. doi:10.1007/s00330-008-0977-y
Wang YX, Schroeder J, Siegmund H, Idee JM, Fretellier N, Jestin-Mayer G, Factor C, Deng M, Kang W, Morcos SK (2015) Total gadolinium tissue deposition and skin structural findings following the administration of structurally different gadolinium chelates in healthy and ovariectomized female rats. Quant Imaging Med Surg 5(4):534–545. doi:10.3978/j.issn.2223-4292.2015.05.03
Semelka RC, Commander CW, Jay M, Burke LM, Ramalho M (2016) Presumed gadolinium toxicity in subjects with normal renal function: a report of 4 cases. Invest Radiol 51(10):661–665. doi:10.1097/RLI.0000000000000318
Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M, Ramalho M (2016) Gadolinium deposition disease: initial description of a disease that has been around for a while. Magn Reson Imaging 34(10):1383–1390. doi:10.1016/j.mri.2016.07.016
Semelka RC, Ramalho M, AlObaidy M, Ramalho J (2016) Gadolinium in humans: a family of disorders. AJR Am J Roentgenol 207(2):229–233. doi:10.2214/AJR.15.15842
Burke LM, Ramalho M, AlObaidy M, Chang E, Jay M, Semelka RC (2016) Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn Reson Imaging 34(8):1078–1080. doi:10.1016/j.mri.2016.05.005
Gathings RM, Reddy R, Santa Cruz D, Brodell RT (2015) Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol 151(3):316–319. doi:10.1001/jamadermatol.2014.2660
Williams SGU (2014) Gadolinium toxicity: a survey of the chronic effects of retained gadolinium from contrast MRIs. https://gdtoxicity.files.wordpress.com/2014/09/gd-symptom-survey.pdf. Accessed 31 May 2016
Gadolinium Toxicity website. www.gadoliniumtoxicity.com. Accessed 10 Jan 2017
Arsenault TM, King BF, Marsh JW Jr, Goodman JA, Weaver AL, Wood CP, Ehman RL (1996) Systemic gadolinium toxicity in patients with renal insufficiency and renal failure: retrospective analysis of an initial experience. Mayo Clin Proc 71(12):1150–1154. doi:10.1016/S0025-6196(11)64695-8
Hui FK, Mullins M (2009) Persistence of gadolinium contrast enhancement in CSF: a possible harbinger of gadolinium neurotoxicity? AJNR Am J Neuroradiol 30(1):E1. doi:10.3174/ajnr.A1205
Maramattom BV, Manno EM, Wijdicks EF, Lindell EP (2005) Gadolinium encephalopathy in a patient with renal failure. Neurology 64(7):1276–1278. doi:10.1212/01.WNL.0000156805.45547.6E
Miller JH, Hu HH, Pokorney A, Cornejo P, Towbin R (2015) MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics 136(6):e1637–1640. doi:10.1542/peds.2015-2222
Balint B, Bhatia KP (2016) T1-weighted basal ganglia hyperintensities due to gadolinium deposition—a cautionary note. Parkinsonism Relat Disord 32:135–136. doi:10.1016/j.parkreldis.2016.09.017
Welk B, McArthur E, Morrow SA, MacDonald P, Hayward J, Leung A, Lum A (2016) Association between gadolinium contrast exposure and the risk of parkinsonism. JAMA 316(1):96–98. doi:10.1001/jama.2016.8096
US Food and Drug Administration FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging (MRI). www.fda.gov/downloads/Drugs/DrugSafety/UCM455390.pdf. Accessed 28 June 2016
Malayeri AA, Brooks KM, Bryant LH, Evers R, Kumar P, Reich DS, Bluemke DA (2016) National Institutes of health perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol 13(3):237–241. doi:10.1016/j.jacr.2015.11.009
AuntMinnieEurope.com EMA continues investigation of gadolinium contrast agents. http://www.auntminnieeurope.com/index.aspx?sec=ser&sub=def&pag=dis&ItemID=613908. Accessed 18 Jan 2017
Quattrocchi CC, van der Molen AJ (2017) Gadolinium retention in the body and brain: is It time for an international joint research effort? Radiology 282(1):12–16. doi:10.1148/radiol.2016161626
Caranci F, Tedeschi E, Leone G, Reginelli A, Gatta G, Pinto A, Squillaci E, Briganti F, Brunese L (2015) Errors in neuroradiology. Radiol Med 120(9):795–801. doi:10.1007/s11547-015-0564-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no funding.
Conflict of interest
All authors (Enrico Tedeschi, Ferdinando Caranci, Flavio Giordano, Valentina Angelini, Sirio Cocozza and Arturo Brunetti) declare that they have no conflict of interest.
Informed consent
The MRI scans shown in the figures were retrospectively selected among MRI studies previously performed according to clinical indications, with informed consent obtained from all individual participants.
Ethical statements
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Tedeschi, E., Caranci, F., Giordano, F. et al. Gadolinium retention in the body: what we know and what we can do. Radiol med 122, 589–600 (2017). https://doi.org/10.1007/s11547-017-0757-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-017-0757-3